Summary
Background/Objective
Limited animal and human studies have shown function, albeit inadequate, of implanted thyroid tissue in muscles. This work aimed to ascertain results in a larger number of patients, finding practical method for implantation, studying the effect of changing weight of implant and effect of passage of time on its function.
Methods
Forty patients had total thyroidectomy for simple multinodular goiters. A piece of the excised gland was finely minced, mixed with saline as emulsion, and injected in thigh muscles. Twelve patients had 5-g implants, while 28 patients had 10-g implants. Four parameters were studied at 2 months, 6 months, and 12 months: technetium isotope uptake by the implant; thyroid stimulating hormone (TSH); free T3 (FT3); and free T4 (FT4).
Results
All autotransplanted thyroid tissue survived and functioned. After 12 months, mean values (± standard deviation) of isotope uptake, TSH, FT3, and FT4 of the 5-g implants were 0.44 ± 0.16%, 27.74 ± 30.4 UI/mL, 3.07 ± 1.10 pg/mL, and 1.01 ± 0.3 ng/dL, repectively. Those for the 10 g implants were 0.71 ± 0.20%, 22.78 ± 19.7 UI/mL, 3.92 ± 1.2 pg/mL, and 1.05 ± 0.3 ng/dL, repectively. Ten-gram implants showed significantly higher isotope uptake than 5-g. TSH, FT3, and FT4 significantly improved over the period of 1 year.
Conclusion
Injection of thyroid tissue suspension is a simple method for thyroid autotransplantation. TSH was elevated in the majority to maintain normal or near normal thyroid hormones. Ten-gram implants showed higher isotope uptake than 5-g, although this difference was not reflected by thyroid hormone profile. The implant seemed to function better with the passage of time from 2 months to 12 months.
Keywords
multinodular goiter;thyroid autotransplantation;thyroidectomy
1. Introduction
Whenever surgery is indicated for a simple multinodular goiter, the current trend is to do total thyroidectomy.1 ; 2 Inevitably this makes the patient dependent on replacement therapy for life. Although it seems relatively easy to control hypothyroidism by levo-thyroxine, from the patients point of view, a daily dependence on it and regular visits to hospital to check hormone levels are burdensome. Other problems that may interfere with reaching a euthyroid status using replacement therapy are malabsorption3 and noncompliance of patients.4
The clinical application of transplantation in the endocrine field, by autotransplantation of endocrine organs for hormone replacement has already been established in the field of parathyroid surgery. Before applying the same principles on the thyroid gland in humans, studies have been done on animals. Autologous transplantations were found to be successful in 70% of cases and histological examinations showed normal thyroid architecture.5; 6; 7 ; 8 Very few studies have addressed this issue in humans. The number of patients in each study was very small,9; 10; 11 ; 12 the largest study including only 15 patients.12 Furthermore, the study methodology was not consistent.
The aims of the current work were to ascertain these results in a larger number of patients, find a practical method of transplanting sizable thyroid tissue, and study the effects of changing weight of implant and passage of time on its function.
2. Methods
This case series study was approved by the Research Ethics Committee of the Faculty of Medicine at Cairo University, Cairo, Egypt. It was conducted at Cairo University Hospital and included 40 patients with simple multinodular goiters who were indicated for total thyroidectomy because of compression manifestations, and where nodularity extended to both lobes. Children, unwilling patients, and those who had any clinical or ultrasound suspicion of malignancy were excluded. Similarly, those with a family history of thyroid cancer and history of neck irradiation were excluded because they constitute a high risk of developing thyroid cancer. The study included 36 women and four men. Written informed consent was obtained from all patients, stressing the importance of regular follow up. Preoperative evaluation followed the same standard protocol and included a thorough history, examination, thyroid function tests, neck ultrasound, and fine-needle aspiration cytology of a dominant or suspicious nodule.
Total thyroidectomies were performed under general anesthesia. During postexcision hemostasis and closure a member of the operating team performed the autotransplantation preparation at a side table. The healthiest-looking part of the thyroid was chosen. The slightest gross suspicion of malignancy led to termination of the implantation procedure. Intraoperatively, two patients were excluded from the study on gross picture suspicion of malignancy. Neither showed cancer on histological examination of postoperative paraffin sections.
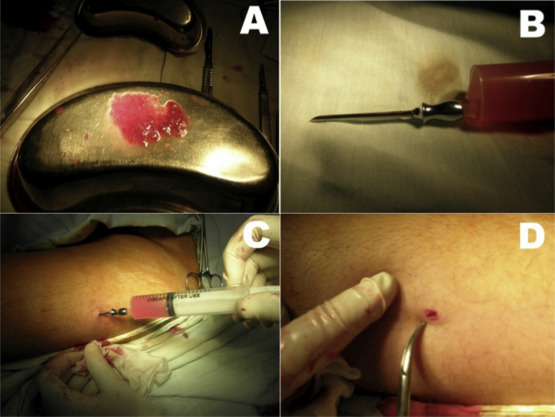
The initial 12 patients received 5-g implants, while the remaining 28 received 10-g implants. The tissues to be transplanted were very finely divided using a pair of scissors and made into an emulsion by adding them to saline in a 20-mL syringe. This was attached to a 2.4 mm-caliber needle. A 3-mm incision was made in the anterolateral aspect of the middle third of the thigh. Through this incision the thyroid tissue emulsion was injected in 8–10 sites in the thigh muscles by changing the direction and depth of needle introduction. Figure 1 shows the steps of this technique.
|
|
|
Figure 1. (A) Finely-minced thyroid tissue. (B) Emulsified thyroid tissue in saline, ready for injection. (C) Injection of the emulsion in the thigh. (D) The 3-mm incision after injection. |
Postoperatively, L-thyroxine replacement was given at a dose of 50 μg/d, pending the take of implanted tissues. Thyroid function tests—free T3 (FT3), free T4 (FT4), thyroid stimulating hormone (TSH), and isotope scan were done at 2 months, 6 months, and 12 months after the operation. Replacement therapy was withheld 3 weeks prior to each occasion in order to strictly assess function of the implanted tissues. Scans were done by intravenous injection of 5–10 mCi of radioactive technetium (99mTc). Imaging was done 20 minutes later and the uptake ratio by the implant was obtained.
Data showed parametric distribution; therefore Student t test was used for comparisons between the 5- and 10-g groups for isotope uptake by the implant, TSH, FT3, and FT4. Analysis of the significance of the time passage on isotope uptake, TSH, FT3, and FT4 was done using analysis of variance test, as well as a comparison of the different means using paired t test. The significance level was set at p ≤ 0.05.
3. Results
Apart from minor complications, postoperative courses were uneventful. Temporary recurrent laryngeal nerve occurred in one patient and resolved spontaneously in 4 weeks. Temporary hypoparathyroidism occurred in 11 patients and was controlled by oral calcium and vitamin D. The condition resolved within 3–7 weeks. There were no complications related to the autotransplantation site. None of the excised thyroids showed histological evidence of malignancy.
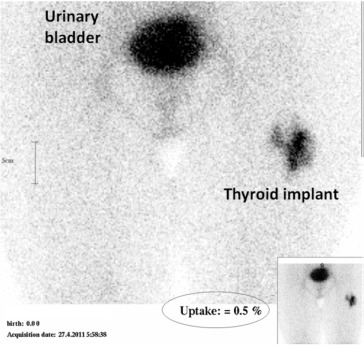
The 5- and 10-g implant patients were statistically matched for age with a mean ± standard deviation of 42.7 ± 12.9 and 37.8 ± 8.8, respectively. In all patients the autotransplanted thyroid tissue showed uptake of 99mTc. The degree of uptake, however, was variable. Figure 2 demonstrates normal isotope uptake of the heterotopically implanted thyroid tissue. Faint isotope uptake in the neck was observed in 18 patients. In all, its mean value was 0.06 ± 0.08%. Table 1 shows 99mTc uptake by the thigh implant. Isotope uptake was significantly higher for the 10-g implants than the 5-g implants at 6 months (p = 0.001) and at 12 months (p = 0.001). Even though uptake tended to increase with passage of time, this did not reach statistical significance.
|
|
|
Figure 2. Normal isotope uptake (0.5%) by the implanted thyroid tissue in the left thigh. |
| 5-g implants (12 patients) | 10-g implants (28 patients) | |||
|---|---|---|---|---|
| Mean ± SD | % Patients achieving normal values | Mean ± SD | % Patients achieving normal values | |
| 2 mo | 0.28 ± 0.16 | 8.3 | 0.44 ± 0.35 | 37 |
| 6 mo | 0.28 ± 0.15 | 16.6 | 0.59 ± 0.27 | 73 |
| 12 mo | 0.44 ± 0.16 | 41.6 | 0.71 ± 0.20 | 88 |
SD = standard deviation.
Table 2 shows TSH levels. There were no statistically significant differences between those with 5- and 10-g implants at 2 months, 6 months, and 12 months. By contrast, TSH levels significantly decreased with time passage for both the 5-g (p = 0.001) and the 10-g implants (p = 0.001).
| 5-g implants (12 patients) | 10-g implants (28 patients) | |||
|---|---|---|---|---|
| Mean ± SD | % Patients achieving normal values | Mean ± SD | % Patients achieving normal values | |
| Preop | 2.26 ± 1.15 | 2.89 ± 1.22 | ||
| 2 mo | 49.56 ± 32 | 0 | 46.0 ± 26.2 | 0 |
| 6 mo | 37.14 ± 29.6 | 0 | 37.04 ± 26.4 | 0 |
| 12 mo | 27.74 ± 30.4 | 16.6 | 22.78 ± 19.7 | 3 |
SD = standard deviation.
Table 3 shows FT3 levels. These levels tended to be higher with the 10-g implants than the 5-g implants, although the difference was statistically significant only at 12 months (p = 0.04). FT3 levels significantly increased by passage of time for the 5-g implants (p = 0.02) and for the 10-g implants (p = 0.01).
| 5-g implants (12 patients) | 10-g implants (28 patients) | |||
|---|---|---|---|---|
| Mean ± SD | % Patients achieving normal values | Mean ± SD | % Patients achieving normal values | |
| Preop | 2.99 ± 0.94 | 3.31 ± 0.8 | ||
| 2 mo | 1.85 ± 1.0 | 58.3 | 2.13 ± 0.6 | 89.2 |
| 6 mo | 2.25 ± 0.9 | 83.3 | 2.61 ± 0.6 | 92.8 |
| 12 mo | 3.07 ± 1.10 | 91.6 | 3.92 ± 1.2 | 100 |
SD = standard deviation.
Table 4 shows FT4 levels. These levels tended to be higher with the 10-g implants than the 5-g implants, although the differences did not reach statistical significance at any time after the procedure. There were, however, significant elevations of FT4 levels with the passage of time for the 5-g implants (p = 0.02) and for the 10-g implants (p = 0.02).
| 5-g implants (12 patients) | 10-g implants (28 patients) | |||
|---|---|---|---|---|
| Mean ± SD | % Patients achieving normal values | Mean ± SD | % Patients achieving normal values | |
| Preop | 1.31 ± 0.37 | 1.08 ± 0.35 | ||
| 2 mo | 0.61 ± 0.3 | 33.3 | 0.68 ± 0.2 | 35.7 |
| 6 mo | 0.78 ± 0.3 | 66.6 | 0.81 ± 0.3 | 67.8 |
| 12 mo | 1.01 ± 0.3 | 83.3% | 1.05 ± 0.3 | 85.7% |
SD = standard deviation.
At 12 months, those patients who achieved normal TSH or near normal levels (up to 8 UI/mL, particularly in the elderly) discontinued eltroxine replacement therapy, but were still kept under regular follow-up every 3 months. These were three patients in the 5-g group and five patients in the 10-g group.
4. Discussion
The purpose of heterotopic thyroid autotransplantation is to leave thyroid tissue in the body that might be able to avoid or reduce severity of post-thyroidectomy hypothyroidism in noncompliant patients. In the meantime, if recurrence occurs it would not be in the neck, thus avoiding compression on the trachea and avoiding dangerous reoperation in the neck. Reports on its clinical application are very scarce in the literature, with few patients in each study. Furthermore, none of these studies focused on simple multinodular goiters; the majority were directed to Graves' disease. The only well-documented study that addressed this issue with a few multinodular cases was that of Roy et al12 who included eight multinodular goiters among their studied 15 patients.
Even though all animal and human studies documented the survival and function of thyroid tissue that was implanted in muscle, the technique seldom achieved a euthyroid status and suffered heterogeneity of tissue preparation (some used frozen, while others used fresh tissue), implant timing, and variability of implant weight. This work was set to study the survival and function of implanted tissues of multinodular goiters, on a larger patient population. It was also a trial at standardizing the amount to be transplanted and to test the applicability of an easy technique for its implementation.
In the best documented technical description, that of Shimizu et al,11 the workers implanted 2.5–3.5 g per patient with Graves' disease. For the current work, the implanted tissue was expected to be less active and hence at the start of the study 5-g implants were implanted for the first 12 patients. As functional results of these patients were found to be suboptimal, the weight of implanted tissue was raised to 10 g. The problem with implanting such sizable amount of tissue was that many muscle pockets are made; they would be over-packed with the implanted thyroid tissue, a factor that may hinder the graft take. The procedure would also take an unacceptably long time. In 2000, Gauger et al13 described the technique of injecting parathyroid emulsion in the sternomastoid. Their technique was adopted in this work for thyroid tissue implantation using a 20-mL syringe with saline. This technique simplifies the implantation of sizable tissues, in a short time (∼15 minutes), and leaves a small, barely visible scar. With this technique all implants survived and functioned.
To avoid the development of hypothyroidism, pending the take of the graft, all patients were given 50 μg levothyroxine daily. Such a relatively low replacement dose was chosen because it was felt that keeping a high TSH would enhance implant survival and function. Replacement therapy was discontinued 3 weeks before assessment of its function. This was judged enough time for the replacement therapy to practically disappear from the circulation, the half-life of T4 being 1 week, and that of T3 1 day. Thus, 3 weeks after discontinuation of replacement therapy, the patient is totally dependent on the implanted thyroid tissue. Although in a number of patients, isotope scans detected residual thyroid tissue in the neck, such cervical uptake was too small to interfere with interpretation of the implant function. The overall implant function was satisfactory but not ideal. At 1 year, the majority of patients showed normal isotope uptake by the implant, normal FT3 and FT4. Actually, all patients with 10-g implants showed normal T3 levels. Meanwhile, the majority maintained a high TSH levels. This means that the majority of patients achieved a euthyroid status but at the expense of hyperactivity of the anterior pituitary gland.
In the literature there is no previous mention of the effect of time passage on the function of thyroid autotransplant. In the current work, however, this point was investigated. Analysis of variance showed that the levels of FT3 and FT4 increased with time, and likewise TSH showed significant move towards normalization, i.e., reduction of its levels. Even though isotope uptake showed also a tendency towards elevation, it did not reach statistical significance.
Since the volume of a normal thyroid gland varies from one country to another,14; 15; 16; 17 ; 18 its weight is also variable from 15 g to 25 g. As far as the effect of tissue weight was concerned, 10-g implants showed significantly higher isotope uptake and higher FT3 than the 5-g implants. It thus looks reasonable to advise that the minimum weight of thyroid autotransplantation for a simple multinodular goiter should be 10 g. Future studies may achieve better function with heavier implants. This sounds logical when the weight of a normal gland is considered. A piece of the excised multinodular goiter, even though it looks grossly normal, is very likely to contain micronodules, and thus may have a lower functional capacity.
The possibility of implanting undetected thyroid cancer in muscle is a concern. In a recent study 23.1% of cervical multinodular goiters that had a benign preoperative fine-needle aspiration were found to have thyroid cancer on final pathology.19 Precautions that have been taken included preoperative and operative measures. Before surgery those with a family history of thyroid cancer or neck irradiation were excluded. Thorough clinical assessment, neck ultrasound, and fine-needle aspiration cytology for any suspicious nodule were done. The slightest suspicion of malignancy disqualified the patient for autotransplantation. At operation the healthiest-looking piece of excised thyroid was chosen for implantation. Cutting of the chosen tissue was too fine to allow for missing any gross change in appearance or texture. Still, the possibility of implanting an occult thyroid cancer is a point to be considered. The possible survival of cancer cells in somatic muscles has to be investigated. However, under the previously mentioned precautions it is a very remote possibility that should not interfere with the clinical application of thyroid autotransplantation. To cite an example, the theoretical possibility of a microscopic carcinoma in a donor organ does not interfere with the transplantation process.
Hypothetically, function depends on the status of the graft (cell density and potential regeneration power), survival of cells in the transplant bed, its size, and the duration of long-term stimulation. Longer follow-up is undoubtedly needed. The principle of the technique relies on the thyroid autotransplant as a robust, life-long substitute for replacement therapy. Such an assumption waits time testing. Another concern is possible goiter recurrence in the thigh muscles. Theoretically a goiter in the thigh is less likely to be visible or to compress important structures. Surgery for such a swelling would certainly be less serious than a second operation in the neck.
References
- 1 J. Moalem, I. Suh, Q. Duh; Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature; World J Surg, 32 (2008), pp. 1301–1312
- 2 G. Agarwal, V. Aggarwal; Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence-based review; World J Surg, 32 (2008), pp. 1313–1324
- 3 D.J. Lips, M.T. van Reisen, V. Voigt, W. Venekamp; Diagnosis and treatment of levothyroxine pseudomalabsorption; Neth J Med, 62 (2004), pp. 114–118
- 4 M.J. Sethi, M. Parr, V. Bhatia; Management strategies for hypothyroidism in noncompliant patients: a case report and review of literature; S. D Med, 61 (2008), pp. 368–369
- 5 K. Shimizu, M. Nagahama, Y. Kitamura, T. Igarashi, N. Aida, S. Tanaka; Improvement of thyroid function after autotransplantation of cryopreserved thyroid tissue in rats: clinical application of the procedure to patients with persistent hypothyroid Graves' disease after thyroidectomy; Thyroidol Clin Exp, 8 (1996), pp. 55–62
- 6 B. Papaziogas, A. Antoniadis, C. Lazaridis, et al.; Functional capacity of the thyroid autograft: an experimental study; J Surg Res, 103 (2002), pp. 223–227
- 7 I. Gál, I. Mikó, I. Furka, D. Nagy; Autotransplantation of cryopreserved thyroid tissue in dogs; Magy Seb, 58 (2005), pp. 93–99 [in Hungarian]
- 8 C. Dobrinja, R. Trevisan, G. Trevisan, G. Liguori; Autotransplantation of thyroid tissue in rats. An experimental study; Ann Ital Chir, 79 (2008), pp. 389–395
- 9 N.S. Pushkar', V.A. Makedonskaia, A.M. Utevskiĭ, V.A. Chuĭko, L.G. Karpenko; Autoimplantation of cryopreserved (−196 degrees C) thyroid gland parenchyma as a treatment method in postoperative hypothyroidism; Probl Endokrinol (Mosk), 30 (1984), pp. 42–46 [in Russian]
- 10 T. Okamoto, Y. Fujimoto, T. Obara, Y. Ito, T. Kodama, K. Kusakabe; Trial of thyroid autotransplantation in patients with Graves' disease whose remnant thyroid has unintentionally been made too small at subtotal thyroidectomy; Endocrinol Jpn, 37 (1990), pp. 95–101
- 11 K. Shimizu, S. Kumita, Y. Kitamura, et al.; Trial of autotransplantation of cryopreserved thyroid tissue for postoperative hypothyroidism in patients with Graves' disease; J Am Coll Surg, 194 (2002), pp. 14–22
- 12 P.G. Roy, M.S. Saund, T.K. Thusoo, D. Roy, R. Sankar; Fate of human thyroid tissue autotransplants; Surg Today, 33 (2003), pp. 571–576
- 13 P.G. Gauger, T.S. Reeve, M. Wilkinson, L.W. Delbridge; Routine parathyroid autotransplantation during total thyroidectomy: the influence of technique; Eur J Surg, 166 (2000), pp. 605–609
- 14 M. Yousef, A. Sulieman, B. Ahmed, A. Abdella, K. Eltom; Local reference ranges of thyroid volume in Sudanese normal subjects using ultrasound; J Thyroid Res, 2011 (2011), p. 935141
- 15 G. Ivanac, B. Rozman, F. Skreb, B. Brkljacić, L. Pavić; Ultrasonographic measurement of the thyroid volume; Coll Antropol, 28 (2004), pp. 287–291
- 16 A. Ahidjo, A. Tahir, M.A. Tukur; Ultrasound determination of thyroid gland volume among adult Nigerians; Internet J Radiol (2006), p. 4 Available at: https://ispub.com/IJRA/4/2/8932
- 17 J.P. Chanoine, V. Toppet, R. Lagasse, M. Spehl, F. Delange; Determination of thyroid volume by ultrasound from the neonatal period to late adolescence; Eur J Pediatr, 150 (1991), pp. 395–399
- 18 A. Adibi, M. Sirous, A. Aminorroaya, et al.; Normal values of thyroid gland in Isfahan, an iodine replete area; J Res Med Sci, 13 (2008), pp. 55–60
- 19 M.J. Campbell, C.D. Seib, L. Candell, et al.; The underestimated risk of cancer in patients with multinodular goiters after a benign fine needle aspiration; World J Surg, 39 (2015), pp. 695–700
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?