Abstract
Background
Although left ventricular (LV) global systolic longitudinal strain (GLS) reliably and accurately assesses LV systolic function and is also a powerful prognostic predictor, the importance and prognostic value of GLS in end-stage renal disease patients receiving maintenance peritoneal dialysis (PD) remain unclear. This study sought to determine the prognostic value of GLS in chronic PD patients.
Methods
This prospective study collected clinical and echocardiographic data from 106 stable PD patients (50.0 ± 13.9 years, 45% male) in a dialysis unit of a university hospital. These patients were enrolled from April 2010 to June 2010 and followed until August 2013 (follow-up duration 30.3 ± 14.3 months). The primary outcomes were the presence of major adverse events (MAEs), defined as all-cause mortality, and major adverse cardiovascular cerebral events (MACCEs), i.e. cardiovascular death, cardiac hospitalization, and stroke.
Results
Twenty-nine patients (27%) reported a primary outcome. Patients with MAEs had worse LV systolic function (MAEs vs. no MAEs, − 14.8 ± 2.8 vs. − 17.1 ± 2.5%, p = 0.003). Using multivariate Cox regression analyses, being male, having a history of heart failure, diabetes mellitus, an increased pulse pressure (≥ 60 mm Hg), and GLS ≥ − 15% were independent predictors of MAEs. The independent risk factors of MACCEs were a history of diabetes mellitus, an increased pulse pressure, and GLS ≥ − 15%. After comparison of the overall log likelihood χ2 of the predictive power, GLS was found to add prognostic information to a model based on traditional risk factors.
Conclusion
GLS ≥ − 15% provided additional prognostic information that allowed for the early identification of high-risk PD patients.
Keywords
Echocardiography;End-stage renal disease;Peritoneal dialysis;Prognosis
1. Introduction
Despite significant advances in dialysis, mortality and morbidity in end-stage renal disease (ESRD) patients receiving maintenance hemodialysis or peritoneal dialysis (PD) remain high and are important unresolved issues [1] ; [2]. The identification of high-risk patients would allow physicians to optimize therapeutic interventions, which may lower morbidity and mortality. Cardiovascular diseases, such as left ventricular (LV) hypertrophy, coronary artery disease (CAD), and heart failure (HF), frequently occur in dialysis patients [3]; [4] ; [5]. Importantly, cardiac structural and functional abnormalities are associated with high mortality in ESRD patients [6]; [7] ; [8]. Although cardiac geometry and function in ESRD patients have been extensively studied using conventional echocardiography, this method provides a semi-quantitative evaluation and cannot detect subclinical cardiac dysfunction [9].
Compared with conventional echocardiographic measurements, speckle-tracking echocardiography (STE) with myocardial deformation analysis (2D strain) is a more accurate, objective, reproducible, and sensitive modality for assessing cardiac function, even among HF patients with preserved LV ejection fraction (LVEF) and chronic kidney disease patients [10]; [11]; [12]; [13] ; [14]. Previous studies indicated that LV global peak systolic longitudinal strain (GLS) is a load-independent measurement of LV systolic function [14]; [15]; [16] ; [17]. In the general population, GLS is a powerful prognostic predictor [18] ; [19]. Nevertheless, the prognostic role of GLS has not been validated in PD patients. Thus, we conducted a prospective observational study to assess the prognostic role of GLS in chronic PD patients.
2. Methods
ESRD patients on continuous PD therapy over 3 months were eligible for this study. These patients were prospectively screened in a single PD unit of the National Cheng Kung University Hospital in Tainan, Taiwan. Patients who were between 18 and 80 years of age, did not have an obvious volume overload, and were willing to join this study were enrolled from April 2010 to June 2010. The exclusion criteria included HF, diagnosed according to the European Society of Cardiology HF criteria [20], presenting with pulmonary edema in the past 6 months, history of acute coronary syndrome in the past 6 months, chronic atrial fibrillation, moderate to severe valvular heart disease (including mitral/aortic regurgitation or stenosis), and inadequate echocardiographic imaging quality. The study adhered to the Declaration of Helsinki and was approved by the Human Research and Ethics Committee of National Cheng Kung University Hospital (IRB number: ER-98-219). All enrolled patients provided informed consent.
Clinical information on co-morbidities, medical history, and current cardiovascular medications was obtained by careful review of each patients medical record and a self-reported questionnaire. Patient compliance with prescribed medication regimens was reliably ascertained. All participants were primarily dialyzed using conventional lactate-buffered glucose-based PD solutions. Residual renal function (RRF) and daily urine volume were measured from a 24-hour urine sample. RRF was calculated as the mean of the 24-hour creatinine clearance and urea clearance normalized to the standard body surface area of 1.73 m2[21]. Patients with a urine volume less than 100 ml/day were assumed to have no RRF. PD adequacy was evaluated by the total weekly Kt/V (product of dialyzer urea clearance and treatment time divided by the urea compartment volume), equal to 7 ∗ (24-hour urea clearance / total body water), with total body water estimated using the Watson formula [22].
After the patient was recumbent for 15 min during the echocardiographic examination, brachial arterial blood pressure was measured by a trained nurse using a validated sphygmomanometer as previously described [23]. Pulse pressure was calculated as the difference between the systolic and diastolic blood pressures. A pulse pressure ≥ 60 mm Hg is associated with an increased risk of mortality in PD patients; therefore, the cut-off point for an increased pulse pressure was defined as 60 mm Hg [24].
2.1. Biochemical analysis
Blood samples were collected upon study enrollment. Serum levels of creatinine, hemoglobin, cholesterol, triglycerides, low-density lipoprotein cholesterol, calcium, phosphate, and albumin were measured using routine methods.
2.2. Echocardiography measurements and analysis
All the patients were examined in the left lateral decubitus position using an ultrasound system with a 3.5-MHz probe (Vivid-i, GE Healthcare, Horten, Norway) by one experienced cardiologist and one well-trained echocardiographer who were blinded to all clinical details of the patients. According to the recommendations of the American Society of Echocardiography [25], quantification of the LV mass index (LVMi), LVEF, and left atrial volume index (LAVi) was performed. LV hypertrophy was defined as LVMi > 115 g/m2 for men and > 95 g/m2 for women. Pulse tissue Doppler imaging of the mitral annulus movement was performed in the apical 4-chamber view when a sample volume was first placed at the septal side and then at the lateral side of the mitral annulus. To obtain the peak systolic (s′) and early diastolic (e′) velocities, we measured 3 end-expiratory beats and averaged these values for further analysis. We used the average e′ velocity acquired from the septal and lateral sides of the mitral annulus to calculate the ratio of the mitral inflow E velocity to the e′ velocity (average E/e′ = E / [(e′septal + e′lateral) / 2]). Two-dimensional gray-scale images in three standard apical views (i.e., apical 4-chamber, apical 2-chamber, and apical long-axis) for three cardiac cycles were acquired and stored digitally with a frame rate of 50–90 frames/s for subsequent off-line analysis.
Using automated function imaging (AFI) software (EchoPAC work station, BT11, GE Healthcare, Israel), off-line image analysis was performed by two cardiologists who were blinded to the patient clinical information. Strain and strain rate were measured using the following protocol [11]; [14] ; [17]. The peak systolic longitudinal strain was obtained from the 3 standard apical views by AFI software, and the average value of peak systolic longitudinal strain from 3 apical views was regarded as GLS (Supplemental figure). Longitudinal systolic strain rate was automatically obtained from the three standard apical views, and six LV segments in the para-sternal short-axis view at the mid-papillary level were then examined to obtain the circumferential strain and systolic strain rate.
2.3. Follow-up and outcome measurements
The patients regularly visited our PD clinic from the day of enrollment until death, cessation of PD, or end of the study. There was only one patient with whom we could not follow up because of immigration to another country. The medical records of enrolled patients during the follow-up period (April 2010 to August 2013) were carefully reviewed. The primary outcomes were major adverse events (MAEs), including all-cause mortality, cardiovascular death, cardiac hospitalization due to cardiovascular events (e.g., decompensated HF with pulmonary congestion, CAD, fatal or non-fatal myocardial infarction (MI), or electrocardiographically documented arrhythmia requiring hospitalization; Supplemental Table 1), scheduled coronary revascularization (i.e., percutaneous transluminal coronary angioplasty and/or coronary artery bypass surgery), thromboembolic or hemorrhagic stroke, or newly diagnosed peripheral artery disease. The secondary outcomes were major adverse cardiovascular cerebral events (MACCEs) [26], i.e., MAEs other than non-cardiovascular death.
2.4. Statistical analysis
Continuous data are presented as the mean ± standard deviation or as the median (interquartile range), depending on the distribution. Dichotomous data are presented as numbers and percentages. Comparisons were conducted using Students t-test or the Mann–Whitney U test for continuous variables, which showed a normal or non-parametric distribution, respectively. A chi-square test or Fishers exact test was used for categorical variables where appropriate. The relationships between continuous variables were evaluated using Pearson correlation analysis.
We did the receiver operating characteristic (ROC) curve analysis and calculated the areas under the ROC curves (AUC) to evaluate the prognostic performance of different LV echocardiographic parameters for MAEs and MACCEs.
Patients were stratified into two groups according to their GLS value. The Kaplan–Meier method was used with a log-rank test to compare event-free rates between strata. In this analysis, patients who received renal transplantation or were permanently transferred to hemodialysis were censored at the time of alternative renal replacement therapy (RRT). A patient was not censored when he or she reached one of the endpoints within 3 months of transferring to another RRT because such an event should be considered as a reflection of health status during the PD period.
A univariate Cox regression analysis was performed to evaluate factors associated with MAEs or MACCEs. Factors with p < 0.1 based on a univariate analysis were used in the multivariate Cox regression analysis to investigate risk factors for MAEs and MACCEs. The final multivariate Cox regression models were validated by a bootstrap resampling procedure with 3000 samples [27] ; [28].
The prognostic value of GLS over the demographic, clinical, and conventional echocardiographic parameters was assessed in 4 modeling steps: model 1 adjusted for demographic parameters, i.e. age and gender. Model 2 adjusted for model 1 factors and LVMi and pulse pressure. Model 3 adjusted for model 2 factors and clinical parameters (co-morbidities). Model 4 adjusted for model 3 factors and GLS. We performed the − 2log likelihood ratio statistic, following a χ2 distribution to evaluate the significance of improvement in model prediction and p value was based on the incremental value compared to the previous model.
Intra- and inter-observer reliabilities of GLS measurement were assessed in 2 sets of 30 randomly selected subjects by performing Bland–Altman analysis [29] of agreement and calculating the interclass correlation coefficient. A two-sided p < 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS software package, version 22.0 (SPSS Inc.).
3. Results
There were 126 PD patients eligible for this study but 13 patients did not provide informed consent. A total of 113 PD patients were prospectively enrolled. However, 7 patients were excluded, including patients who did not undergo echocardiographic examination (n = 3), had poor echocardiographic images for analysis (n = 2), were unable to undergo 2D strain analysis because of chronic atrial fibrillation with a variable heart rate (n = 1), and were lost to follow-up due to immigration to another country (n = 1). Therefore, 106 patients were included in the final analyses. The baseline demographic, clinical, and biochemical data and echocardiographic results are summarized in Table 1 ; Table 2. Fifty-four patients (51%) had no RRF. The mean total Kt/V and hemoglobin values were 2.18 ± 0.31 and 10.8 ± 1.7 g/dl, respectively.
| Total (n = 106) | No MAEs (n = 77) | MAEs (n = 29) | pa | |
|---|---|---|---|---|
| Age (years) | 50.0 ± 13.9 | 48.3 ± 12.5 | 54.5 ± 16.2 | 0.07 |
| Male, n (%) | 48 (45%) | 28 (36%) | 20 (69%) | 0.003 |
| Body mass index (kg/m2) | 22.9 ± 3.7 | 23.1 ± 3.9 | 22.5 ± 3.4 | 0.48 |
| Total Kt/V | 2.18 ± 0.31 | 2.19 ± 0.32 | 2.17 ± 0.27 | 0.76 |
| PD duration (years)b | 4.1 (1.3, 7.3) | 4.2 (1.3, 8.5) | 3.1 (1.4, 5.0) | 0.15 |
| SBP (mm Hg) | 133.2 ± 18.4 | 128.9 ± 16.8 | 141.8 ± 19.8 | 0.006 |
| DBP (mm Hg) | 77.8 ± 13.1 | 78.4 ± 12.0 | 76.1 ± 15.9 | 0.49 |
| Pulse pressure (mm Hg) | 55.4 ± 14.3 | 51.5 ± 11.8 | 65.7 ± 15.4 | < 0.001 |
| Increased pulse pressure (≥ 60 mm Hg), n (%) | 36 (34%) | 18 (23%) | 18 (62%) | < 0.001 |
| Heart rate (beats/min) | 77.7 ± 13.1 | 77.5 ± 13.4 | 78.6 ± 10.8 | 0.67 |
| Presence of RRF, n (%) | 52 (49%) | 34 (44%) | 18 (62%) | 0.10 |
| Daily urine amount (ml)b | 0 (0, 600) | 0 (0, 575) | 180 (0, 625) | 0.28 |
| Clinical background comorbidities, number (%) | ||||
| Heart failure | 6 (6%) | 0 (0%) | 6 (6%) | < 0.001 |
| Coronary artery disease | 4 (4%) | 0 (0%) | 4 (4%) | < 0.001 |
| Diabetes mellitus | 23 (22%) | 10 (13%) | 13 (45%) | < 0.001 |
| Hypertension | 95 (90%) | 68 (88%) | 27 (93%) | 0.72 |
| Hypercholesterolemia | 61 (58%) | 45 (58%) | 16 (55%) | 0.76 |
| Peripheral arterial disease | 17 (16%) | 13 (17%) | 4 (14%) | > 0.99 |
| Left ventricular hypertrophyc | 64 (60%) | 41 (53%) | 23 (79%) | 0.02 |
| Cardiovascular drugs, number (%) | ||||
| ACEIs/ARBs | 35 (33%) | 21 (27%) | 14 (48%) | 0.06 |
| β-Blocker | 46 (46%) | 33 (43%) | 13 (45%) | 0.86 |
| CCB | 49 (46%) | 35 (45%) | 14 (48%) | 0.80 |
| Statin | 40 (38%) | 29 (38%) | 11 (38%) | 0.98 |
| Serum biochemical parameters | ||||
| Calcium (mg/dl) | 9.4 ± 0.9 | 9.5 ± 1.0 | 9.4 ± 0.7 | 0.56 |
| Phosphate (mg/dl) | 5.1 ± 1.1 | 5.0 ± 1.1 | 5.2 ± 1.2 | 0.43 |
| Albumin (g/dl) | 4.0 ± 0.4 | 4.0 ± 0.4 | 3.9 ± 0.4 | 0.54 |
| Cholesterol (mg/dl) | 183.1 ± 37.1 | 183.4 ± 35.3 | 182.2 ± 42.3 | 0.89 |
| Triglyceride (mg/dl)b | 131.5 (91.0, 201.0) | 137.0 (91.5, 192.0) | 118.0 (87.0, 211.0) | 0.73 |
| LDL-C (mg/dl) | 104.7 ± 34.6 | 106.0 ± 33.9 | 101.1 ± 36.6 | 0.53 |
| Hemoglobin (g/dl) | 10.8 ± 1.7 | 10.9 ± 1.8 | 10.5 ± 1.4 | 0.22 |
| CCr (ml/min/1.73 m2)b | 0 (0, 1.4) | 0 (0, 1.3) | 0.4 (0, 1.4) | 0.30 |
| Baseline echocardiographic measurements | ||||
| LV EDVi (ml/m2)b | 65.5 (53.0, 81.7) | 61.1 (50.9, 79.6) | 73.3 (59.0, 84.1) | 0.03 |
| LVMi (gm/m2)b | 113.2 (91.1, 133.4) | 102.7 (86.1, 132.2) | 127.7 (113.6, 162.4) | < 0.001 |
| IVCe diameter (cm) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.4 ± 0.3 | 0.002 |
| LV EF (%) | 66.8 ± 7.7 | 68.1 ± 6.0 | 63.4 ± 10.5 | 0.03 |
| s′ (cm/s) | 8.9 ± 2.1 | 9.2 ± 1.9 | 8.1 ± 2.5 | 0.03 |
| LV GLS (%) | − 16.5 ± 2.8 | − 17.1 ± 2.5 | − 14.8 ± 2.8 | 0.003 |
| Longitudinal SRs (s− 1) | − 0.92 ± 0.21 | − 0.96 ± 0.20 | − 0.82 ± 0.20 | < 0.001 |
| Patients with reduced GLS (≥− 15%), n (%) | 31 (29%) | 14 (18%) | 17 (59%) | < 0.001 |
| CS (%) | − 14.5 ± 3.9 | − 15.1 ± 3.9 | − 13.0 ± 3.5 | 0.01 |
| Circumferential SRs (s− 1) | − 1.04 ± 0.39 | − 1.08 ± 0.42 | − 0.91 ± 0.26 | 0.02 |
| E (m/s)c | 0.65 (0.57, 0.82) | 0.63 (0.57, 0.76) | 0.69 (0.59, 0.98) | 0.12 |
| A (m/s) | 0.89 ± 0.23 | 0.86 ± 0.22 | 0.97 ± 0.25 | 0.04 |
| E/Ab | 0.74 (0.65, 0.92) | 0.78 (0.66, 0.92) | 0.71 (0.60, 1.02) | 0.68 |
| e′ (cm/s) | 7.5 ± 2.5 | 7.7 ± 2.3 | 6.9 ± 2.9 | 0.19 |
| E/e′b | 9.3 (7.3 11.5) | 8.7 (7.0, 11.4) | 10.2 (8.0, 14.4) | 0.02 |
| LAVi (ml/m2) | 28.6 ± 11.3 | 26.9 ± 11.0 | 33.0 ± 11.1 | 0.02 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II-receptor blocker; CCB, calcium channel blocker; CCr, creatinine clearance rate; CS, circumferential strain; DBP, diastolic blood pressure; EDVi, end-diastolic volume index; EF, ejection fraction; E/A, early to late diastolic trans-mitral velocity ratio; E/e′, early trans-mitral velocity to tissue Doppler mitral annular early diastolic velocity ratio; e′, tissue Doppler mitral annular early diastolic velocity; GLS, global left ventricular peak systolic longitudinal strain; IVCe, end-expiratory inferior vena cava diameter; LAVi, left atrial volume index; LDL-C, low density lipoprotein of cholesterol; LV, left ventricular; LVMi, left ventricular mass index; MAE, major adverse event; PD, peritoneal dialysis; RRF, residual renal function; SBP, systolic blood pressure; SRs, systolic strain rate; s′, left ventricular systolic myocardial velocity.
Unless specified otherwise, data are expressed as mean ± SD or number (%).
a. p value for comparison between patients with and without MAEs by Students t-test for normal distributed continuous data, nonparametric test for non-normal distributed continuous data, and chi-square test or Fishers exact test for categorical variables.
b. Median (interquartile range).
c. Left ventricular hypertrophy was diagnosed by echocardiography.
| Variables | MAEs | MACCEs | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age (years) | 1.03 (1.002–1.06) | 0.03 | 1.01 (0.97–1.04) | 0.80 | 1.02 (0.99–1.05) | 0.18 | 0.99 (0.96–1.03) | 0.71 |
| Male | 3.15 (1.43–6.93) | 0.004 | 2.36 (1.05–5.31) | 0.04 | 3.18 (1.38–7.33) | 0.007 | 2.55 (1.09–5.98) | 0.03 |
| Background history of HF | 7.13 (2.85–17.8) | < 0.001 | 2.88 (1.11–7.46) | 0.03 | 6.38 (2.37–17.2) | < 0.001 | 2.44 (0.88–6.82) | 0.09 |
| Coronary artery disease | 5.28 (1.81–15.4) | 0.002 | – | – | 5.92 (2.01–17.4) | 0.001 | – | – |
| Diabetes mellitus | 3.57 (1.70–7.46) | 0.001 | 2.41 (1.13–5.13) | 0.02 | 3.75 (1.72–8.15) | 0.001 | 2.30 (1.03–5.14) | 0.04 |
| Increased pulse pressure (≥ 60 mm Hg) | 3.89 (1.83–8.24) | < 0.001 | 2.56 (1.18–5.56) | 0.02 | 4.03 (1.83–8.90) | 0.001 | 2.84 (1.27–6.35) | 0.01 |
| Presence of RRF | 1.85 (0.87–3.92) | 0.11 | – | – | 1.82 (0.83–4.01) | 0.14 | – | – |
| Albumin | 0.57 (0.20–1.63) | 0.29 | – | – | 0.60 (0.20–1.85) | 0.38 | – | – |
| LVMi | 1.01 (1.01–1.02) | 0.001 | 1.00 (0.99–1.01) | 0.64 | 1.01 (1.01–1.02) | 0.002 | 1.00 (0.98–1.01) | 0.55 |
| Reduced GLS (≥− 15%) | 3.95 (1.88–8.29) | < 0.001 | 2.26 (1.06–4.78) | 0.03 | 5.23 (2.33–11.7) | < 0.001 | 3.15 (1.38–7.18) | 0.006 |
Abbreviations: CI, confidence interval; HF, heart failure; HR, hazard ratio; GLS, global left ventricular peak systolic longitudinal strain; LVMi, left ventricular mass index; RRF, residual renal function.
–: not enrolled.
The median follow-up duration was 30.3 ± 14.3 months (range 1–42 months). During the follow-up period, 22 patients were permanently transferred to maintenance hemodialysis, 7 patients underwent renal transplantation, and 9 patients died. Seven of the deaths were not due to cardiovascular causes (pneumonia in 2 patients, other infections in 4 patients, and malignancy in 1 patient). Twenty-two patients had one or more cardiovascular cerebral events, including paroxysmal supraventricular tachycardia (suspicion of Wolff–Parkinson–White syndrome) in 1 patient, non-fatal MI in 2 patients, angina in 5 patients, decompensated HF with pulmonary edema in 7 patients, scheduled revascularization in 7 patients, cerebrovascular events in 4 patients, and peripheral artery disease with/without percutaneous transluminal angioplasty in 8 patients.
Overall, 29 patients (27.3%) experienced MAEs. The enrolled patients were stratified into two groups, namely, the MAE and MAE-free groups. Compared with the MAE-free group, the MAE group had an increased pulse pressure and higher rates of CAD, HF, diabetes mellitus, and LVH. In addition, there were more male patients in the MAE group (Table 1).
3.1. Evaluation of cardiac function (Table 1)
Sixty-four patients (60%) had LVH, and 4 patients had reduced LVEF (LVEF < 50%). The MAE group had a slightly deteriorated LV systolic function, represented by changes in the LVEF, s′, and GLS. Moreover, both groups had a reverse ratio between early and late LV filling velocity (E/A), high E/e′, and high LAVi, all of which are consistent with diastolic dysfunction.
3.2. Prognostic indicator stratification
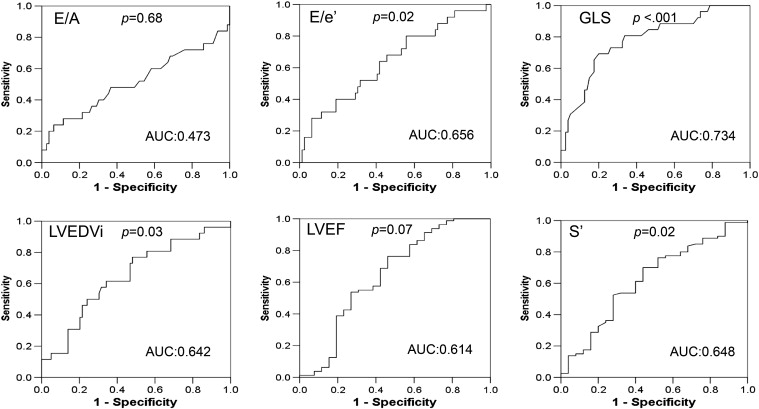
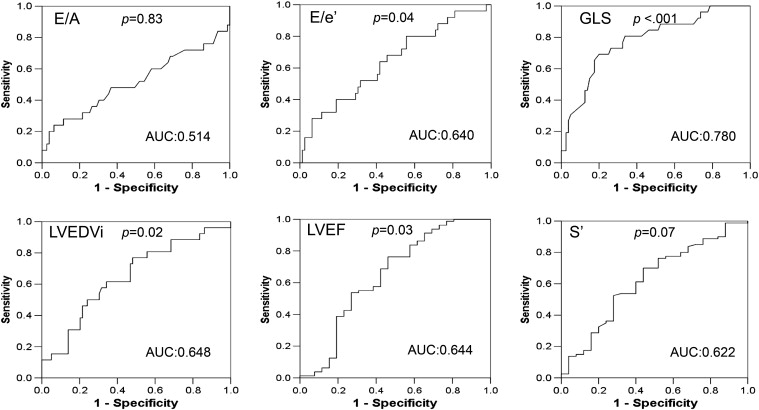
Because there were many echocardiographic parameters with significant differences between the MAE and MAE-free groups, we used Pearson correlation analyses to determine the correlation between GLS and these parameters, which could serve as potential prognostic factors. We found a significant correlation between GLS and the majority of echocardiographic measurements, including LVEDVi, LVMi, LVEF, s′, E/e′, systolic circumferential strain, and systolic circumferential/longitudinal strain rate (Supplemental Table 2). Compared to the LV conventional and TDI echocardiographic parameters, GLS had the highest AUC values of MAEs and MACCEs (Fig. 1 ; Fig. 2). We subsequently did multivariate Cox regression analyses to identify the independent echocardiographic parameters of MAEs and MACCEs and found that GLS was more powerful than the LV conventional and TDI measurements (Supplemental Table 3). Therefore, we did not include these parameters in the following Cox regression analyses.
|
|
|
Fig. 1. Receiver operating characteristic (ROC) curves and corresponding area under the curve (AUC) of major adverse events (MAEs) for left ventricular (LV) echocardiographic parameters: LV end-diastolic volume index (LVEDVi), LV ejection fraction (LVEF), early to late diastolic trans-mitral velocity ratio (E/A), early trans-mitral velocity to tissue Doppler mitral annular early diastolic velocity ratio (E/e′), LV systolic myocardial velocity (s′), and global LV peak systolic longitudinal strain (GLS). Only GLS had AUC value more than 0.7. |
|
|
|
Fig. 2. Receiver operating characteristic (ROC) curves and corresponding area under the curve (AUC) of major adverse cardiovascular cerebral events (MACCEs) for left ventricular (LV) echocardiographic parameters: LV end-diastolic volume index (LVEDVi), LV ejection fraction (LVEF), early to late diastolic trans-mitral velocity ratio (E/A), early trans-mitral velocity to tissue Doppler mitral annular early diastolic velocity ratio (E/e′), LV systolic myocardial velocity (s′), and global LV peak systolic longitudinal strain (GLS). Only GLS had AUC value more than 0.7. |
In a recent study, we demonstrated that GLS ≥ − 15% was an independent prognostic predictor of all-cause mortality in clinically stable hemodialysis patients with preserved LVEF [17]. In addition, several studies have shown that GLS ≥ − 15% should be considered pathological [23]; [24] ; [25]. Therefore, we defined the cutoff point of GLS to be − 15%.
Using a univariate Cox regression analysis, we subsequently found several potential MAE and MACCE risk factors, including old age, male gender, history of HF, CAD, diabetes, increased LVMi, increased pulse pressure (≥ 60 mm Hg), and GLS ≥ − 15% (Table 2). However, many covariates in the multivariate Cox model may provide an unreliable estimate because there were only 29 patients who reached a primary outcome. Therefore, we also used 5 multivariate Cox regression models to assess independent prognostic predictors of MAEs and MACCEs (Supplemental Tables 4 and 5). First, we performed multivariate Cox regression analysis with well-known prognostic risk factors of PD patients (model 1). We did not include CAD in model 2 because it was not a significant marker in model 1 and there were only 4 enrolled PD patients who had a history of CAD; thus, the regression analysis result was consistent with model 1. We added the factor of GLS in model 3 and the factors of GLS ≥ − 15% and CAD in model 4 (Table 2, Supplemental Tables 4 and 5). We also performed an analysis of model 5 because LAVi was reported as a prognostic indicator in PD patients; however, we found that LAVi was not significant in our PD patients. From these multivariate Cox regression models, we determined that male gender, history of HF and diabetes, increased pulse pressure, and GLS ≥ − 15% are significantly independent prognostic predictors of MAEs in PD patients (Table 2). We also found that male gender, diabetes mellitus, increased pulse pressure, and GLS ≥ − 15% were significantly independent MACCE predictors in PD patients (Table 2).
To test the stability of the final multi-variate Cox regression models, we performed a bootstrap investigation with 3000 samples using the same 7 variables. The bootstrap validation showed that the significant variables of MAEs were those selected in the original analysis with confidence intervals slightly larger than those from the original model; nevertheless, the significance of male gender for MACCEs was borderline (95% confidence interval [CI]: 0.99 to 5.58, p = 0.052).
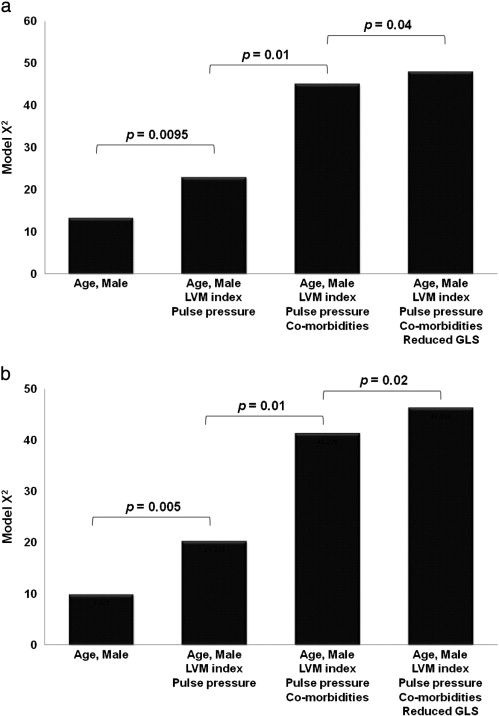
PD patients with GLS ≥ − 15% had a worse prognosis, including higher numbers of cardiac hospitalization episodes, scheduled revascularization, MACCEs, and MAEs (Table 3). Moreover, Kaplan–Meier survival curves demonstrated significant differences with respect to MAEs, MACCEs, and cardiovascular events (Fig. 3). GLS added incremental prognostic information for MAEs and MACCEs based on a comparison of the overall log likelihood χ2 of the predictive power (Fig. 4).
| Reduced GLS group (GLS ≥ − 15%, n = 31) | Preserved GLS group (GLS < − 15%, n = 75) | p | |
|---|---|---|---|
| Primary outcome | |||
| MAEs, n (%) | 17 (55%) | 12 (16%) | < 0.001 |
| Secondary outcomes | |||
| All-cause mortality, n (%) | 5 (16%) | 4 (5%) | 0.12 |
| Cardiovascular death, n (%) | 2 (6%) | 0 | 0.08 |
| Revascularization, n (%) | 5 (16%) | 2 (3%) | 0.02 |
| Admission due to heart failure, n (%) | 7 (23%) | 0 (0%) | < 0.001 |
| Admission due to cardiovascular events, n (%) | 12 (39%) | 2 (3%) | < 0.001 |
| Admission due to stroke, n (%) | 3 (10%) | 1 (1%) | 0.07 |
| New onset PAD with/without PTA, n (%) | 3 (10%) | 5 (7%) | 0.69 |
| MACCEs, n (%) | 17 (%) | 9 (12%) | < 0.001 |
Abbreviations: GLS, global left ventricular peak systolic longitudinal strain; MACCEs, major adverse cardiovascular cerebral events (including cardiovascular death, revascularization, and admission due to cardiovascular events, [i.e. heart failure, angina, arrhythmia, and fatal/non-fatal myocardial infarction], stroke); MAEs, major adverse events (including MACCEs and all-cause mortality); PAD, peripheral artery occlusive disease; PTA, percutaneous transluminal angioplasty.
|
|
|
Fig. 3. Kaplan–Meier estimates of (a) major adverse events, (b) major adverse cardiovascular cerebral events, and (c) cardiovascular events in peritoneal dialysis patients using the cutoff value for left ventricular global peak systolic longitudinal strain (GLS). |
|
|
|
Fig. 4. The incremental prognostic information of age, male gender, left ventricular mass index (LVMi), pulse pressure, underlying co-morbidities, and left ventricular global peak systolic longitudinal strain (GLS) for (a) major adverse events and (b) major adverse cardiovascular cerebral events. |
3.3. Inter- and intra-rater variability
The intra- and inter-observer correlation coefficients of the average measures for GLS were 0.98 (95% CI: 0.95 to 0.99) and 0.97 (95% CI: 0.94 to 0.99), respectively. A Bland–Altman analysis revealed no systemic bias of GLS between intra- and inter-rater agreements. The mean intra- and inter-rater differences [mean ± standard deviation (95% limits of agreement)] for GLS were − 0.02 ± 0.61 (− 1.24 to 1.21) and − 0.26 ± 0.72 (− 1.71 to 1.19), respectively.
4. Discussion
This is the first study to demonstrate that GLS is a prognostic predictor of MAEs and MACCEs in stable PD patients; furthermore, GLS adds incremental prognostic information for clinically stable ESRD patients receiving maintenance PD. In this prospective study of 106 clinically stable PD patients, we identified powerful prognostic predictors of MAEs and MACCEs, including a history of HF or diabetes, male gender, an increased pulse pressure (≥ 60 mm Hg), and GLS ≥ − 15%. It is noteworthy that GLS provided addictive prognostic information to a model based on the predictors of male gender, history of HF and diabetes, and increased pulse pressure. Based on these data, we have validated the clinical application of GLS for risk assessment in clinically stable chronic PD patients.
Despite significant improvement in dialysis modalities and medical care, the high mortality and morbidity of ESRD patients remain important and unresolved issues. Therefore, early identification of high-risk patients may enable physicians to optimize therapeutic interventions and improve the prognosis of dialysis patients. Several studies have focused on this issue and discovered multiple prognostic predictors, including a history of HF [30], increased pulse pressure [24], increased LVMi [31], and serum biomarkers, such as hemoglobin [2], albumin [2], cardiac troponin T (cTnT) [31] ; [32], and N-terminal pro-brain natriuretic peptide (NT-pro-BNP) [33]. Moreover, increased NT-pro-BNP or cTnT levels may suggest pathological cardiac structures and/or cardiac dysfunction [31] ; [34]. Although cardiac function in ESRD patients has been extensively studied by measuring LVEF, LVMi, LAVi, and E/A, these conventional echocardiographic parameters only provide a semi-quantitative evaluation and cannot detect subclinical cardiac dysfunction [9]. Furthermore, LV hypertrophy and reduced LVEF are recognized as prognostic predictors in dialysis patients; however, most dialysis patients have LV hypertrophy and preserved LVEF (EF ≥ 50%), which may limit the clinical application of LV hypertrophy and LVEF as prognostic factors [1]; [7] ; [9]. As a result, the detection of subtle LV systolic dysfunction is important to accurately assess cardiac function in dialysis patients.
GLS has been shown to be a more accurate, reliable, and sensitive parameter for the assessment of LV systolic function in both HF patients with preserved LVEF and chronic hemodialysis patients [11]; [12] ; [14]. Importantly, our recent work also demonstrated the prognostic power of GLS in clinically stable hemodialysis patients with preserved LVEF [17]. Abnormal GLS (GLS ≥ − 15%) may be the precursor of overt uremic cardiomyopathy in dialysis patients. Although the mechanisms of abnormal GLS in ESRD patients are not fully elucidated, abnormal GLS represents subtle LV systolic dysfunction that has been shown to be significantly associated with poor prognosis in many studies [17]; [18]; [35]; [36] ; [37]. Furthermore, GLS ≥ − 15% may also be associated with microvascular ischemia caused by the reduction in the density of myocardial capillaries, myocardial fibrosis, or dialysis-related myocardial stunning [13]; [38]; [39] ; [40]. The predictive value of GLS for long-term prognosis in clinically stable ESRD patients undergoing chronic PD may be associated with the myocardial abnormalities in ESRD patients that lead to a poor prognosis.
This study has some limitations. First, although prevalent CAD was not a prognostic predictor in our study population, we recognize that the prognostic power of prevalent CAD may be underestimated because of the relatively small number of included patients. Second, all the PD patients were from a university hospital. We acknowledge that this population may not represent the general PD patient population; thus, our data should be cautiously applied to the overall PD population. We did not measure NT-pro-BNP or cTnT levels in this study. Although emerging evidence indicates the clinical relevance of NT-pro-BNP or cTnT in PD patients, neither NT-pro-BNP nor cTnT can replace echocardiography in the evaluation of cardiac function and cardiovascular risk profile. More importantly, echocardiography can provide more information than these serum biomarkers. At last, because of the limited outcome size and a significant correlation between GLS and the majority of conventional echocardiographic measurements, i.e. LVEDVi, LVMi, LVEF, s′, and E/e′, we did not include these parameters in multi-variate Cox regression analysis. We recognized that there might be interactions that cannot be assessed in the present study.
5. Conclusions
Among clinically stable PD patients, male gender, a history of HF, increased pulse pressure, and GLS ≥ − 15% are predictive of MAEs and MACCEs. The use of GLS added prognostic information to the clinical predictors and may allow for the early identification of high-risk PD patients. These findings highlight the application of GLS in clinical practice.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
This work was supported by the National Cheng Kung University Hospital (NCKUH) in Tainan, Taiwan (NCKUH research grant No. 20100074).
Appendix A. Supplementary data
Supplementary material.
References
- [1] C. deFilippi, S. Wasserman, S. Rosanio, E. Tiblier, H. Sperger, M. Tocchi, et al.; Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis; JAMA, 290 (2003), pp. 353–359
- [2] P. Vejakama, A. Thakkinstian, A. Ingsathit, P. Dhanakijcharoen, J. Attia; Prognostic factors of all-cause mortalities in continuous ambulatory peritoneal dialysis: a cohort study; BMC Nephrol, 14 (2013), p. 28
- [3] A.Y. Wang, M. Wang, C.W. Lam, I.H. Chan, S.F. Lui, J.E. Sanderson; Heart failure in long-term peritoneal dialysis patients: a 4-year prospective analysis; Clin J Am Soc Nephrol, 6 (2011), pp. 805–812
- [4] S. Silaruks, D. Sirivongs, D. Chunlertrith; Left ventricular hypertrophy and clinical outcome in CAPD patients; Perit Dial Int, 20 (2000), pp. 461–466
- [5] P.S. Parfrey, R.N. Foley, J.D. Harnett, G.M. Kent, D.C. Murray, P.E. Barre; Outcome and risk factors for left ventricular disorders in chronic uraemia; Nephrol Dial Transplant, 11 (1996), pp. 1277–1285
- [6] A.Y. Wang, C.W. Lam, C.M. Yu, M. Wang, I.H. Chan, S.F. Lui, et al.; Troponin T, left ventricular mass, and function are excellent predictors of cardiovascular congestion in peritoneal dialysis; Kidney Int, 70 (2006), pp. 444–452
- [7] R. Sharma, D.C. Gaze, D. Pellerin, R.L. Mehta, H. Gregson, C.P. Streather, et al.; Cardiac structural and functional abnormalities in end stage renal disease patients with elevated cardiac troponin T; Heart, 92 (2006), pp. 804–809
- [8] M. Goicoechea, S.G. de Vinuesa, F. Gomez-Campdera, J. Luno; Predictive cardiovascular risk factors in patients with chronic kidney disease (CKD); Kidney Int Suppl, 93 (2005), pp. S35–S38
- [9] N.C. Edwards, A. Hirth, C.J. Ferro, J.N. Townend, R.P. Steeds; Subclinical abnormalities of left ventricular myocardial deformation in early-stage chronic kidney disease: the precursor of uremic cardiomyopathy?; J Am Soc Echocardiogr, 21 (2008), pp. 1293–1298
- [10] H. Thibault, G. Derumeaux; Assessment of myocardial ischemia and viability using tissue Doppler and deformation imaging: the lessons from the experimental studies; Arch Cardiovasc Dis, 101 (2008), pp. 61–68
- [11] Y.W. Liu, W.C. Tsai, C.T. Su, C.C. Lin, J.H. Chen; Evidence of left ventricular systolic dysfunction detected by automated function imaging in patients with heart failure and preserved left ventricular ejection fraction; J Card Fail, 15 (2009), pp. 782–789
- [12] J. Wang, D.S. Khoury, Y. Yue, G. Torre-Amione, S.F. Nagueh; Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure; Eur Heart J, 29 (2008), pp. 1283–1289
- [13] Y.W. Liu, C.T. Su, C.C. Chou, S.P.H. Wang, C.S. Yang, Y.Y. Huang, et al.; Association of subtle left ventricular systolic dysfunction with elevated cardiac troponin T in asymptomatic hemodialysis patients with preserved left ventricular ejection fraction; Acta Sin Cardiol, 28 (2012), pp. 95–102
- [14] Y.W. Liu, C.T. Su, Y.Y. Huang, C.S. Yang, J.W. Huang, M.T. Yang, et al.; Left ventricular systolic strain in chronic kidney disease and hemodialysis patients; Am J Nephrol, 33 (2011), pp. 84–90
- [15] A.T. Burns, A. La Gerche, J. D'hooge, A.I. MacIsaac, D.L. Prior; Left ventricular strain and strain rate: characterization of the effect of load in human subjects; Eur J Echocardiogr, 11 (2010), pp. 283–289
- [16] L. Mendes, R. Ribeiras, T. Adragão, A.I. MacIsaac, D.L. Prior; Load-independent parameters of diastolic and systolic function by speckle tracking and tissue Doppler in hemodialysis patients; Rev Port Cardiol, 27 (2008), pp. 1011–1025
- [17] Y.W. Liu, C.T. Su, J.M. Sung, S.P. Wang, Y.R. Su, C.S. Yang, et al.; Association of left ventricular longitudinal strain with mortality among stable hemodialysis patients with preserved left ventricular ejection fraction; Clin J Am Soc Nephrol, 8 (2013), pp. 1564–1574
- [18] T. Stanton, R. Leano, T.H. Marwick; Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring; Circ Cardiovasc Imaging, 2 (2009), pp. 356–364
- [19] A. Mignot, E. Donal, A. Zaroui, P. Reant, A. Salem, C. Hamon, et al.; Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study; J Am Soc Echocardiogr, 23 (2010), pp. 1019–1024
- [20] J.J. McMurray, S. Adamopoulos, S.D. Anker, A. Auricchio, M. Bohm, K. Dickstein, et al.; ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC; Eur Heart J, 33 (2012), pp. 1787–1847
- [21] K.D. Nolph, H.L. Moore, Z.J. Twardowski, R. Khanna, B. Prowant, M. Meyer, et al.; Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis; ASAIO J, 38 (1992), pp. M139–M142
- [22] R.W. van Olden, R.T. Krediet, D.G. Struijk, L. Arisz; Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis; J Am Soc Nephrol, 7 (1996), pp. 745–750
- [23] M.C. Wang, W.C. Tsai, J.Y. Chen, J.J. Huang; Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease; Am J Kidney Dis, 45 (2005), pp. 494–501
- [24] W. Fang, X. Yang, J.M. Bargman, D.G. Oreopoulos; Association between pulse pressure and mortality in patients undergoing peritoneal dialysis; Perit Dial Int, 29 (2009), pp. 163–170
- [25] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, et al.; Recommendations for chamber quantification: a report from the American Society of Echocardiographys Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology; J Am Soc Echocardiogr, 18 (2005), pp. 1440–1463
- [26] A.J. IJsselmuiden, P.W. Serruys, A. Scholte, F. Kiemeneij, T. Slagboom, L.R. vd Wieken, et al.; Direct coronary stent implantation does not reduce the incidence of in-stent restenosis or major adverse cardiac events: six month results of a randomized trial; Eur Heart J, 24 (2003), pp. 421–429
- [27] P. Ortolani, A. Marzocchi, C. Marrozzini, T. Palmerini, F. Saia, N. Taglieri, et al.; Predictive value of high sensitivity C-reactive protein in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention; Eur Heart J, 29 (2008), pp. 1241–1249
- [28] B. Richter, L. Koller, P.J. Hohensinner, G. Zorn, M. Brekalo, R. Berger, et al.; A multi-biomarker risk score improves prediction of long-term mortality in patients with advanced heart failure; Int J Cardiol, 168 (2013), pp. 1251–1257
- [29] J.M. Bland, D.G. Altman; Statistical methods for assessing agreement between two methods of clinical measurement; Lancet, 1 (1986), pp. 307–310
- [30] A.G. Stack, D.A. Molony, N.S. Rahman, A. Dosekun, B. Murthy; Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States; Kidney Int, 64 (2003), pp. 1071–1079
- [31] D. Duman, S. Tokay, A. Toprak, A. Oktay, I.C. Ozener, O. Unay; Elevated cardiac troponin T is associated with increased left ventricular mass index and predicts mortality in continuous ambulatory peritoneal dialysis patients; Nephrol Dial Transplant, 20 (2005), pp. 962–967
- [32] A.Y. Wang, C.W. Lam, M. Wang, I.H. Chan, W.B. Goggins, C.M. Yu, et al.; Prognostic value of cardiac troponin T is independent of inflammation, residual renal function, and cardiac hypertrophy and dysfunction in peritoneal dialysis patients; Clin Chem, 53 (2007), pp. 882–889
- [33] A.Y. Wang, C.W. Lam, C.M. Yu, M. Wang, I.H. Chan, Y. Zhang, et al.; N-terminal pro-brain natriuretic peptide: an independent risk predictor of cardiovascular congestion, mortality, and adverse cardiovascular outcomes in chronic peritoneal dialysis patients; J Am Soc Nephrol, 18 (2007), pp. 321–330
- [34] M. Haapio, E. Honkanen, C. Ronco; Brain natriuretic peptide in peritoneal dialysis patients; Contrib Nephrol, 163 (2009), pp. 110–116
- [35] P. Lancellotti, E. Donal, J. Magne, M. Moonen, K. O'Connor, J.C. Daubert, et al.; Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay; Heart, 96 (2010), pp. 1364–1371
- [36] J.S. Dahl, L. Videbaek, M.K. Poulsen, T.R. Rudbaek, P.A. Pellikka, J.E. Moller; Global strain in severe aortic valve stenosis: relation to clinical outcome after aortic valve replacement; Circ Cardiovasc Imaging, 5 (2012), pp. 613–620
- [37] J. Koyama, R.H. Falk; Prognostic significance of strain Doppler imaging in light-chain amyloidosis; JACC Cardiovasc Imaging, 3 (2010), pp. 333–342
- [38] J.O. Burton, H.J. Jefferies, N.M. Selby, C.W. McIntyre; Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function; Clin J Am Soc Nephrol, 4 (2009), pp. 1925–1931
- [39] Y.W. Liu, C.T. Su, S.P. Wang, C.S. Yang, J.W. Huang, K.Y. Hung, et al.; Application of speckle-tracking echocardiography in detecting coronary artery disease in patients with maintenance hemodialysis; Blood Purif, 32 (2011), pp. 38–42
- [40] S.J. Kang, H.S. Lim, B.J. Choi, S.Y. Choi, G.S. Hwang, M.H. Yoon, et al.; Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension; J Am Soc Echocardiogr, 21 (2008), pp. 907–911
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?