Summary
The object of this study is to report a rare case of explosion during laparotomy where diathermy ignited intraperitoneal gas from a spontaneous stomach perforation. Fortunately, the patient survived but the surgeon experienced a finger burn. A literature review demonstrates other examples of intraoperative explosion where gastrointestinal gases were the fuel source. Lessons learned from these cases provide recommendations to prevent this potentially lethal event from occurring.
Keywords
gastrointestinal gases;intraoperative explosion;laparotomy;stomach perforation
1. Introduction
Fires and explosions in the operating theater are rare events. When they do occur they have the potential to cause significant harm and even death to patients as well as injury to staff and structural damage to equipment and theaters. The ingredients for an explosion are found in every operating theater.
Gastrointestinal gases have been reported as the fuel for explosions in surgery at all levels of the alimentary tract, at colonoscopy, at laparoscopy and after opening the peritoneal cavity in a patient with a perforated viscous. The following report highlights a case of an explosion that occurred on opening the peritoneal cavity with electrocautery in a patient who had a stomach perforation. Only one other case could be found in the literature of an explosion on entering the peritoneal cavity and that patient died.1 The paper will also review the literature with respect to explosions in the operating theater, where gastrointestinal gases were the fuel source and summarize the advice to minimize the risk of this most devastating event from occurring.
2. Case report
A 75-year-old obese lady presented to the emergency department via Queensland Ambulance Service with sudden onset of generalized abdominal pain. The pain had started approximately half an hour after dinner. She had eaten pork chops and vegetables. Her past medical history included Barretts esophagus with gastroesophageal reflux disease having had a laparoscopic fundoplication 4 years previously at a different hospital. She had received yearly endoscopy since then with the most recent being 12 months previously. The patient had a remote history of a gastric ulcer and admitted to being poorly compliant with proton pump inhibitors. There was no other significant medical history and the patient was not taking any non-steroidal anti-inflammatory medications. She reported being well up until that evening with no vomiting prior to the onset of pain.
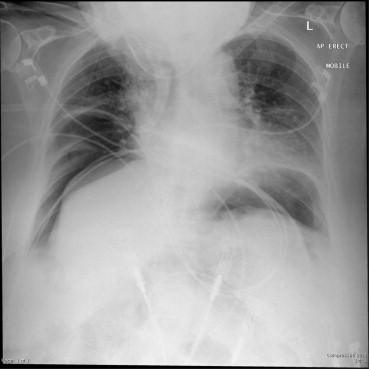
On examination the patient looked unwell. She was afebrile. Her heart rate (HR) was fluctuating 120–140 with periodic bradycardic episodes to a rate of 50. She was hypertensive with oxygen saturations initially 90% on room air but 100% on high flow oxygen via a non-rebreather mask. She was tachypneic with respiratory rate of 28. Abdominal examination demonstrated a distended abdomen with generalized involuntary guarding and percussion tenderness. An urgent chest X-ray revealed extensive free gas (Fig. 1). The patient was transferred to the operating theater with a presumptive diagnosis of gastric or duodenal perforation, with a differential diagnosis of diverticular perforation.
|
|
|
Figure 1. Mobile erect chest X-ray demonstrating free gas. |
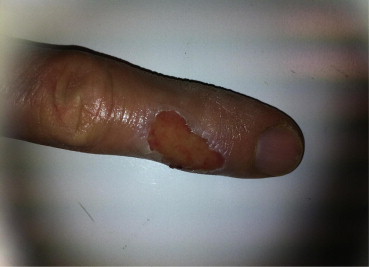
The patient had a general anesthetic and an endotracheal tube was placed. A nasogastric tube was not inserted. A midline laparotomy was performed with diathermy cautery through the subcutaneous fat and linea alba. The peritoneum was also entered using diathermy with vertical traction on the abdominal wall from both the surgeon and the assistant. At the instant the peritoneal cavity was breached, a low pitch roar was heard and a jet of blue flame was identified coming from the peritoneal defect. The flame was extinguished with an abdominal pack with no evidence of thermal injury to the patient. No change in the patients heart rate, blood pressure or electrocardiogram (ECG) trace was noted. The surgeon experienced a second-degree burn to his left index finger (Fig. 2). An initial examination of the abdomen failed to demonstrate any visceral injury related to the explosion.
|
|
|
Figure 2. Finger of the surgeon on Day 1 following a burn from the intra-abdominal explosion. |
The operation proceeded and after division of the gastrocolic ligament a 5-cm linear gastric perforation in the posterior wall of the stomach was identified and primarily repaired. A large amount of partially digested food was retrieved from the stomach and lesser sac. There was no evidence of ulceration or malignancy on the mucosal surface or adjacent to the gastric defect. During the case, blood was noted to be pooling in the left upper quadrant of the abdomen. The spleen was examined and found to have a 3-cm laceration in the lower pole despite no handling in this region. A splenectomy was performed. The patient was transferred to the intensive care unit. The postoperative course was slow. The patient was treated with intravenous antibiotics for pneumonia and had a superficial wound dehiscence requiring daily dressings. A computed tomography-guided percutaneous drain was placed on Postoperative Day 11 for a fluid collection in the splenic bed. The patient was discharged from hospital on Day 21. The surgeons finger healed with simple dressings in 2 weeks with no scarring.
3. Discussion
Three ingredients are necessary for a fire to occur: fuel, oxygen and a source of ignition. Oxygen is ubiquitous in the operating theater from either atmospheric air, supplemented oxygenation or as a byproduct of nitrous oxide. The ignition source may be electrocautery, faulty electric equipment, or laser with the former making up the majority. Gastrointestinal gases, gases produced in the bladder during electroresection, skin-sterilizing agents, and disposable materials (including drapes, swabs, tracheal tubes, nebulizers) are all fuels that have resulted in fires or explosions. This review will focus on fires that have occurred with gastrointestinal gases as the principle fuel.
Flammable gases are produced at all levels in the gastrointestinal tract. An explosion will occur when a suitable concentration of flammable gases are ignited by a spark. Methane and hydrogen are the principle flammable gases produced. Methane production occurs in a constant fashion from endogenous enteric bacteria and is unrelated to diet. This mostly occurs in the colon but may occur in the small bowel and stomach in the setting of obstruction. Approximately one-third of the population are “methane producers”,2 and these patients are at greater risk of developing potentially explosive gas mixtures in the colon.
Hydrogen is dependent on bacterial metabolism of ingested material and the fermentation of non-absorbable or incompletely absorbed carbohydrates. Other factors thought to affect bowel gas concentration are diffusion of gas from blood and swallowed air. Hydrogen concentration more than 4% and less than 75% will potentially cause an explosion if the oxygen concentration is greater than 4%.
The corresponding range of methane is 4.4–16.3% if the oxygen concentration is greater than 10.7%.3
Explosion during colonoscopy has been widely reported in the literature since the first case in 1954.4
Manner et al3 reported on eight cases of colonic explosion during argon plasma coagulation (APC) over a 10-year period. They found seven of the eight cases occurred when enemas were used rather than full bowel preparation and concluded meticulous full bowel cleansing should be carried out before any APC treatment of the colon. Mannitol and sorbitol preparations were formerly considered the reference agents for bowel preparation. Bacterial fermentation of these agents was thought to be responsible for the production of explosive gas mixtures. Polyethylene glycol electrolyte solutions were introduced to avoid the use of sugar compounds in bowel preparation and this has seen a reduction in the reported cases of explosion. Other methods reported to reduce the risk of explosion during colonoscopy are insufflation of carbon dioxide at the time of the procedure and preadministration of antibiotics. The administration of antibiotics prior to colonoscopy is not practical and its benefit in the post mannitol era is debated.
Opening the bowel with electrocautery, particularly if the bowel is obstructed, may lead to an explosion.
The most commonly reported region is the colon. Five cases of colonic explosion on opening the colon with diathermy were identified between 1961 and 1993.5; 6; 7; 8 ; 9 In all cases, colonic distension was noted at the time of surgery.
Three cases have been reported of explosion on opening the small bowel with electrocautery.10; 11 ; 12
All authors concluded that diathermy should not be used to open the bowel in the setting of obstruction. Of note, in all cases where the anesthetic agent was reported, nitrous oxide (NO) was used to maintain anesthesia. Patients ventilated on NO have been shown to increase their intestinal gas volumes.13 NO can also provide oxygen for combustion.
Up to 50% of normal individuals have combustible concentrations of gases in the unprepared colon.14 As both methane and hydrogen are dependent on bacterial actions, the relative sterility of both the stomach and small bowel would preclude high concentrations of these gases. However, in the setting of obstruction, bacterial overgrowth may occur. Pyloric stenosis has long been reported to produce combustible gas concentration in the stomach. There are several sensational incidents of patients “breathing fire” when belching in close proximity to a lit cigarette.15 The mechanism is thought to be prolonged fermentation of foodstuffs within the stomach. Explosions on entering the stomach with diathermy have been reported.16 ; 17 In both cases, gastric outlet obstruction was present. In one case, the explosion occurred despite full nasogastric decompression.
Only one previous report of an explosion on entering the abdominal cavity with diathermy was identified.1 In this case, the patient had perforated their stomach within a large hiatus hernia. Prolonged cardiopulmonary resuscitation was required for a ventricular fibrillation (VF) cardiac arrest prior to entering the operating theater.
Immediately after the explosion, the patient had severe hemodynamic compromise and laparotomy identified significant injuries to the diaphragm, spleen, and anterior abdominal wall musculature. The operation proceeded, but the patient died 3 hours after the procedure.
Explosions during laparoscopy have been reported when oxygen and NO were used for peritoneal insufflation. No such reports have occurred with carbon dioxide. Greilich et al18 reported on a case of intra-abdominal explosion during laparoscopic cholecystectomy in which combined oxygen and carbon dioxide were accidentally insufflated rather than pure CO2. The ignition was caused by electrocautery. The use of CO2 has added benefits of high blood solubility and, therefore, reduces the likelihood of a gas embolus.
In our case, there was no evidence of obstruction, ulceration or malignancy and the cause of the perforation is presumed to be spontaneous. It would appear that the explosive gas mixture was produced by fermentation of food in the stomach. The time of ingestion of dinner to the time of operation was approximately 3 hours. It is also probable that preoxygenation of the patient increased the gastric and subsequent peritoneal oxygenation concentration, producing a more volatile gas mixture. The significance of this patients past fundoplication is uncertain. Posterior stomach perforations may cause free intraperitoneal gas via the epiploic foramen and there are multiple reports of this in stomach perforations during cardiopulmonary resuscitation.19
The splenic laceration was considered most likely to be iatrogenic due to downward traction on the stomach rather than directly related to the explosion; this is because there was no injury evident on initial examination. A splenectomy was favored over a splenic preserving repair due to the laceration not being amenable to splenorrhaphy, patient age, the degree of abdominal contamination, unfavorable patient body habitus, and the decision to finish the operation so the patient could be sent to the intensive care unit for warming and further resuscitation. It is interesting that no reports exist of explosions following laparotomy for colonic diverticular perforations given the greater gas volatility within the colon. This may be explained by the lack of oxygen normally present in the colon (5%) as opposed to the stomach (10%) and the fact that oxygen administered by the anesthetists would not contribute to gas in the lower alimentary tract.15
We provide the following recommendations for preventing explosions in the operating theater where gastrointestinal gases are the fuel source:
- Colonoscopy
- Avoid preparation solutions containing mannitol or other malabsorbed sugars
- Only perform APC following full bowel preparation
- Limit time or avoid electrocautery during snaring of colonic polyps without full bowel preparation
- Use CO2 insufflation
- Enterotomy
- Avoid electrocautery enterotomy to any obstructed bowel at all levels of alimentary tract
- When performing stricturoplasty of small bowel for Crohn disease, first decompress the bowel with a knife or scissors before extending the enterotomy with diathermy
- Avoid nitrous oxide as anesthetic agent where bowel obstruction is suspected
- Laparotomy with free gas or suspected perforated viscous
- Open peritoneum with knife or scissors
- Avoid excessive preoxygenation
- Laparoscopy
- Ensure pure CO2 insufflation
4. Conclusion
Explosions in the operating theater are rare but potentially devastating events. In this case of an explosion on entering the peritoneum in a patient with a stomach perforation, the patient was fortunately uninjured but the surgeon suffered a partial thickness burn to his finger. Unfortunately, other patients suffering similar explosions have fared far worse with numerous deaths reported. We have provided a list of recommendations to prevent explosions of intestinal gases and specifically in this case recommend opening the peritoneum with scissors or a knife to decompress the peritoneal cavity before diathermy is used.
References
- 1 A. Dhebri, S. Afify; Free gas in the peritoneal cavity: the final hazard of diathermy; Postgrad Med J, 78 (2002), pp. 496–497
- 2 J. Bond, R. Ingal, M. Levitt; Explosion of hydrogen gas in the colon during proctosigmoidoscopy; Gastrointest Endosc, 23 (1976), pp. 41–42
- 3 H. Manner, N. Plum, O. Pech, et al.; Colon explosion during argon plasma coagulation; Gastrointest Endosc, 67 (2008), pp. 1123–1127
- 4 Levy El; Explosions during lower bowel electrosurgery; Am J Surg, 88 (1954), pp. 754–758
- 5 N. Shinagawa, Y. Mizuno, Y. Shibata, et al.; Gas explosion during diathermy colostomy; Br J Surg, 72 (1985), p. 306
- 6 M. Altomare, V. Memeo; Colonic explosion during diathermy colostomy; Dis Colon Rectum, 36 (1993), pp. 291–292
- 7 M. Barrkman; Intestinal explosion after opening a caecostomy with diathermy; BMJ, 1 (1965), pp. 1594–1595
- 8 C. Bellmore; Colostomy explosion; ANZ J Surg, 31 (1962), pp. 325–326
- 9 J. Hussey, A. Pois; Bowel-gas explosion – an unusual surgical complication; Am J Surg, 120 (1970), pp. 103–105
- 10 J. Branday; Jejunal gas explosion resulting from the use of diathermy; Br J Surg, 69 (1982), p. 728
- 11 D. Vellar, R. Pucius, I. Vellar; Explosion injury of the proximal jejunum caused by diathermy in a patient with obstructing sclerosing peritonitis; Br J Surg, 73 (1986), pp. 157–158
- 12 T. Brown, J.M. Church; Small bowel explosion: a complication of strictureplasty; Int J Colorect Dis, 9 (1994), pp. 5–7
- 13 E. Eger; Hazards of nitrous oxide anesthesia in bowel obstruction and pneumothorax; Anesthesiology, 26 (1965), p. 61
- 14 H. Ragins, H. Shinya, W. Wolfe; The explosive potential of colonic gas during colonoscopic electrosurgical polypectomy; Surg Gynecol Obstet, 138 (1974), pp. 554–556
- 15 A. Macdonald; A brief historical review of non-anaesthetic causes of fires and explosions in the operating theatre; Br J Anaesth, 73 (1994), pp. 847–856
- 16 J. Earnshaw, T. Keane; Gastric explosion: a cautionary tale; BMJ, 298 (1989), pp. 93–94
- 17 G. Mills, R. Jones; Explosion during surgery for gastric outlet obstruction; Anaesthesia, 48 (1993), p. 544
- 18 P. Greilich, N. Greilich, E. Froelich; Intraabdominal fire during laparoscopic cholecystectomy; Anesthesiology, 83 (1995), pp. 871–874
- 19 I. Spoormans, K. Van Hoorenbeeck, L. Balliu, P.G. Jerens; Gastric perforation after cardiopulmonary resuscitation: review of literature; Resuscitation, 81 (2010), pp. 272–280
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?