Abstract
Chlorella vulgaris was isolated from sewerage treatment plant and grown in the presence of sodium bicarbonate as carbon source at 0.25, 0.5 and 1.0 g L− 1 . Highest specific growth rate (0.653 μ d− 1 ) was obtained with 1 g L− 1 bicarbonate followed by 0.5 g L− 1 (0.641 d− 1 ) on 15th day culturing. Total chlorophyll content of microalgae has increased in a dose dependent fashion with bicarbonate addition and maximum level recorded in 1 g L− 1 (0.769 ± 0.09 g L− 1 ). The biomass productivity was in the range of 0.237–0.996 g d− 1 L− 1 . Rate of CO2 fixation and carbon content, in terms of quantity was estimated. Results showed that at 1 g L− 1 sodium bicarbonate concentration, maximum CO2 fixation (0.497 g/dry weight) and carbon content (0.69 g mL− 1 day− 1 ) was found. Biomass concentration was significantly higher (p < 0.05) in cultures (1.54 g L− 1 ) supplemented with 1 g L− 1 bicarbonate whereas there was no much difference in cellular lipid concentration (16 mg mL− 1 ). GC–MS analysis of fatty acids showed highest amounts of palmitic acid, myristic and stearic acid. In summary, the addition of sodium bicarbonate increases cellular abundance, chlorophyll content and to some extent in the case of lipid content in C. vulgaris integrated with CO2 sequestration.
Keywords
Microalgae ; Bicarbonate ; Chlorella ; Biomass ; Lipid production
Introduction
Among the atmospheric pollutants, CO2 contributes significantly to the greenhouse effect. Global warming attributed primarily to the elevated CO2 level in the atmosphere has led to various CO2 mitigation strategies. As chemical reaction based CO2 capturing is relatively costly and energy consuming, it is necessary to develop cost effective and sustainable alternatives. Biological CO2 mitigation leads to the production of biomass energy and is an alternative strategy of CO2 fixation through photosynthesis (Kondili and Kaldellis, 2007 ; de Morais and Costa, 2007 ). Microalgae are more efficient than terrestrial plants in photosynthesis from ambient air and are important for the prevention of increase in atmospheric CO2 concentration. It converts CO2 into biomass energy and thus recycles CO2 (Demirbas, 2004 ). Coupling of CO2 sequestration with algal cultivation reduces the carbon footprint and sustainable environment.
Microalgae have drawn more attention as a promising source for the production of biodiesel because they possess high growth rate and provide lipid fraction for biofuel production. Microalgal growth and biochemical composition are governed by environmental conditions (Guiheneuf et al ., 2008 ; Pal et al ., 2011 ). Sodium bicarbonate has been demonstrated to enhance lipid accumulation in both freshwater and marine microalgae (Gardner et al ., 2012 ; Gardner et al ., 2013 ; White et al ., 2013 ; Peng et al ., 2014 ). Microalgae utilize bicarbonate as the external source of carbon for photosynthesis and derive CO2 via the action of carbonic anhydrase (Dixon et al ., 1987 ; Nimer et al ., 1997 ; Bozzo et al ., 2000 ). This work established the growth of Chlorella vulgaris in a medium where sodium bicarbonate serves as a source of inorganic carbon by assessing growth rate, chlorophyll content, biomass and lipid production in batch cultures. Further, rates of inorganic carbon concentration and CO2 fixation were determined for CO2 sequestration using bicarbonate.
Materials and Method
Sample Collection and Identification
Algal samples were collected from Bangalore Water Supply and Sewerage Board (BWSSB), Bengaluru (13°04′N, 77°58′E) and poured into a closed 250 mL bottle and exposed in sunlight for 3 weeks. The upper layer of the water was inoculated in agar plates enriched with BG11 medium containing 200 μg mL− 1 ampicillin to control the growth of bacteria as the sample used was sewage water. Agar plating technique was used to isolate the microalgae and the plates were incubated at 25 ± 2 °C under cool white fluorescent light (40 μmol photons m− 2 s− 1 ; 15 h light/9 h dark) until algal growth was detected. The isolates were purified by streak plating and individual colonies were diluted in distilled water. Species of single cells were obtained using capillary pipette under a microscope followed by inoculation into fresh media. After appropriate growth, cells were observed to confirm the single culture and the capillary method was repeated as many times as required to obtain axenic cultures. Identification of Chlorella was using standard protocols as described by Anderson (2005) , Stanier et al. (1971) and the database http://web.biosci.utexas.edu/utex/default .
Growth Under Different Concentrations of Sodium Bicarbonate
Analytical grade sodium bicarbonate was used as the source of bicarbonate in all experiments. Batch cultures (100 mL) of C. vulgaris (BG 11 medium; n = 3) were grown under different levels of bicarbonate supplementation (0.25, 0.5 and 1 g L− 1 ) into early stationary growth phase (10–15 days), where samples were taken for growth rate, biomass productivity, chlorophyll content and cellular lipid analyses. Media without the addition of bicarbonate were served as control.
Analytical Methods
Biomass Concentration and Productivity
Biomass concentration (g L− 1 ) of C. vulgaris grown under different bicarbonate concentrations was determined by measuring the optical density (OD680 ) using UV–Vis spectrophotometer. The result was converted to biomass concentration using the calibration curve relating OD680 (Xia et al., 2014 ) using the following Eq. (1)
|
|
( 1) |
The biomass productivity (mg L− 1 d− 1 ) was calculated according to Eq. (2) .
|
|
( 2) |
where B2 and B1 represent the dry weight biomass densities at the time T (days), at the end and start of the experiment, respectively.
Specific Growth Rate
Specific growth rate (μ) of the microalgae was calculated (Guillard and Ryther, 1962 ) according to the following formula.
|
|
where, Nt and N0 are the total cells at the end of log phase (Tt ) and start of log phase (T0 ), respectively.
Chlorophyll Estimation
Chlorophyll a and b of microalgae were estimated according to Mackinney (1941) . Algal suspension was filtered and extracted with methanol in water bath at 60 °C for 30 min. The suspension was cooled, added equal volume of 96% methanol and centrifuged for 6500 g for 10 min. Pigment content of the supernatant was analyzed in a UV–Vis spectrophotometer at 650 nm and 665 nm using 96% methanol as blank and the total chlorophyll was determined using Eq. (3)
|
|
( 3) |
where, E650 and E665 are the absorbance at 650 and 665 nm wavelengths respectively.
Estimation of Carbon Content and Carbon Dioxide Fixation Rate
Carbon content of the dried algal biomass was estimated by Walkley and Black (1934) . Dried algal sample was mixed with 1 N potassium dichromate (10 mL) and conc. H2 SO4 (20 mL) mixture diluted with distilled water (200 mL) followed by addition of H3 PO4 (10 mL) and diphenyl amine (1 mL). The reaction mixture was titrated against 4 N ferrous ammonium sulfate (FAS) until the appearance of brilliant green color and the carbon content was estimated using Eq. (4) .
|
|
( 4) |
where, a is the carbon content, g is the weight of algal sample, T and S are the ferrous ammonium sulfate with blank and sample (mL), respectively.
The amount of carbon dioxide fixation rate was estimated using Eq. (5) . (Yun et al., 1997 )
|
|
( 5) |
where, RCO2 and μL are the CO2 fixation rate (g CO2 m− 3 h− 1 ) and the volumetric growth rate (g dry weight m− 3 h− 1 ), respectively, CC is the average carbon content (algal dry weight [g]). MCO2 and MC are the molecular weights of CO2 and elemental carbon, respectively.
Lipid Extraction
Algal cultures were pelleted and added with 10 mL of ice cold 0.2 N HClO4 . After 15 min at 4 °C, the sample was centrifuged and the supernatant was added with 10 mL of 0.2 N HClO4 followed by centrifugation. To the pellet, 10 mL of chloroform–methanol (2:1) solution was added, centrifuged and 0.2 volumes of distilled water were added to the supernatant. The mixture was centrifuged and the lower organic phase was collected, evaporated under a stream of nitrogen to a final volume of 2 mL (Folch et al., 1956 ).
Lipid Estimation
Standard solutions of palmitic acid (5, 10, 20 and 30 mg mL− 1 ) and algal lipid samples were prepared in chloroform, added with 2 mL of dichromate solution (2.5 g of K2 Cr2 O7 in 1 L of conc. H2 SO4 ) and placed in a boiling water bath for 45 min. The mixture was cooled, removed 1 mL from each and diluted to 100 mL with distilled water followed by measuring the absorbance at 350 nm (Marsh and Weinstein, 1966 ).
Fatty Acid Methyl Ester Preparation
The lipid extract was saponified with 5 mL of 0.5 N methanolic solution of potassium hydroxide by boiling for 3–5 min (Hartman and Lago, 1973 ). To this, 15 mL of esterification reagent (2 g of ammonium chloride in 60 mL of methanol and 3 mL of conc. H2 SO4 ) was added and refluxed for 3 min. Subsequently, it was transferred to a separating funnel using 25 mL of diethyl ether and 50 mL of saturated sodium chloride solution and the aqueous layer discarded. The diethyl ether layer was washed twice with 25 mL of NaCl solution and the solvent was evaporated under a stream of nitrogen for GC–MS analysis.
GC–MS Analysis
GC–MS analysis of lipid extract was performed using an Agilent GC comprising a Thermo DSQII auto-sampler equipped with a DB 5 MS column (30 m × 0.25 mm ID × 0.25 μm). For GC–MS detection, an electron ionization system was operated in electron impact mode with ionization energy of 70 eV. Helium gas (99.999%) was used as a carrier gas at a constant flow rate of 1.0 mL min− 1 and an injection volume of 1 μL was employed in a split mode (1:10). The initial temperature was 40 °C with a hold time of 2 min and the ramp was 310 °C at a rate of 10 °C/min for a hold period of 10 min. Mass spectra were taken at a scan mass range of 30–600 m/z. The solvent delay was 0 to 2 min and the total GC/MS running time was 32 min. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. Quadrupole mass analyzer along with Xcalibur and AMDIS software was adopted to handle mass spectra and chromatograms were compared with NIST 2011.
Statistical Analysis
All the experiments were carried out in triplicates and the results were expressed as mean values and the standard deviation (SD). The statistical differences were obtained through one-way analysis of variance (ANOVA) (p < 0.05).
Results and Discussion
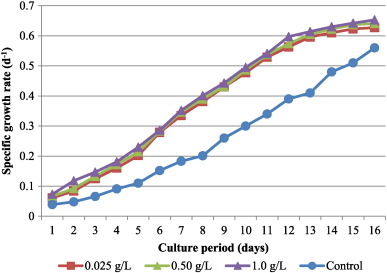
The effect of bicarbonate concentration on specific growth rate (μ) of C. vulgaris is shown in Fig. 1 . Specific growth rate was not significantly different within the concentrations of NaHCO3 . It showed a similar trend in growth rate with 1 g L− 1 bicarbonate maintaining the highest specific growth rate (0.653 d− 1 ) at the end of 15 days culture period followed by 0.5 g L− 1 (0.641 d− 1 ).
|
|
|
Fig. 1. Specific growth rate of C. vulgaris culture supplemented with different levels of bicarbonate. |
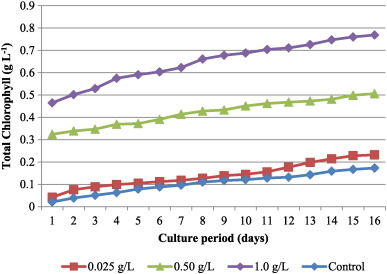
Significant positive effect on the levels of chlorophyll content with an approximate doubling or more with the addition of 1 g L− 1 bicarbonate when compared to treatments received 0.25 g L− 1 and 0.5 g L− 1 (Fig. 2 ) was observed. At the same time, both Chl a and b were significantly varied between lower concentrations of bicarbonate. The total chlorophyll content increased in a dose dependent fashion with bicarbonate addition and maximum level recorded in 1 g L− 1 (0.769 ± 0.09 g L− 1 ).
|
|
|
Fig. 2. Chlorophyll content of C. vulgaris culture supplemented with different levels of bicarbonate. |
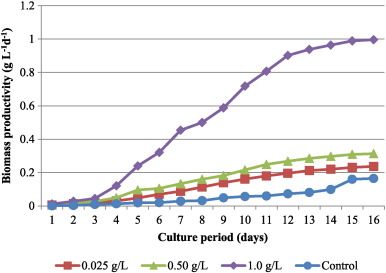
The first equation gives the biomass concentration of the culture. In the 2nd equation, B1 is the early stationary phase and B2 is the end of stationary phase. Stationary phase was considered rather than exponential, as the data is valid at the end of stationary phase in terms of biomass productivity. The addition of bicarbonate had significant effects on the biomass productivity in C. vulgaris ranged from 0.237–0.996 g d− 1 L− 1 . As depicted in Fig. 3 , biomass productivity at 1 g L− 1 (0.996 ± 0.06 g d− 1 L− 1 ) was significantly higher (p < 0.05) than those cultures with lower bicarbonate addition. Reduced biomass productivity was observed with lower concentrations of sodium bi carbonate throughout the culturing period.
|
|
|
Fig. 3. Biomass productivity of C. vulgaris culture supplemented with different levels of bicarbonate. |
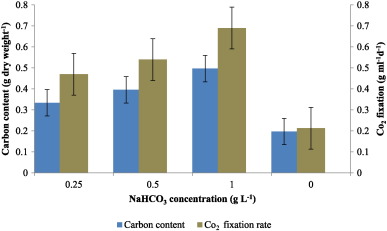
Carbon content of the dried algal biomass was estimated and increasing carbon content enhanced the CO2 fixation. At 1 g L− 1 sodium bicarbonate concentration, carbon content was maximum (0.497 g dw− 1 ) and CO2 fixation was 0.69 g mL− 1 day− 1 . The lowest level of CO2 fixation was observed in 0.25 g L− 1 bicarbonate addition (Fig. 4 ).
|
|
|
Fig. 4. Carbon content and CO2 fixation rate in C. vulgaris supplemented with different levels of bicarbonate. |
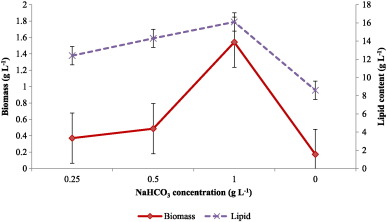
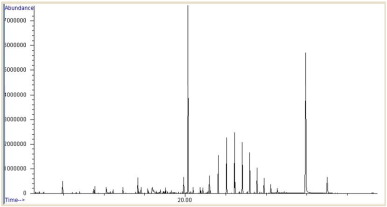
The results of biomass concentration and lipid production at the end of lag phase are compared in Fig. 5 . At 1 g L− 1 of sodium bicarbonate levels, biomass concentration was significantly higher (1.542 g L− 1 ) which was triple the amount obtained at 0.5 g L− 1 bicarbonate addition. However, there was no much difference in lipid production at varying bicarbonate concentrations. Cellular lipid concentration was determined and the total cellular lipid was highest in cultures supplemented with 1 g L− 1 bicarbonate (16.1 ± 0.07 mg mL− 1 ) compared to cultures with 0.5 and 0.25 g L− 1 bicarbonate (14.3 ± 0.14 and 12.4 ± 0.21 mg mL− 1 ). Based on highest total lipid production, fatty acid profiles in C. vulgaris grown under 1.0 g L− 1 bicarbonate addition were determined post-conversion to fatty acid methyl esters by GC–MS (Fig. 6 ). Sodium bicarbonate has promoted the fatty acid profiles significantly and the key fatty acids were palmitic, myristic and stearic acid alongside several minor fatty acid components with a carbon number of C16 and C18.
|
|
|
Fig. 5. Biomass and cellular lipid production in C. vulgaris supplemented with different levels of bicarbonate. |
|
|
|
Fig. 6. GC–MS profile of fatty acid methyl esters from C. vulgaris at 1.0 g L− 1 bicarbonate concentration. |
Cultivation conditions can maximize the algal biomass production rates and lipid concentrations. Carbon limitations due to the poor solubility of CO2 in water can lead to growth inhibition (Smith and Bidwell, 1989 ; Giordano et al ., 2005 ; Aishvarya et al ., 2012 ). NaHCO3 has greater solubility than CO2 and could be an alternative inorganic carbon source (Hsueh et al., 2007 ) for microalgal cultivation. Further, gaseous CO2 is costly to store and transport, whereas bicarbonate salts can easily be transported to algal facilities and stored until needed. Enhanced inorganic carbon uptake due to the addition of bicarbonate allows cellular material production and thereby achieving maximum productivity. In this study, maximum biomass of 0.996 g L− 1 d− 1 was observed in 1 g L− 1 sodium bicarbonate addition and this is in agreement with Yeh et al. (2010) who have demonstrated that 1 g L− 1 sodium bicarbonate was found optimum for biomass production in C. vulgaris . In another study, 1 M NaHCO3 has produced higher biomass concentration (Chi et al., 2013 ).
Conventionally, chlorophyll is considered as a reliable and standard algal biomass measurement (Wiltshire et al ., 1998 ; Knefelkamp et al ., 2007 ). Physiological state of microalgae is indicated by carbon to chlorophyll ratio and in this study total chlorophyll content was taken as the indicator of physiology of C. vulgaris influenced by inorganic carbon (sodium bicarbonate). Total chlorophyll content of 0.769 g L− 1 was determined in cultures grown in the highest concentration of bicarbonate and was lower in decreasing concentrations. Higher chlorophyll biosynthesis was observed in the presence of sodium bicarbonate at 1 g L− 1 in C. vulgaris ( Kong et al., 2011 ) where as it was 0.005 M in Chlorella salina ( Jayasankar and Valsala, 2008 ). Growth rate of microalgal population is another measure of increase in biomass and increase in specific growth rate was observed at 1 g L− 1 over time. The duration of exponential phase in terms of specific growth rate was longer which explains impact of sodium bicarbonate in the culture medium. It was clear that higher photosynthetic efficiency due to increase in chlorophyll content indicates sodium bicarbonate would promote the growth rate as well as biomass production. Biofixation of CO2 using microalgae and converting it into biomass by way of photosynthesis is a promising way to sequester CO2 ( Watanabe and Hall, 1996 ; Ono and Cuello, 2006 ; Ryu et al ., 2009 ). High CO2 tolerant Chlorella sp. with good growth rate was reported ( Sung et al ., 1998 ; Sung et al ., 1999 ; Yue and Chen, 2005 ).The amount of carbon content and CO2 fixation rates were determined in C. vulgaris and carbon content in the range of 0.334–0.497 g dry weight− 1 was obtained. Similar carbon content in C. vulgaris was reported in earlier studies ( Yun et al ., 1997 ; Pandian and Ravindran, 2012 ). It was observed that at higher bicarbonate levels, increased carbon sequestration was found.
Bicarbonate induces carbon storage metabolic activity and triggers lipid accumulation (Gardner et al ., 2012 ; Gardner et al ., 2013 ; White et al ., 2013 ). Higher neutral lipid production in microalgae with the addition of bicarbonate was reported by Bywaters and Fritsen (2015) . Sodium bicarbonate has been used as a carbon source for the study of growth and biochemical composition in a range of microalgae species (Guiheneuf et al ., 2008 ; Guiheneuf et al ., 2009 ; Sostaric et al ., 2009 ; Pimolrat et al ., 2010 ; Yeh et al ., 2010 ) and has been shown to stimulate triacylglycerol accumulation in microalgal species. The length of carbon chain and the number of double bonds in fatty acids affect its fuel properties in which C16:1 and C18:1 are the ideal biodiesel feedstock (Knothe, 2008 ; Stansell et al ., 2012 ). One of the main objectives of this study is to enhance lipid content of microalgae for biodiesel production hence the lipid class which are suitable for biodiesel production was considered. In this study, triglycerides with carbon chain lengths of C14–C17 were obtained revealing the potential of bicarbonate addition in microalgal cultivation for biofuel production. It was noted that although increased bicarbonate concentration has increased lipid productivity, the values were still in the lower range of those reported for selected species.
Conclusion
This study has the potential to aid industrial algal biofuel production with the use of sodium bicarbonate as an alternative inorganic carbon source to trigger biomass and lipid production. From the results, it was clear that bicarbonate addition had significant effects on pigment, biomass and lipid production in C. vulgaris isolated from sewage treatment plant. Microalgae exposed to 1 g L− 1 concentration of bicarbonate expressed three times higher biomass concentration compared to other treatments. Further, the amount of total chlorophyll content, CO2 fixation, biomass and lipid productivity were significantly influenced by the addition of sodium bicarbonate.
Acknowledgement
The authors are grateful to the Karnataka State Biofuel Development Board and the Karnataka State Council for Science and Technology with Grant No. (38S_B_MSc_015 ) for the financial support.
References
- Aishvarya et al., 2012 V. Aishvarya, N. Pradhan, R.R. Nayak, L.B. Sukla, B.K. Mishra; Enhanced inorganic carbon uptake by Chlorella sp. IMMTCC-2 under autotrophic conditions for lipid production and CO2 sequestration ; J. Appl. Phycol., 24 (2012), pp. 1455–1463
- Anderson, 2005 R.A. Anderson (Ed.), Algal Culturing Techniques, Elsevier Inc., USA (2005)
- Bozzo et al., 2000 G.G. Bozzo, B. Colman, Y. Matsuda; Active transport of CO2 and bicarbonate is induced in response to external CO2 concentration in the green alga Chlorella kessleri; J. Exp. Bot., 51 (2000), pp. 1341–1348
- Bywaters and Fritsen, 2015 K.F. Bywaters, C.H. Fritsen; Biomass and neutral lipid production in geothermal microalgal consortia; Front. Bioeng. Biotechnol., 2 (2015), pp. 1–11
- Chi et al., 2013 Z. Chi, Y. Xie, F. Elloy, Y. Zheng, Y. Hu, S. Chen; Bicarbonate-based integrated carbon capture and algae production system with alkalihalophilic cyanobacterium; Bioresour. Technol., 1333 (2013), pp. 513–521
- de Morais and Costa, 2007 M.G. de Morais, J.A.V. Costa; Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide; Energy Convers. Manag., 48 (2007), pp. 2169–2173
- Demirbas, 2004 A. Demirbas; Current technologies for the thermo conversion of biomass into fuels and chemicals; Energy Sources, 26 (2004), pp. 715–730
- Dixon et al., 1987 G.K. Dixon, B.N. Patel, M.J. Merrett; Role of intracellular carbonic anhydrase in inorganic-carbon assimilation by Porphiridium purpureum; Planta, 172 (1987), pp. 508–513
- Folch et al., 1956 J. Folch, M. Lees, G.H.S. Stanley; A simple method for the isolation and purification of total lipids from animal tissues; J. Biol. Chem., 226 (1956), pp. 497–509
- Gardner et al., 2012 R.D. Gardner, K.E. Cooksey, F. Mus, R. Macur, K. Moll, E. Eustance, R.P. Carlson, R. Gerlach, M.W. Fields, B.M. Peyton; Use of sodium bicarbonate to stimulate triacylglycerol accumulation in the chlorophyte Scenedesmus sp. and the diatom Phaeodactylum tricornutum; J. Appl. Phycol., 24 (2012), pp. 1311–1320
- Gardner et al., 2013 R.D. Gardner, E. Lohman, R. Gerlach, K.E. Cooksey, B.M. Peyton; Comparison of CO2 and bicarbonate as inorganic carbon sources for triacylglycerol and starch accumulation in Chlamydomonas reinhardtii; Biotechnol. Bioeng., 110 (2013), pp. 87–96
- Giordano et al., 2005 M. Giordano, J. Beardall, J.A. Raven; CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution ; Annu. Rev. Plant Biol., 56 (2005), pp. 99–131
- Guiheneuf et al., 2008 F. Guiheneuf, V. Mimouni, L. Ulmann, G. Tremblin; Environmental factors affecting growth and omega 3 fatty acid composition in Skeletonema costatum . The influences of irradiance and carbon source ; Diatom Res., 23 (2008), pp. 93–103
- Guiheneuf et al., 2009 F. Guiheneuf, V. Mimouni, L. Ulmann, G. Tremblin; Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture ; J. Exp. Mar. Biol. Ecol., 369 (2009), pp. 136–143
- Guillard and Ryther, 1962 R.R.L. Guillard, J.H. Ryther; Studies on marine planktonic diatoms I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran ; Can. J. Microbiol., 8 (1962), pp. 229–239
- Hartman and Lago, 1973 L. Hartman, R.C.A. Lago; Rapid preparation of fatty acid methyl esters from lipids; Lab. Pract., 22 (1973), pp. 475–476
- Hsueh et al., 2007 H.T. Hsueh, H. Chu, S.T. Yu; A batch study on the biofixation of carbon dioxide in the absorbed solution from a chemical wet scrubber by hot spring and marine algae; Chemosphere, 66 (2007), pp. 878–886
- Jayasankar and Valsala, 2008 R. Jayasankar, K.K. Valsala; Influence of different concentrations of sodium bicarbonate on growth rate and chlorophyll content of Chlorella salina; J. Mar. Biol. Assoc. India, 50 (2008), pp. 74–78
- Knefelkamp et al., 2007 B. Knefelkamp, K. Carstens, K.H. Wiltshire; Comparison of different filter types on chlorophyll-a retention and nutrient measurements; J. Exp. Mar. Biol. Ecol., 345 (2007), pp. 61–70
- Knothe, 2008 G. Knothe; “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties; Energy Fuel, 22 (2008), pp. 1358–1364
- Kondili and Kaldellis, 2007 E.M. Kondili, J.K. Kaldellis; Biofuel implementation in East Europe: current status and future prospects; Renew. Sustain. Energy Rev., 11 (2007), pp. 2137–2151
- Kong et al., 2011 W. Kong, H. Song, Y. Cao, H. Yang, A. Hua, C. Xia; The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation ; Afr. J. Biotechnol., 10 (2011), pp. 11620–11630
- Mackinney, 1941 J. Mackinney; Absorption of light by chlorophyll; J. Biol. Chem., 140 (1941), pp. 315–322
- Marsh and Weinstein, 1966 J.B. Marsh, D.B. Weinstein; Simple charring method for determination of lipids; J. Lipid Res., 7 (1966), pp. 574–576
- Nimer et al., 1997 N.A. Nimer, M.D. Iglesias-Rodriguez, M.J. Merrett; Bicarbonate utilization by marine phytoplankton species; J. Phycol., 33 (1997), pp. 625–631
- Ono and Cuello, 2006 E. Ono, J.L. Cuello; Feasibility assessment of microalgal carbon dioxide sequestration technology with photobioreactor and solar collector; Biosyst. Eng., 95 (2006), pp. 597–606
- Pal et al., 2011 D. Pal, I. Khozin-Goldberg, Z. Cohen, S. Boussiba; The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. ; Appl. Microbiol. Biotechnol., 90 (2011), pp. 1429–1441
- Pandian and Ravindran, 2012 P. Pandian, A.D. Ravindran; Lipid extraction and CO2 mitigation by microalgae ; J. Biochem. Technol., 4 (2012), pp. 469–472
- Peng et al., 2014 X. Peng, S. Liu, W. Zhang, Y. Zhao, L. Chen, H. Wang, T. Liu; Triacylglycerol accumulation of Phaeodactylum tricornutum with different supply of inorganic carbon ; J. Appl. Phycol., 26 (2014), pp. 131–139
- Pimolrat et al., 2010 P. Pimolrat, S. Direkbusarakom, C. Chinajariyawong, S. Powtongsook; The effect of sodium bicarbonate concentrations on growth and biochemical composition of Chaetoceros gracilis Schutt ; Kasetsart Univ. Fish. Res. Bull., 34 (2010), pp. 40–47
- Ryu et al., 2009 H.J. Ryu, K.K. Oh, Y.S. Kim; Optimization of the influential factors for the improvement of CO2 utilization efficiency and CO2 mass transfer rate ; J. Ind. Eng. Chem., 15 (2009), pp. 471–475
- Smith and Bidwell, 1989 R.G. Smith, R. Bidwell; Mechanism of photosynthetic carbondioxide uptake by the red macroalga, Chondrus crispus; Plant Physiol., 89 (1989), pp. 93–99
- Sostaric et al., 2009 M. Sostaric, J. Golob, M. Bricelj, D. Klinar, A. Pivec; Studies on the growth of Chlorella vulgaris in culture media with different carbon sources ; Chem. Biochem. Eng. Q., 23 (2009), pp. 471–477
- Stanier et al., 1971 R.Y. Stanier, R. Kunisawa, M. Mandel, G. Cohen-Bazire; Purification and properties of unicellular blue-green algae (order Chroococcales); Bacteriol. Rev., 35 (1971), pp. 171–205
- Stansell et al., 2012 R.G. Stansell, M.G. Gray, D.S. Stuart; Microalgal fatty acid composition: implications for biodiesel quality; J. Appl. Phycol., 24 (2012), pp. 791–801
- Sung et al., 1998 K.D. Sung, J.S. Lee, C.S. Shin, S.C. Park; Isolation of a new highly CO2 tolerant fresh water microalga Chlorella sp. KR-1 ; Korean J. Chem. Eng., 15 (1998), pp. 449–450
- Sung et al., 1999 K.D. Sung, J.S. Lee, C.S. Shin, S.C. Park, M.J. Choi; CO2 fixation by Chlorella sp. KR-1 and its cultural characteristics ; Bioresour. Technol., 68 (1999), pp. 269–273
- Walkley and Black, 1934 A. Walkley, I.A. Black; An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method; Soil Sci., 37 (1934), pp. 29–37
- Watanabe and Hall, 1996 Y. Watanabe, D.O. Hall; Photosynthetic CO2 conversion technologies using a photobioreactor incorporating microalgae - energy and material balances ; Energy Conserv. Manag., 37 (1996), pp. 1321–1326
- White et al., 2013 D.A. White, A. Pagarette, P. Rooks, S.T. Ali; The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures; J. Appl. Phycol., 25 (2013), pp. 153–165
- Wiltshire et al., 1998 K.H. Wiltshire, S. Harsdorf, B. Smidt, G. Blocker, R. Reuter, F. Schroeder; The determination of algal biomass (as chlorophyll) in suspended matter from the Elbe estuary and the German Bight: a comparison of high-performance liquid chromatography, delayed fluorescence and prompt fluorescence methods; J. Exp. Mar. Biol. Ecol., 222 (1998), pp. 113–131
- Xia et al., 2014 L. Xia, J. Rong, H. Yang, Q. He, D. Zhang, C. Hu; NaCl as an effective inducer for lipid accumulation in freshwater microalgae Desmodesmus abundans; Bioresour. Technol., 161 (2014), pp. 402–409
- Yeh et al., 2010 K.L. Yeh, J.S. Chang, W.M. Chen; Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31 ; Eng. Life Sci., 10 (2010), pp. 201–208
- Yue and Chen, 2005 L. Yue, W. Chen; Isolation and determination of cultural characteristics of a new highly CO2 tolerant fresh water microalgae ; Energy Convers. Manag., 46 (2005), pp. 1868–1876
- Yun et al., 1997 Y.S. Yun, B.S. Lee, T.M. Park, C. Lee, J.W. Yang; Carbon dioxide fixation by algal cultivation using waste water nutrients; J. Chem. Technol. Biotechnol., 69 (1997), pp. 451–455
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?