Abstract
Cashew nuts are an increasingly common cause of food allergy. We compare the soluble protein profile of cashew nuts following heating. SDS-PAGE indicate that heating can alter the solubility of cashew nut proteins. The 11S legumin, Ana o 2, dominates the soluble protein content in ready to eat and mildly heated cashew nuts. However, we found that in dark-roasted cashew nuts, the soluble protein profile shifts and the 2S albumin Ana o 3 composes up to 40% of the soluble protein. Analysis of trypsin-treated extracts by LC/MS/MS indicate changes in the relative number and intensity of peptides. The relative cumulative intensity of the 5 most commonly observed Ana o 1 and 2 peptides are altered by heating, while those of the 5 most commonly observed Ana o 3 peptides remaine relatively constant. ELISA experiments indicate that there is a decrease in rabbit IgG and human serum IgE binding to soluble cashew proteins following heating. Our findings indicate that heating can alter the solubility of cashew allergens, resulting in altered IgE binding. Our results support the use of both Ana o 2 and Ana o 3 as potential cashew allergen diagnostic targets.
Keywords
Cashew nut ; Food allergy ; Immunoglobulin E ; Mass-spectrometry ; Peptide ; Solubility
1. Introduction
Cashew trees (Anacardium occidentale ) are native to South and Central America, but are now widely grown in several tropical regions including Vietnam, India, Nigeria, Cote d'Ivoire, and Brazil [18] and [38] . The U.S. is the largest individual importer of shelled cashew nuts [40] . Cashew nuts are in fact seeds, and are harvested after developing along with a brightly colored cashew apple [32] . Cashew nuts, replete with beneficial fatty acids, anti-oxidants, and proteins [1] , are consumed in various forms including cashew nut butter, ingredients in bakery products, savory dishes, and as whole nuts. Cashews and other nuts are considered excellent sources of nutrients whose consumption has been linked to numerous health benefits [2] and [6] .
Cashew nut processing involves several steps to shell the edible nut and clear the nut of undesirable solids and oils. Cashew nuts contain anacardic acid and other irritants that must be removed before they can be consumed [12] and [17] . Under a general protocol of cashew nut processing, the raw nut undergoes several rounds of heating and cooling to facilitate extraction of the nut from the shell and skin. After harvesting and cleaning, nuts are usually dried in the sun or in a roaster to remove excess moisture. Next, in-shell nut roasting or steaming is performed to make the shell brittle and therefore easier to remove along with associated cashew nut shell liquids [38] . The cashew nut shell liquids, their anacardic acid, and other acid compounds are being investigated for use in therapeutic and other applications [21] and [42] . Once the shell is cut open the nut is removed and humidified, often with steam, in order to loosen and aid in the removal of the skin encasing the nut. Once the skin is peeled away the nut is ready to eat, but the cashew nuts are often heated or flushed with air again to attain an optimal moisture content of 3–5% prior to grading, packaging, and shipping to commercial outlets.
Cashew nuts are considered major food allergens and are included in a list of 8 foods that most commonly cause food allergy. Importantly, the prevalence of allergy to cashew nut appears to be increasing [15] , [37] and [45] , and reactions to cashew nuts are often severe [14] and [16] . Characterized cashew allergens include 3 seed storage proteins: Ana o 1, 2, and 3 [31] , [41] , [50] and [51] . The Ana o 2 legumin accounts for approximately 50% of the soluble cashew nut protein [34] , while the Ana o 1 and Ana o 3 proteins are less abundant [35] .
Food processing steps can alter the nutritional, sensory, and immunological properties of food proteins [24] , [25] , [29] , [33] and [47] . Although effects vary depending upon conditions, thermal processing of peanuts and tree nuts can alter the profile of extractable proteins and their immunological properties [4] , [5] , [9] , [10] , [11] , [22] , [23] , [29] , [30] , [36] , [43] , [44] , [48] and [49] . Previous work has investigated the effects of processing on the stability and IgE binding of cashew nut allergens using several methods including autoclaving, boiling, microwaving, roasting, and irradiation [39] and [46] . The authors concluded that cashew allergens are generally refractive to denaturation and that there is little change in cashew allergen stability when assessed with antibodies directed towards individual cashew allergens [39] and [46] . Our studies investigated the utility of enzymatic digestion or chemical treatment to reduce IgE binding to cashew allergens in vitro[26] and [28] . The abundance of Ana o 2, as well as its relative stability during heating and other processing, suggests that the 11S legumin Ana o 2 may serve as a useful protein marker to detect cashew nuts in foods [46] .
Several novel approaches for cashew nut detection have been described, including those targeting cashew protein and DNA. For example, sandwich ELISAs using polyclonal antibodies directed against total cashew protein that can detect small amounts of cashew protein in food samples have been developed [19] and [53] . Similarly, PCR based methods using primers specific for the Ana o 3 cashew allergen gene have been used to detect cashew nut in foods [8] , and primers targeting cashew ribosomal sequences have been described for detection of cashew as an adulterant in marzipan [7] and [20] . Multiplex platforms including thin-film biosensor chips targeting the Ana o 3 gene sequence [52] , immuno-magnetic beads [13] , and a competitive multi-ELISA format for use on chocolate samples [3] have been developed for the detection of cashew nut as well as other food allergens.
Diagnostic tests for food allergens are an important tool in the food manufacturing and clinical arenas. Several factors, including food manufacturing processes have the potential to change food immunogenicity and allergenicity. Alterations in food allergen secondary or tertiary structure could have detrimental effects on the specificity and sensitivity of cashew allergen detection methods. Here, we characterize changes in cashew allergen solubility and antibody binding following cashew nut roasting. Our findings may enable improvements in cashew allergen detection in the food industry and clinical allergy settings.
2. Materials and methods
2.1. Cashew nut preparation
Ready to eat cashew nuts (designated raw for this study) were purchased from Nutsonline.com. Aluminum trays containing 20 g of raw cashews in a single layer were heated at 300 °F/149 °C (or 350 °F/177 °C) for the following times: 12 min for mild roast, 20 min for medium roast, and 24 min for dark roast. An equal amount of cashew nuts from the same sample was left untreated as a control, unheated sample. After heating, cashew extracts were prepared by grinding nuts in a coffee grinder, followed by defatting with petroleum ether using a BUCHI B-811 Standard Extraction Unit (BUCHI Labortechnik, AG, Flawil, Switzerland). The defatted cashew protein from each sample was dried in a fume hood to completely remove any residual ether residue. The defatted cashew powder was re-ground to fine particles and resuspended at a 1:10 (w/v) ratio for 1 h in borate buffered saline (BBS) solution (100 mM H3 BO4 , 25 mM NaB4 O7 , 75 mM NaCl, pH 8.6) [35] with constant mixing at 4 °C. During this time, each sample was sonicated twice on ice for 15 s using a Sonic Dismembrator (Fisher Scientific Co., Orlando, FL, USA). Clarified cashew extract solutions were prepared by centrifugation for 30 min at 14,000 rpm at 4 °C. Protein solutions from the clarified extracts were collected by pipette and protein concentrations were determined using a NanoDrop (ThermoFisher, Pittsburgh, PA, USA) device. Collected samples were dispensed into 1 ml aliquots and stored at −80 °C prior to use.
2.2. SDS-PAGE
Sample buffer with reducing agent 4X NuPAGE LDS (Life Technologies, Carlsbad, CA, USA) was added to the protein samples in a 1:4 (v/v) ratio, and a Novex Mini Cell gel rig (Life Technologies, Carlsbad, CA, USA) was used for electrophoresis. Pre-stained Precision Plus molecular weight markers (Bio-Rad, Hercules, CA, USA) were used as size indicators. Prior to loading, samples were heated at 65 °C for 15 min, electrophoresed, and protein bands were visualized using Safe Stain (Invitrogen, Grand Island, NY, USA). Gel images were captured and the protein load in each lane was quantified using the 680 nm signal channel of an Odyssey CLx infrared imaging system (LI-COR, Lincoln, NE, USA). Equivalent amounts of protein were empirically pre-determined by normalizing the signal from each lane with the IRDye680 channel on the Odyssey CLx, and load volumes were adjusted accordingly.
2.3. Liquid chromatography–mass-spectrometry (LC–MS/MS)
Cashew extract samples were prepared and characterized by LC–MS/MS in a manner similar to that described in previous work [27] . However, in these experiments equivalent amounts of protein (50 ng) from raw or roasted cashew nuts were digested with 0.2 ng trypsin, and samples were acidified with formic acid before being analyzed with an Agilent 1200 LC system, an Agilent Chip Cube interface, and an Agilent 6520 Q-TOF tandem mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The raw data files were extracted, sequenced, and searched against a custom database containing cashew allergen protein sequences to identify matching peptides using Spectrum Mill software (Agilent Technologies, Santa Clara, CA, USA) and determine relative abundance. Relative quantification of individual peptide intensity was accomplished by integrating the extracted ion chromatogram from the MS data specifically for the respective ion indicated.
2.4. ELISA
Polyclonal rabbit anti-cashew antisera, used previously in cashew allergen binding studies [26] , was purchased from Pierce Biotechnology Inc. (Rockford, IL, USA). Samples containing 250 ng of cashew extract were diluted with half-log serial dilutions in PBS with 0.1% Tween-20 (PBST), and 50 μl was added to microtiter plate wells. After incubating overnight at 4 °C, cashew extract was removed and 50 μl of PBST containing 2% BSA was added for 1 h at room temperature to block remaining binding sites within the wells. After washing 4 times with 200 μl of PBST, rabbit anti-cashew serum (diluted 1:5,000 in PBST) was added to wells and incubated at room temperature for 1 h. Rabbit antisera was removed and wells were washed as above followed by the addition of a secondary anti-rabbit antibody labeled with IRdye-800 (LI-COR, Lincoln, NE, USA) diluted 1:20,000 in PBST. Wells were washed 4 times again with PBST and antibody binding was visualized and quantified using with an Odyssey CLx infrared imaging system (LI-COR, Lincoln, NE, USA). ELISA assays were performed in quadruplicate, and the data for each treatment (raw, medium, and dark) was compared for statistical analysis. The rabbit anti-cashew antibody data was analyzed using a typical saturation kinetics model:
|
|
where S is the signal, S0 is the background signal, P is the sample (protein) concentration, f is the fraction of active protein (defined by affinity for the antibody), Ab is the effective antibody concentration, and Kd is the dissociation constant. We converted this to a modified Scatchard equation by taking the reciprocal of the above equation:
|
|
The background S0 was determined by averaging the signals at the 2 lowest sample concentrations. To avoid further complications of the background, the data point immediately above the 2 background points in each treatment set was excluded from the analysis. Because the equations above are only valid for protein concentrations well above the antibody concentration, the 2 points at the highest concentrations were excluded as well. For each treatment, 1/(S − S0 ) was plotted vs. 1/P , and the least-squares slope of each plot was determined, corresponding to Kd /(f Ab ). Although the intercepts appeared to be well behaved in this particular data examination, in general this determination is more subject to error than the determination of slope for a Scatchard analysis, and therefore they were not included in the analysis. IgG binding data was evaluated using a one-way ANOVA analysis comparing 3 of the treatments (“Raw,” “Medium” and “Dark”).
For ELISA using human sera samples, microplate wells were coated with 1 μg of raw, mild, medium, or dark roast cashew extract and left at 4 °C overnight. The following morning, PBST containing 2% BSA was added to the well and incubated for 1 h at room temperature. Wells were washed 4 times with 200 μl of PBST and pooled human serum samples from cashew allergic patients previously characterized [26] and [28] were diluted 1:5 with PBST and added to microplate wells. After 1 h at room temperature they were washed 4 times with 200 μl of PBST, and secondary anti-human IgE antibody labeled with IRdye-800 (diluted 1:5,000 in PBST) was added for 1 h at room temperature. Following incubation of the membranes for 1 h at room temperature, the membranes were washed and visualized as above using an Odyssey CLx infrared imaging system (LI-COR, Lincoln, NE, USA). The average of 4 human sera IgE binding experiments for each treatment was used for analysis. The average of IgE binding to raw cashews was set to 100% and the average of the other treatments was converted as a percentage of the raw cashew signal using the formula (B /A ) × 100, where B is the average of the treatment in question and A is the average of the raw treatment antibody binding value. The average percent binding for each of the samples was plotted with standard deviation indicated in the error bars.
3. Results
3.1. Solubility profile of heated cashews
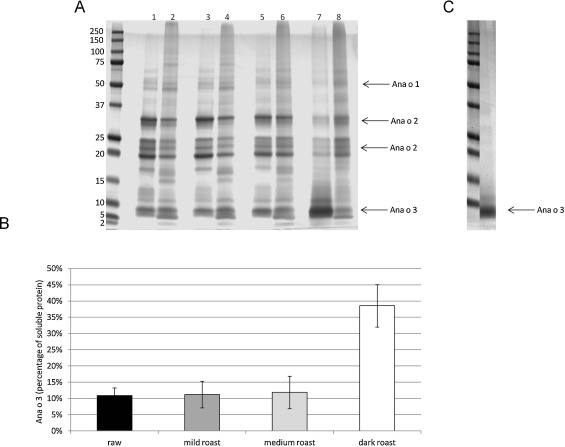
Food processing and preparation steps are meant to enhance flavor, but they can alter several other food characteristics. To determine the effect that dry heat has on cashew nuts, we began by characterizing the solubility profile of heated cashew nut extracts using SDS-PAGE. Protein extracts were made from untreated cashew nuts (raw) or cashews heated in a single layer at 300 °F/149 °C for the following times: 12 min for mild roast, 20 min for medium roast, and 24 min for dark roast (Fig. 1 ). Normalized soluble and insoluble protein loading was empirically determined by adjusting loading volume based upon previous analysis of Coumassie stained SDS-PAGE and quantification using the IRDye 680 channel of a Odyssey CLx instrument. Equivalent amounts of protein (25 μg) from the soluble and insoluble portions of ready to eat (raw) or heated cashew were then electrophoresed in SDS-PAGE, stained with Coumassie, and the protein content in whole lanes was again quantified using the IRDye 680 channel of a Odyssey CLx instrument to ensure equivalent loading. As shown in Fig. 2 A, heating for either 12 (mild roast) or 20 (medium roast) min at 300 °F did not appreciably alter the profile of soluble and insoluble protein compared to that of raw cashews. In contrast, heating cashews for 24 min (dark roast) resulted in a visible change in the extraction profile (Fig. 2 A, lanes 7, 8). Most notably, we observed an increase in the relative amount of Ana o 3 in the soluble protein fraction of the dark roast sample. Quantification of the Ana o 3 band from each individual lane indicated that the level of Ana o 3 in the soluble fraction from the dark roast sample was increased by approximately 40%, and that there was a corresponding decrease in the insoluble Ana o 3 fraction (Fig. 2 A and B). Conversely, soluble levels of both Ana o 1 and Ana o 2 were decreased, respectively, in the dark roast cashew nuts heated for 24 min (Fig. 2 ). More intense heating of cashew nuts at 350 °F (177 °C) lead to an even greater proportion of Ana o 3 in the soluble extract fraction. Heating cashew nuts for greater than 22 min at 350 °F resulted in Ana o 3 being essentially the only protein extracted from the heated nuts (Fig. 2 C).
|
|
|
Fig. 1. Representative images of cashew nuts following heating at 300 °F for 12 min (mild), 20 min (medium), 24 min (dark), or un-heated (raw). |
|
|
|
Fig. 2. SDS-PAGE analysis of the soluble and insoluble fractions of raw and heated (300 °F) cashew nut extracts (A) and quantification of soluble Ana o 3 level (B). Lane 1: soluble raw cashew, lane 2: insoluble raw cashew, lane 3: soluble mild roast (12 min) cashew, lane 4: insoluble mild roast cashew, lane 5: soluble medium roast (20 min) cashew, lane 6: insoluble medium roast cashew, lane 7: soluble dark roast (24 min), lane 8: insoluble dark roast cashew. Soluble protein extracted from cashew nuts heated at 350 °F for 24 min (soluble 350 °F dark roast) (C). Molecular weight markers are indicated on the left-most lane of the gels, and the arrows indicate the migration position of the Ana o 1, Ana o 2, and Ana o 3 proteins. |
3.2. Liquid chromatography mass–spectrometry of raw and heated cashew extracts
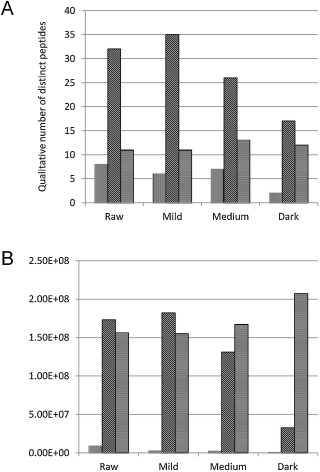
To further characterize the changes in soluble protein profiles, we assessed the raw and heated cashew samples by mass-spectrometric analysis. Following treatment of samples with trypsin and LC/MS/MS, we detected a qualitative shift in the relative number and intensity of peptides surveyed by the mass-spectrometric analysis (Fig. 3 A). For example, the number of Ana o 1 and 2 peptides observed in the soluble fraction from dark roast cashew nuts was reduced compared to the raw and mildly roasted samples. Further, there was a reduction in the qualitative intensity of Ana o 1 and Ana o 2 peptides detected in the dark roast sample compared with the raw and mildly roasted samples (Fig. 3 B). Conversely, the qualitative number and intensity of Ana o 3 peptides was relatively constant among the various samples (Fig. 3 B). While these results are qualitative, they are consistent with our observations from our SDS-PAGE analysis.
|
|
|
Fig. 3. Qualitative mass-spectrometric analysis of observed peptides from trypsin-digested soluble extracts of raw and heated cashew nuts. Qualitative number of distinct peptides (A) and Qualitative intensity of observed peptides (B) from Ana o 1 (vertical bars), Ana o 2 (diagonal bars), and Ana o 3 (horizontal bars). |
To further characterize the changes in the observed mass-spectrometry signals, we assembled the 5 most commonly observed peptides for each of the cashew allergens and integrated the respective peak areas for each of these peptides. We noticed changes in the intensity of peptides detected as cashew nut heating progressed (see Table 1 , Table 2 and Table 3 ). The most commonly observed Ana o 1 and Ana o 2 peptides varied as heating progressed; however, the same 5 most common Ana o 3 peptides were observed independent of heating duration. Further, there was an increase in the signal intensity of the Ana o 3 peptides observed in the dark roast sample. These data further illustrate the differences in soluble proteins and peptide profiles following cashew nut heating.
| Ana o1 | Peptide sequence | AA | RT | Precursor | Mass | Peak area |
|---|---|---|---|---|---|---|
| Start | (min) | m /z | [MH]+ | |||
| raw | (K)IWPFTEESTGSFK(L) | 342 | 7.546 | 764.867 | 1528.73 | 3,77,89,982 |
| raw | (R)AFSWEILEAALK(T) | 293 | 18.561 | 689.371 | 1377.74 | 4,31,72,441 |
| raw | (R)IDYPPLEK(L) | 375 | 10.786 | 487.763 | 974.519 | 4,40,20,857 |
| raw | (K)QDEEFFFQGPEWR(K) | 517 | 16.058 | 857.88 | 1714.75 | 2,09,49,306 |
| raw | (R)QGDIVSISSGTPFYIANNDENEK(L) | 238 | 14.711 | 833.391 | 2498.168 | 2,25,95,486 |
| mild | (K)IWPFTEESTGSFK(L) | 342 | 7.719 | 764.866 | 1528.73 | 3,09,02,244 |
| mild | (R)EREHEEEEEEWGTGGVDEPSTHEPAEK(H) | 68 | 9.658 | 625.268 | 3122.31 | 3,17,53,066 |
| mild | (R)AFSWEILEAALK(T) | 171 | 18.422 | 689.371 | 1377.74 | 3,07,82,590 |
| mild | (K)YGQLFAER(I) | 366 | 10.768 | 556.77 | 1112.54 | 2,16,28,229 |
| mild | (R)IDYPPLEK(L) | 375 | 10.733 | 487.237 | 974.519 | 2,16,28,229 |
| med | (k)IDPELK(Q) | 28 | 7.98 | 357.705 | 714.403 | 2,50,10,289 |
| med | (K)IWPFTEESTGSFK(L) | 342 | 7.453 | 764.868 | 1528.73 | 3,19,13,750 |
| med | (R)AFSWEILEAALK(T) | 293 | 18.546 | 689.365 | 1377.74 | 3,48,39,007 |
| med | (K)QDEEFFFQGPEWR(K) | 517 | 15.979 | 857.878 | 1714.75 | 1,90,94,254 |
| med | (K)YGQLFEAER(I) | 366 | 10.77 | 556.77 | 1112.54 | 2,26,51,363 |
| dark | (k)IDPELK(Q) | 28 | 7.98 | 357.705 | 714.403 | 20,93,985 |
| dark | (R)QYDEQQKEQCVK(E) | 44 | 14.549 | 791.862 | 1582.72 | 27,14,972 |
| dark | (R)AFSWEILEAALK(T) | 293 | 12.022 | 689.375 | 1377.74 | 29,36,063 |
| dark | (K)HLSQCMR(Q) | 95 | 7.138 | 466.217 | 931.424 | 15,53,769 |
| dark | (K)QDEEFFFQGPEWR(K) | 517 | 16.179 | 857.866 | 1714.75 | 5,60,779 |
| Ana o2 | Peptide sequence | AA | RT | Precursor | Mass | Peak area |
|---|---|---|---|---|---|---|
| Start | (min) | m /z | [MH]+ | |||
| raw | (R)LDALEPDNRVEYEAGTVEAWDPNHEQFR(C) | 30 | 14.25 | 825.886 | 3300.519 | 37,56,82,411 |
| raw | (R)ADIYTPEVGR(L) | 292 | 10.344 | 560.786 | 1120.563 | 28,81,83,427 |
| raw | (R)LKENINDPAR(A) | 282 | 7.851 | 390.548 | 1169.627 | 51,56,99,539 |
| raw | (R)EGQMLVVPQNFAVVK(R) | 369 | 15.35 | 829.956 | 1658.893 | 23,01,82,192 |
| raw | (R)KFHLAGNPK(D) | 175 | 7.58 | 337.863 | 1011.573 | 42,99,69,213 |
| mild | (R)LDALEPDNRVEYEAGTVEAWDPNHEQFR(C) | 30 | 14.179 | 825.883 | 3300.519 | 60,47,44,767 |
| mild | (R)LKENINDPAR(A) | 282 | 7.773 | 390.548 | 1169.627 | 82,32,15,133 |
| mild | (R)ADIYTPEVGR(L) | 292 | 10.282 | 560.783 | 1120.563 | 51,51,30,530 |
| mild | (R)KFHLAGNPK(D) | 175 | 7.46 | 337.864 | 1011.573 | 57,39,18,793 |
| mild | (R)EGQMLVVPQNFAVVK(R) | 369 | 15.151 | 829.956 | 1658.893 | 34,84,03,379 |
| med | (R)LDALEPDNRVEYEAGTVEAWDPNHEQFR(C) | 30 | 14.099 | 825.878 | 3300.519 | 41,07,69,443 |
| med | (R)LKENINDPAR(A) | 282 | 7.717 | 390.548 | 1169.627 | 59,26,46,710 |
| med | (R)ADIYTPEVGR(L) | 292 | 10.218 | 560.783 | 1120.563 | 33,88,91,251 |
| med | (R)KFHLAGNPK(D) | 175 | 7.47 | 337.866 | 1011.573 | 37,01,33,067 |
| med | (R)EGQMLVVPQNFAVVK(R) | 369 | 15.177 | 829.956 | 1658.893 | 24,87,50,019 |
| dark | (R)LDALEPDNRVEYEAGTVEAWDPNHEQFR(C) | 30 | 14.324 | 825.878 | 3300.519 | 5,78,65,998 |
| dark | (R)LKENINDPAR(A) | 282 | 7.6 | 390.548 | 1169.627 | 4,70,39,354 |
| dark | (R)NLFSGFDTELLAEAFQVDER(L) | 198 | 20.489 | 767.697 | 2301.103 | 9,83,21,604 |
| dark | (R)FEWISFK(T) | 390 | 15.487 | 478.474 | 956.488 | 3,41,28,938 |
| dark | (R)VEYEAGTVEAWDPNHEQFR(C) | 39 | 2.937 | 759.674 | 2277.021 | 2,90,55,243 |
| Ana o3 | Peptide sequence | AA | RT | Precursor | Mass | Peak area |
|---|---|---|---|---|---|---|
| Start | (min) | m /z | [MH]+ | |||
| raw | (R)ELYETASELPR(I) | 112 | 11.189 | 654.321 | 1306.628 | 1,31,29,09,658 |
| raw | (R)CQNLEQMVR(Q | 89 | 10.263 | 589.277 | 1176.539 | 1,11,34,56,233 |
| raw | (R)ECCQELQEVDRR(C) | 75 | 8.518 | 541.24 | 540.233 | 99,79,74,248 |
| raw | (R)QLQQQEQIKGEEVR(E) | 98 | 8.344 | 856.948 | 1711.882 | 9,04,92,853 |
| raw | (R)ICSISPSQGCQFQSSY(−) | 123 | 11.942 | 924.893 | 1847.783 | 1,41,52,444 |
| med | (R)ELYETASELPR(I) | 112 | 11.057 | 654.33 | 1306.645 | 1,63,77,45,040 |
| med | (R)CQNLEQMVR(Q | 89 | 10.147 | 589.274 | 1176.534 | 1,29,45,05,423 |
| med | (R)ECCQELQEVDRR(C) | 75 | 8.591 | 541.236 | 540.229 | 1,26,10,18,230 |
| med | (R)QLQQQEQIKGEEVR(E) | 98 | 8.234 | 856.949 | 1711.884 | 13,14,47,629 |
| med | (R)ICSISPSQGCQFQSSY(−) | 123 | 11.912 | 924.901 | 1847.783 | 3,07,14,199 |
| mild | (R)ELYETASELPR(I) | 112 | 11.137 | 654.326 | 1306.638 | 1,82,76,46,498 |
| mild | (R)CQNLEQMVR(Q | 89 | 10.254 | 589.277 | 1176.539 | 1,29,52,54,642 |
| mild | (R)ECCQELQEVDRR(C) | 75 | 8.632 | 541.24 | 540.233 | 1,44,99,74,404 |
| mild | (R)QLQQQEQIKGEEVR(E) | 98 | 8.27 | 856.95 | 1711.885 | 21,28,84,939 |
| mild | (R)ICSISPSQGCQFQSSY(−) | 123 | 11.898 | 924.899 | 1847.783 | 1,39,84,077 |
| dark | (R)ELYETASELPR(I) | 112 | 10.876 | 654.331 | 1306.647 | 2,45,13,14,832 |
| dark | (R)CQNLEQMVR(Q | 89 | 9.844 | 589.269 | 1176.524 | 1,50,65,80,462 |
| dark | (R)ECCQELQEVDRR(C) | 75 | 8.518 | 541.24 | 540.233 | 1,81,38,01,039 |
| dark | (R)QLQQQEQIKGEEVR(E) | 98 | 8.038 | 856.953 | 1711.891 | 30,48,64,976 |
| dark | (R)ICSISPSQGCQFQSSY(−) | 123 | 11.919 | 924.897 | 1847.783 | 5,42,88,703 |
3.3. IgG and IgE binding to raw and heated cashew extracts
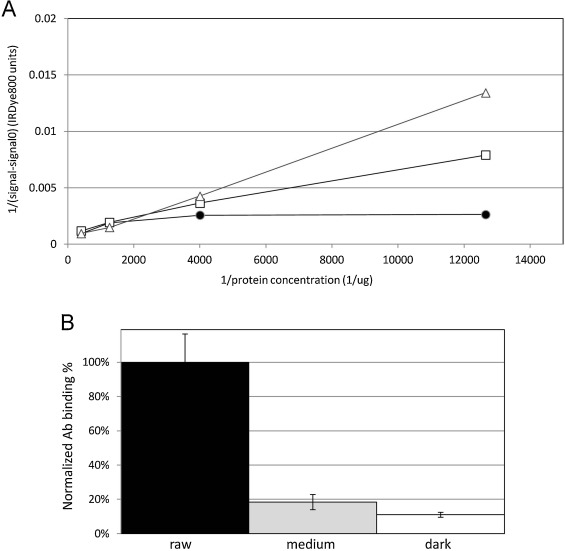
To evaluate the immunological significance of the changes in solubility we observed, we tested the binding of rabbit anti-cashew polyclonal antisera to the cashew samples. Because the SDS-PAGE and mass-spectrometry results were so similar between the raw and mild samples, we did not analyze the mild roasted samples using the polyclonal sera. Antibody binding to serial half-log dilutions of the samples starting with a concentration of 0.25 μg/ml was analyzed by ELISA. IgG binding to the dark roast cashew extract had lower initial and final values compared to the raw and medium roast samples. We evaluated the data using a one-way ANOVA analysis and found that there was significant difference between the 3 treatments (“Raw,” “Medium”, and “Dark”) with a confidence level greater than 99.9% (F (2, 5) = 41.3). A representative plot of the slopes for each treatment is shown in Fig. 4 A. Assuming the effective antibody concentration and the dissociation constant to be stable between treatments, the reciprocal of the slope is proportional to the active (antibody binding) fraction of protein within each sample. Normalizing to the raw samples, we show that the fraction of active protein drops more than 5-fold going from raw to medium, and drops another 50% going from medium to dark roast (Fig. 4 B).
|
|
|
Fig. 4. Direct IgG ELISA using serial half-log dilutions of soluble extract from raw cashew (dark circles), medium roast cashew (open squares), dark roast cashew (open triangles); and rabbit anti-cashew polyclonal sera. Plot of antibody binding to extracts from each treatment (A), where the inverse of protein concentration is indicated on the x -axis and the y axis is 1/(signal—background). Determination of percentage of active antibody binding protein from each sample (B) with raw (dark bar), medium (dark grey bar), and dark roast extract (white bar). |
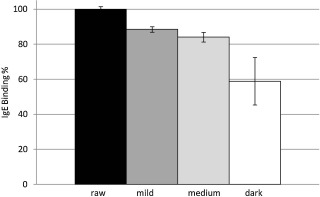
ELISA assays using a pool of 6 cashew-allergic patient sera were also used to characterize the antigenic differences between the raw and roasted cashew extracts. Pooled sera were diluted 1:4 with PBS and added to wells containing 1 μg of soluble protein from the raw, mild, medium, or dark roasted cashew nuts. There was reduced IgE binding to 1 μg of protein made from heated cashew extracts. While IgE binding to the mild and medium roasted cashews was reduced to 88% and 84%, respectively, compared with the raw extract, IgE binding to the dark roast cashew extract was reduced to less than 60% of the raw extract (Fig. 5 ). These data are consistent with the reduced solubility of the higher molecular weight bands observed in the SDS-PAGE gel and suggest a reduction in IgE binding epitopes within the roasted samples.
|
|
|
Fig. 5. Direct IgE ELISA with 1 μg of soluble protein extract from raw cashew (dark bar), mild roast cashew (dark grey bar), medium roast cashew (light grey bar), dark roast cashew (white bar); and a pool of 6 human sera from patients with clinically defined cashew allergy. Cashew sample is indicated on the X -axis, percent of IgE binding relative to that of raw cashew nut is indicated on the Y -axis, and standard deviation is used for errors. |
4. Discussion
In the present study, we describe changes in the soluble protein profile of heated cashew nuts, and we propose that these results are likely to have food allergen detection and immunological consequences. While Ana o 2 makes up roughly 50% of the soluble cashew protein in raw or ready-to-eat nuts, our SDS-PAGE results indicate that heating can alter the relative amount of soluble immunoreactive Ana o 2. This reduction in protein solubility leads to a greater relative percentage of the Ana o 3 allergen. This is consistent with previously published analysis of extracts from cashew nuts heated for 20 min at 170 °C or 15 min at 200 °C in which SDS-PAGE analysis indicated a change in relative cashew allergen solubility [46] . Our results support targeting both Ana o 2 and Ana o 3 for cashew allergen detection-methods development. Ana o 1 makes up a smaller percentage of soluble cashew protein, and we found that its level was reduced in heated cashews, making it less useful as a target for allergen detection in processed foods.
We report that among the cashew allergen peptides detected using LC/MS/MS analysis, the Ana o 3 peptides are relatively abundant and intense. In fact, the list of 5 most common Ana o 3 peptides was unaltered in the raw and heated cashew extracts. Furthermore, the overall accumulated intensity of these peptides was increased in heated cashew samples. The most commonly observed Ana o 3 peptides we found included amino acid sequences from a previously mapped strongly reacting Ana o 3 IgE epitope, epitope 10-72-SLRECCQELQEV-83 [31] . Although Ana o 2 peptides were relatively well conserved among the samples, the overall intensity was decreased, consistent with the SDS-PAGE results. In contrast, the 5 most commonly observed Ana o 1 peptides changed in heated cashews and the overall relative intensity decreased.
There is a large body of evidence indicating that peanut and tree protein solubility and immunological characteristics are altered following thermal processing [4] , [5] , [9] , [10] , [11] , [22] , [29] , [30] , [36] , [43] , [44] , [48] and [49] . Similarly, our findings suggest that heating can alter the solubility of cashew allergens and antibody binding. Our findings are consistent with previous studies showing that monoclonal antibodies to Ana o 1 and Ana o 2 exhibited reduced binding to heated cashew extracts relative to unheated cashew extracts [46] . The results for each cashew allergen from the Venkatachalam et al. [46] study varied depending upon the monoclonal antibody used and the test being performed, and this may reflect changes in allergen structure or heating-induced modification. Our finding that the relative amount of Ana o 3 is increased after heating is also consistent with the Venkatachalam et al. [46] study that reported an increase in monoclonal antibody binding to Ana o 3 by immunoblot in extracts made from cashews heated for 20 min at 170 °C or 15 min at 200 °C [46] . Although boiling or blanching has been shown to reduce soluble Ana o 2 and Ana o 3 levels [46] , collectively these findings suggest that diagnostic approaches targeting both Ana o 2 and Ana o 3 may be advantageous.
Our findings indicate that heating can alter the solubility of cashew allergens and thus change the relative amount of cashew allergens such as Ana o 2 and Ana o 3 present in the extract. Cashew nuts are processed in several steps using processing systems and machinery that can vary regionally before they reach consumers. The methods and equipment used could introduce variation in the amount of heating that cashew nuts are exposed to resulting in variations in allergen solubility. Further studies characterizing different processing steps and methods from various regions may identify differences that affect allergen solubility. Similarly, cashew nuts are used in various foods from stir-fry to confections and heating steps are likely to alter the soluble allergen profile. Our findings could be applied to the conception and design of improved diagnostic tools and methods for detection of cashew nut allergens in mislabeled or contaminated foods.
Transparency document
Transparency Document.
Acknowledgments
We would like to thank Si-Yin Chung, Michael Santiago, and Peter Bechtel for helpful discussion and critical evaluation of the material presented. This research was supported by funds from the U.S. Department of Agriculture-Agricultural Research Service and the Allergy Partners of North Texas Research . Mention of trade names, commercial products, or companies in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
- [1] C. Alasalvar, B.W. Bolling; Review of nut phytochemicals fat-soluble bioactives, antioxidant components and health effects; Br. J. Nutr., 113 (Suppl. 2) (2015), pp. S68–78
- [2] J.A. Barbour, P.R. Howe, J.D. Buckley, J. Bryan, A.M. Coates; Nut consumption for vascular health and cognitive function; Nutr. Res. Rev., 27 (2014), pp. 131–158
- [3] S. Ben Rejeb, M. Abbott, D. Davies, C. Cleroux, P. Delahaut; Multi-allergen screening immunoassay for the detection of protein markers of peanut and four tree nuts in chocolate; Food Addit. Contam., 22 (2005), pp. 709–715
- [4] K. Beyer, E. Morrow, X.M. Li, L. Bardina, G.A. Bannon, A.W. Burks, H.A. Sampson; Effects of cooking methods on peanut allergenicity; J. Allergy Clin. Immunol., 107 (2001), pp. 1077–1081
- [5] F. Blanc, Y.M. Vissers, K. Adel-Patient, N.M. Rigby, A.R. Mackie, A.P. Gunning, N.K. Wellner, P.S. Skov, L. Przybylski-Nicaise, B. Ballmer-Weber, L. Zuidmeer-Jongejan, Z. Szepfalusi, J. Ruinemans-Koerts, A.P. Jansen, H. Bernard, J.M. Wal, H.F. Savelkoul, H.J. Wichers, E.N. Mills; Boiling peanut Ara h 1 results in the formation of aggregates with reduced allergenicity; Mol. Nutr. Food Res., 55 (2011), pp. 1887–1894
- [6] R. Blomhoff, M.H. Carlsen, L.F. Andersen, D.R. Jacobs Jr.; Health benefits of nuts: potential role of antioxidants; Br. J. Nutr., 96 (Suppl. 2) (2006), pp. S52–60
- [7] P. Bruning, I. Haase, R. Matissek, M. Fischer; Marzipan: polymerase chain reaction-driven methods for authenticity control; J. Agric. Food Chem., 59 (2011), pp. 11910–11917
- [8] J.L. Brzezinski; Detection of cashew nut DNA in spiked baked goods using a real-time polymerase chain reaction method; J. AOAC Int., 89 (2006), pp. 1035–1038
- [9] B. Cabanillas, C. Cuadrado, J. Rodriguez, J. Hart, C. Burbano, J.F. Crespo, N. Novak; Potential changes in the allergenicity of three forms of peanut after thermal processing; Food Chem., 183 (2015), pp. 18–25
- [10] B. Cabanillas, S.J. Maleki, J. Rodriguez, H. Cheng, S.S. Teuber, M.L. Wallowitz, M. Muzquiz, M.M. Pedrosa, R. Linacero, C. Burbano, N. Novak, C. Cuadrado, J.F. Crespo; Allergenic properties and differential response of walnut subjected to processing treatments; Food Chem., 157 (2014), pp. 141–147
- [11] B. Cabanillas, M.M. Pedrosa, J. Rodriguez, M. Muzquiz, S.J. Maleki, C. Cuadrado, C. Burbano, J.F. Crespo; Influence of enzymatic hydrolysis on the allergenicity of roasted peanut protein extract; Int. Arch. Allergy Immunol., 157 (2012), pp. 41–50
- [12] A.L. Carvalho, R. Annoni, P.R. Silva, P. Borelli, R.A. Fock, M.T. Trevisan, T. Mauad; Acute, subacute toxicity and mutagenic effects of anacardic acids from cashew (Anacardium occidentale Linn.) in mice ; J. Ethnopharmacol., 135 (2011), pp. 730–736
- [13] C.Y. Cho, W. Nowatzke, K. Oliver, E.A. Garber; Multiplex detection of food allergens and gluten; Anal. Bioanal. Chem., 407 (2015), pp. 4195–4206
- [14] A.T. Clark, K. Anagnostou, P.W. Ewan; Cashew nut causes more severe reactions than peanut: case-matched comparison in 141 children; Allergy, 62 (2007), pp. 913–916
- [15] A. Cox, S.H. Sicherer; Peanut and tree nut allergy; Chem. Immunol. Allergy, 101 (2015), pp. 131–144
- [16] M. Davoren, J. Peake; Cashew nut allergy is associated with a high risk of anaphylaxis; Arch. Dis. Child., 90 (2005), pp. 1084–1085
- [17] M.A. ElSohly, P.D. Adawadkar, D.A. Benigni, E.S. Watson, T.L.J. Little; Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n -alkylbenzenes ; J. Med. Chem., 29 (1986), pp. 606–611
- [18] FAOSTAT, Cashew nut production quantities by country, 2015.
- [19] F.E. Gaskin, S.L. Taylor; Sandwich enzyme-linked immunosorbent assay (ELISA) for detection of cashew nut in foods; J. Food Sci., 76 (2011), pp. T218–226
- [20] I. Haase, P. Bruning, R. Matissek, M. Fischer; Real-time PCR assays for the quantitation of rDNA from apricot and other plant species in marzipan; J. Agric. Food Chem., 61 (2013), pp. 3414–3418
- [21] F.B. Hamad, E.B. Mubofu; Potential biological applications of bio-based anacardic acids and their derivatives; Int. J. Mol. Sci., 16 (2015), pp. 8569–8590
- [22] S.J. Koppelman, C.A. Bruijnzeel-Koomen, M. Hessing, H.H. de Jongh; Heat-induced conformational changes of Ara h 1, a major peanut allergen, do not affect its allergenic properties; J. Biol. Chem., 274 (1999), pp. 4770–4777
- [23] S.J. Maleki, S.Y. Chung, E.T. Champagne, J.P. Raufman; The effects of roasting on the allergenic properties of peanut proteins; J. Allergy Clin. Immunol., 106 (2000), pp. 763–768
- [24] S.J. Maleki, B.K. Hurlburt; Structural and functional alterations in major peanut allergens caused by thermal processing; J. AOAC Int., 87 (2004), pp. 1475–1479
- [25] L.J. Masthoff, R. Hoff, K.C. Verhoeckx, H. van Os-Medendorp, A. Michelsen-Huisman, J.L. Baumert, S.G. Pasmans, Y. Meijer, A.C. Knulst; A systematic review of the effect of thermal processing on the allergenicity of tree nuts; Allergy, 68 (2013), pp. 983–993
- [26] C.P. Mattison, W.A. Desormeaux, R.L. Wasserman, M. Yoshioka-Tarver, B. Condon, C.C. Grimm; Decreased immunoglobulin E (IgE) binding to cashew allergens following sodium sulfite treatment and heating; J. Agric. Food Chem., 62 (2014), pp. 6746–6755
- [27] C.P. Mattison, J. Dinter, M.J. Berberich, S.Y. Chung, S.S. Reed, S. Le Gall, C.C. Grimm; In vitro evaluation of digestive and endolysosomal enzymes to cleave CML-modified Ara h 1 peptides; Food Sci. Nutr., 3 (2015), pp. 273–283
- [28] C.P. Mattison, C.C. Grimm, R.L. Wasserman; In vitro digestion of soluble cashew proteins and characterization of surviving IgE-reactive peptides; Mol. Nutr. Food Res., 58 (2014), pp. 884–893
- [29] E.N. Mills, A.I. Sancho, N.M. Rigby, J.A. Jenkins, A.R. Mackie; Impact of food processing on the structural and allergenic properties of food allergens; Mol. Nutr. Food Res., 53 (2009), pp. 963–969
- [30] R. Noorbakhsh, S.A. Mortazavi, M. Sankian, F. Shahidi, S.J. Maleki, L.R. Nasiraii, R. Falak, H.R. Sima, A. Varasteh; Influence of processing on the allergenic properties of pistachio nut assessed in vitro; J. Agric. Food Chem., 58 (2010), pp. 10231–10235
- [31] J.M. Robotham, F. Wang, V. Seamon, S.S. Teuber, S.K. Sathe, H.A. Sampson, K. Beyer, M. Seavy, K.H. Roux; Ana o 3: an important cashew nut (Anacardium occidentale L.) allergen of the 2S albumin family ; J. Allergy Clin. Immunol., 115 (2005), pp. 1284–1290
- [32] F. Rosengarten; The Book of Edible Nuts; Dover Publications, Inc., New York (1984)
- [33] S.K. Sathe, G.M. Sharma; Effects of food processing on food allergens; Mol. Nutr. Food Res., 53 (2009), pp. 970–978
- [34] S.K. Sathe, K.W.C. Sze-Tao, W.J. Wolf, B.R. Hamaker; Biochemical characterization and in vitro digestibility of the major globulin in cashew nut (Anacardium occidentale ) ; J. Agric. Food Chem., 45 (1997), pp. 2854–2860
- [35] S.K. Sathe, M. Venkatachalam, G.M. Sharma, H.H. Kshirsagar, S.S. Teuber, K.H. Roux; Solubilization and electrophoretic characterization of select edible nut seed proteins; J. Agric. Food Chem., 57 (2009), pp. 7846–7856
- [36] D.A. Schmitt, J.B. Nesbit, B.K. Hurlburt, H. Cheng, S.J. Maleki; Processing can alter the properties of peanut extract preparations; J. Agric. Food Chem., 58 (2010), pp. 1138–1143
- [37] S.H. Sicherer, A. Munoz-Furlong, J.H. Godbold, H.A. Sampson; US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up; J. Allergy Clin. Immunol., 125 (2010), pp. 1322–1326
- [38] G. Srivatsava; Cashew Handbook 2014—Global Perspective; J (Ed.) (4th ed.)Foretell Business Solutions Private Limited, Kodihalli, Bengaluru-560 048, India (2014) www.cashewinfor.com
- [39] M. Su, M. Venkatachalam, S.S. Teuber, K.H. Roux, S.K. Sathe; Impact of g-irradiation and thermal processing on the antigenicity of almond, cashew nut, and walnut proteins; J. Sci. Food Agric., 84 (2004), pp. 1119–1125
- [40] T. SWAP, Cashew Sector: Supply Capacity Constraints and Technical Assistance Strategy, in: P.C.n.T. team (Ed.). 2008.
- [41] S.S. Teuber, S.K. Sathe, W.R. Peterson, K.H. Roux; Characterization of the soluble allergenic proteins of cashew nut (Anacardium occidentale L.) ; J. Agric. Food Chem., 50 (2002), pp. 6543–6549
- [42] A.H. Tullo; A nutty chemical; Chem. Eng. News, 96 (2008), pp. 26–27
- [43] E.L. van Boxtel, S.J. Koppelman, L.A. van den Broek, H. Gruppen; Determination of pepsin-susceptible and pepsin-resistant epitopes in native and heat-treated peanut allergen Ara h 1; J. Agric. Food Chem., 56 (2008), pp. 2223–2230
- [44] E.L. van Boxtel, L.A. van den Broek, S.J. Koppelman, H. Gruppen; Legumin allergens from peanuts and soybeans: effects of denaturation and aggregation on allergenicity; Mol. Nutr. Food Res., 52 (2008), pp. 674–682
- [45] J.P. van der Valk, A.E. Dubois, R. Gerth van Wijk, H.J. Wichers, N.W. de Jong; Systematic review on cashew nut allergy; Allergy, 69 (2014), pp. 692–698
- [46] M. Venkatachalam, E.K. Monaghan, H.H. Kshirsagar, J.M. Robotham, S.E. O'Donnell, M.S. Gerber, K.H. Roux, S.K. Sathe; Effects of processing on immunoreactivity of cashew nut (Anacardium occidentale L.) seed flour proteins ; J. Agric. Food Chem., 56 (2008), pp. 8998–9005
- [47] K.C. Verhoeckx, Y.M. Vissers, J.L. Baumert, R. Faludi, M. Feys, S. Flanagan, C. Herouet-Guicheney, T. Holzhauser, R. Shimojo, N. van der Bolt, H. Wichers, I. Kimber; Food processing and allergenicity; Food Chem. Toxicol., 80 (2015), pp. 223–240
- [48] Y.M. Vissers, F. Blanc, P.S. Skov, P.E. Johnson, N.M. Rigby, L. Przybylski-Nicaise, H. Bernard, J.M. Wal, B. Ballmer-Weber, L. Zuidmeer-Jongejan, Z. Szepfalusi, J. Ruinemans-Koerts, A.P. Jansen, H.F. Savelkoul, H.J. Wichers, A.R. Mackie, C.E. Mills, K. Adel-Patient; Effect of heating and glycation on the allergenicity of 2S albumins (Arah 2/6) from peanut; PLoS One, 6 (2011), p. e23998
- [49] Y.M. Vissers, M. Iwan, K. Adel-Patient, P. Stahl Skov, N.M. Rigby, P.E. Johnson, P. Mandrup Muller, L. Przybylski-Nicaise, M. Schaap, J. Ruinemans-Koerts, A.P. Jansen, E.N. Mills, H.F. Savelkoul, H.J. Wichers; Effect of roasting on the allergenicity of major peanut allergens Ara h 1 and Ara h 2/6: the necessity of degranulation assays; Clin. Exp. Allergy, 41 (2011), pp. 1631–1642
- [50] F. Wang, J.M. Robotham, S.S. Teuber, S.K. Sathe, K.H. Roux; Ana o 2: a major cashew (Anacardium occidentale L.) nut allergen of the legumin family ; Int. Arch. Allergy Immunol., 132 (2003), pp. 27–39
- [51] F. Wang, J.M. Robotham, S.S. Teuber, P. Tawde, S.K. Sathe, K.H. Roux; Ana o 1, a cashew (Anacardium occidental ) allergen of the vicilin seed storage protein family ; J. Allergy Clin. Immunol., 110 (2002), pp. 160–166
- [52] W. Wang, J. Han, Y. Wu, F. Yuan, Y. Chen, Y. Ge; Simultaneous detection of eight food allergens using optical thin-film biosensor chips; J. Agric. Food Chem., 59 (2011), pp. 6889–6894
- [53] Y. Wei, S.K. Sathe, S.S. Teuber, K.H. Roux; A sensitive sandwich ELISA for the detection of trace amounts of cashew (Anacardium occidentale L.) nut in foods ; J. Agric. Food Chem., 51 (2003), pp. 3215–3221
Document information
Published on 02/05/17
Accepted on 02/05/17
Submitted on 02/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?