Summary
Perivascular epithelioid cell tumor (PEComa) is a rare tumor. Here, we present data regarding clinical presentations, diagnoses, management, and prognosis of five cases of hepatic PEComa between January 2002 and December 2008. Ultrasonography showed hyperechoic masses in all patients. Precontrast computed tomography (CT) showed that all lesions scanned were heterogeneous in density and were heterogeneously enhanced in arterial phase images. In two cases, magnetic resonance imaging showed hypointensity on T1-weighted images and hyperintensity on T2-weighted images. In enhanced scanning, lesions showed asymmetrical enhancement during arterial phase imaging. All tumors were composed of varying proportions of smooth muscle, adipose tissue, and thick-walled blood vessels, and showed positive immunohistochemical staining for Human Melanoma Black-45. All patients underwent hepatectomy, and there was no evidence of recurrence or metastasis during the follow-up period.
Keywords
hepatectomy;Human Melanoma Black-45;liver;perivascular epithelioid cell tumor
1. Introduction
Perivascular epithelioid cell tumor (PEComa) is a rare tumor that derives from mesenchyma. PEComa occurs most commonly in the kidneys. Hepatic PEComa represents the second most common site of involvement. Rare occurrences at many other sites have been reported, including the uterus, retroperitoneum, mediastinum, renal capsule, the nasopharyngeal cavity, the buccal mucosa, the hard palate, penis, vagina, fallopian tube, abdominal wall, skin, stomach, and spinal cord.1; 2 ; 3 Most hepatic PEComas are benign. Malignant hepatic PEComa is extremely rare; only four cases have been reported.4 Hepatic PEComa is composed of varying amounts of smooth muscle cells, adipose tissue, and blood vessels. It shows typical immunophenotypic features of both smooth muscle and melanocytic differentiation.5 This heterogeneity makes the preoperative diagnosis by imaging, needle biopsy, and other techniques difficult. Because of low morbidity, there has been a lack of comprehensive understanding of this hepatic lesion.
In this article, we will present five pathologically proven cases of hepatic PEComa in our hospital between 2002 and 2008, retrospectively discuss their clinical and histologic features, and review the literature.
2. Patients and methods
2.1. Clinical history and presentation
Five surgically resected specimens of hepatic PEComa were available at the Shengjing Hospital, China Medical University, from five individual cases between January 2002 and December 2008. The specimens were from one male and four female patients, ranging in age from 26 to 57 years. In all patients, liver function and α-fetoprotein were normal. None of the patients were complicated with tuberous sclerosis complex (TSC), hepatitis B virus (HBV), or hepatitis C virus (HCV). One female patient was complicated with renal PEComa. None of the patients had received preoperative therapy. Two patients had symptoms caused by the large space-occupying lesion, but the other three patients were asymptomatic and were discovered incidentally during routine physical examination. One case had multiple tumors and the others had a sporadic tumor.
All tissues were reviewed independently by two pathologists. Histopathological diagnosis was made according to the World Health Organization classification of tumors of the liver and intrahepatic bile ducts.6 The most important diagnostic criterion was the presence of Human Melanoma Black-45 (HMB-45)-positive myoid cells.
2.2. Imaging representation
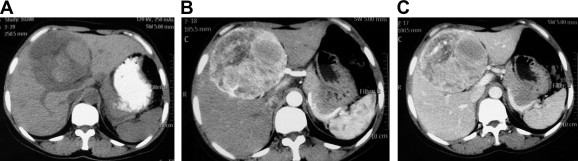
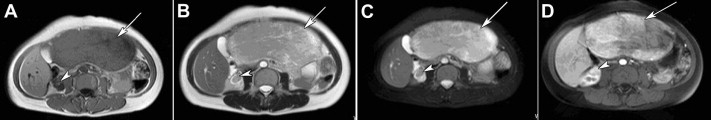
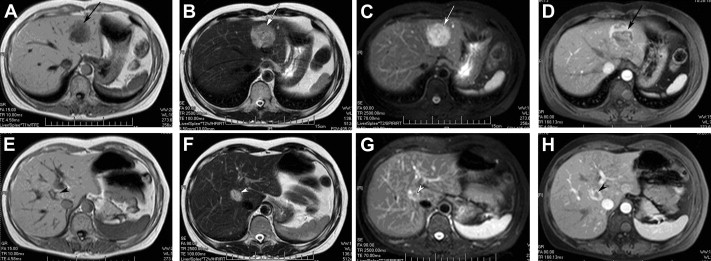
All patients received ultrasonography and computed tomography (CT) examinations, but only two patients received magnetic resonance imaging (MRI). On sonography, the tumors were seen as a hyperechoic mass, either homogeneous or heterogeneous. The lesions exhibited well-demarcated mass with heterogeneous density in plain CT scan. In arterial CT, the lesions were heterogeneously enhanced. In the portal venous phase, the lesions returned to an isoattenuating state (Fig. 1). In a 37-year-old woman complicated with renal PEComa, MRI showed heterogeneous hypointensity on T1-weighted images (T1WIs) and hyperintensity on T2-weigthed images (T2WI). After fat saturation, hyperintensity on T2WI in the liver lesion remained, but T2WI in the renal lesion showed hypointensity. In enhanced scanning, the lesions showed asymmetrical enhancement in arterial phase images (Fig. 2). In a 52-year-old woman with multiple liver lesions, MRI showed a lesion located on the left lobe and S8 of the liver. The lesions showed heterogeneous hypointensity on T1WI and heterogeneous hyperintensity on T2WI. After fat saturation, hyperintensity on T2WI remained. In enhanced scanning, the lesions showed asymmetrical enhancement in arterial phase images (Fig. 3). Prior to operation, one case was diagnosed with hepatic PEComa, three cases with hepatocellular carcinoma, and one case with hepatic adenomata.
|
|
|
Figure 1. CT image of hepatic PEComa. (A) Plain CT scan showed a well-demarcated mass with heterogeneous density in the liver. (B) Contrast-enhanced CT scan of the same lesion showed a heterogeneously enhanced lesion in the arterial phase. (C) Contrast-enhanced CT scan of the same lesion showed the lesion returned to an isoattenuating state in the portal phase. CT = computed tomography. |
|
|
|
Figure 2. MR image of hepatic PEComa (arrows) complicated with renal PEComa (arrow heads) in a 37-year-old woman. (A) T1-weighted MR image showed a heterogeneous hypointense mass with well-demarcated tumor margins. (B) T2-weighted MR image showed the same lesions with heterogeneous hyperintensity. (C) T2-weighted fat-suppressed MR image showed moderately heterogeneous hyperintensity in the liver lesion and moderate hypointensity in the renal lesion. (D) During the hepatic arterial phase, the lesions showed asymmetrical enhancement. MR = magnetic resonance; PEComa = perivascular epithelioid cell tumor. |
|
|
|
Figure 3. MR image of multiple hepatic perivascular epithelioid cell tumors in a 52-year-old woman. (A) T1-weighted MR image showed a heterogeneous hypointense mass in the left lobe (arrows). (B) T2-weighted MR image showed the same lesions in the left lobe with heterogeneous hyperintensity. (C) T2-weighted fat-suppressed MR image showed heterogeneous hyperintensity in the liver lesion in the left lobe. (D) During the hepatic arterial phase, the lesions in the left lobe showed asymmetrical enhancement. (E) T1-weighted MR image showed a heterogeneous hypointense mass in S8 (arrowheads). (F) T2-weighted MR image showed the same lesions in S8 with heterogeneous hyperintensity. (G) T2-weighted fat-suppressed MR image showed heterogeneous hyperintensity in the liver lesion in S8. (H) During the hepatic arterial phase, the lesions in S8 showed asymmetrical enhancement. MR = magnetic resonance. |
2.3. Pathologic findings
2.3.1. Gross
The tumors were all circumscribed, either unencapsulated or encapsulated. The cut surface was described as soft, with color descriptions ranging from yellow to dark red, and areas of hemorrhage were present in three cases. In a case of multiple PEComa, two lesions were 2.4 and 4 cm in their greatest dimension. The largest lesion was 23 × 16 × 6 cm3 and had a capsule.
2.3.2. Histopathology
In all cases, interpretation of frozen sectioning performed during surgery suggested benign hepatic neoplasm; the final diagnosis was deferred to the analysis of permanent sections. Routine histopathological examination with hematoxylin and eosin staining was performed. Immunohistochemical studies were performed by streptavidin peroxidase conjugated method, using a panel of antibodies [HMB-45, smooth muscle actin (SMA), melan-A, vimentin, CK8/18, hepatocyte, and S100] in all of the tumor tissues.
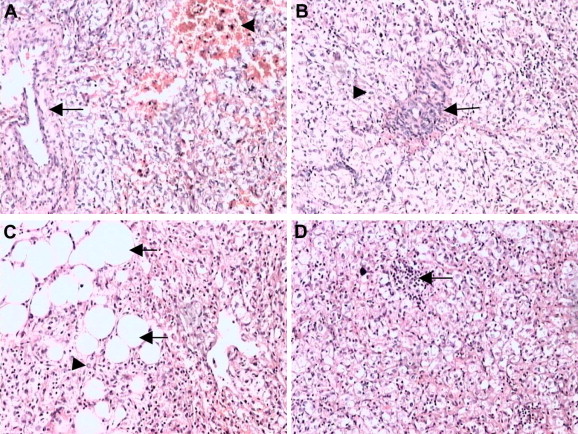
Microscopically, the tumor was composed of a mixture of thick-walled blood vessels, mature adipose tissue, and irregularly arranged bundles of smooth muscle. Extramedullary hematopoiesis was focally noted. Some cells were large, with clear cytoplasm and prominent perinuclear eosinophilic condensations (Fig. 4).
|
|
|
Figure 4. Microscopic appearances of hepatic perivascular epithelioid cell tumor. (A) Thick-walled blood vessels and smooth muscle; hemorrhage in smooth muscle (H&E 100×). (B) Smooth muscle cell surrounding thick-walled blood vessels (H&E 100×). (C) Sheets of adipocytes webbed among smooth muscle cells (H&E 100×). (D) Extramedullary hematopoiesis (H&E 100×). H&E = hematoxylin and eosin. |
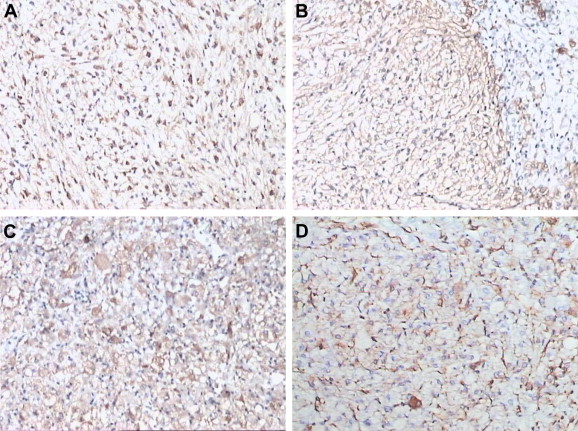
Immunohistochemically, the tumor cells were immunoreactive for HMB-45 in all five cases, for SMA in four cases, for vimentin in one case, and for melan-A in two cases (Fig. 5). The tumor cells were negative for S100, hepatocyte, and CK8/18.
|
|
|
Figure 5. Immunopathological characteristics of hepatic perivascular epithelioid cell tumor. (A) Human Melanoma Black-45 (SP method, 100×). (B) Smooth muscle actin (SP method, 100×). (C) Melan-A (SP method, 100×). (D) Vimentin (SP method, 100×). SP = streptavidin peroxidase. |
2.4. Treatment and follow-up
All patients received hepatectomy. Partial hepatectomy was performed in three patients, left hepatic lobectomy and partial right hepatectomy in a case of multiple hepatic PEComa, and partial hepatectomy and partial nephrectomy in a case complicated with renal PEComa. Prior to this study, all patients received a follow-up examination. The time of follow-up ranged from 29 to 101 months. No recurrence or metastasis was found in any patient, and liver function was normal during the follow-up period.
3. Discussion
The term of “perivascular epithelioid cell” (PEC) was first proposed by Bonetti et al in 1992.7 After several years, Zamboni et al8 coined the term “PEComa” for the family of related mesenchymal neoplasms containing the distinctive “PEC.” This type of tumor consists of three components: thick-walled, often hyalinized blood vessels; smooth muscle cells; and adipose tissue. Angiomyolipoma of the kidney9 ; 10 and liver,11 clear cell “sugar” tumor of the lung,12 ; 13 and lymphangiomyomatosis14 ; 15 are all PEComas.
Hepatic PEComa most commonly occurs in females and is partially associated with TSC in 6–10% of cases.11 ; 16 TSC is a group of autosomal-dominant genetic disorders caused by germ-line mutations in the TSC1 or TSC2 genes, which encode the proteins hamartin and tuberin, respectively. 17 ; 18
The majority of the hepatic lesions are solitary. Multiple PEComas of liver are extremely rare and usually occur in tuberous sclerosis patients. In 1995, Nonomura et al19 first reported two cases of multiple hepatic PEComas without any neurologic manifestations or any radiologic features suggestive of tuberous sclerosis. In recent years, analogous cases have been reported.20 In our study, a 52-year-old woman with multiple hepatic PEComas had two lesions distributed in the left lobe and S8 of the liver. The size of tumors was 4 cm × 4 cm × 2.5 cm, and 2.4 cm × 2.0 cm × 1.6 cm, respectively. The patient did not have TSC and was asymptomatic.
Regardless of their location, this family of tumors possesses similar immunohistochemical profiles, including positivity for melanocytic (HMB-45) and smooth muscle (SMA) markers. The HMB-45 marker was first reported in both renal and hepatic PEComas in 1991.21 ; 22 Soon afterward, additional studies confirmed these findings. Both melanocytic (HMB-45 and/or melan-A) and smooth muscle (muscle-specific actin and/or desmin) markers are helpful for confirming the diagnosis.23 ; 24 In the past, researchers assumed that PEComas expressed both melanocytic and smooth muscle markers at the same time. However, studies show that only 80% of cases express both, so negative expression of smooth muscle markers do not necessarily exclude diagnosis of PEComas.25 Besides HMA-45 and SMA, additional immunohistochemical markers including S-100, CD34, and CD117 were detected. Detection of these markers was not completely coincidental.1; 26 ; 27 Li et al26 have reported myoid cells positive for S-100 and CD34 in hepatic PEComa. However, Paiva et als27 resultswere negative. In our study, the tumor cells were immunoreactive for HMB-45 in five cases, for SMA in four cases, for vimentin in one case, and for melan-A in two cases. The tumor cells were negative for S100, hepatocyte, and CK8/18.
The variable proportions of the different components of hepatic PEComa make diagnosis difficult prior to surgery. In our study, one case was diagnosed with hepatic PEComa, three cases with hepatocellular carcinoma, and one case with hepatic adenomata. Histologically, the chief differential diagnostic considerations for hepatic PEComa are metastatic gastrointestinal stromal tumor and malignant melanoma, which include epithelioid cell, spindle cell, and positive CD117 expression.28 The vast majority of PEComas are benign, and the malignant form is extremely rare. Since relatively few malignant PEComas have been reported, firm criteria for malignancy have yet to be established. To date, there are only four case reports documenting the malignant form of hepatic PEComa,5; 29 ; 30 In a recent study, taking into account their data and a review of the literature, Nguyen et al summarize the similarities and differences between benign and malignant hepatic PEComas. The consistent features observed in all four cases include cytologic atypia and coagulative necrosis.5 Although cytologic atypia is usually a feature of malignant neoplasms, it is also present in benign hepatic PEComa.31 Coagulative necrosis is a consistent feature of malignant hepatic PEComa and appears to be a reliable indicator of malignancy. In proposing the features for malignant hepatic PEComa, this analysis shows that cytologic atypia and an infiltrative growth pattern are less important, whereas coagulative necrosis, larger tumor size (>10 cm), negativity for CD117, and clinical evidence of aggressive disease are more important features.31
If the diagnosis of hepatic PEComa has been made definitively by imaging techniques, fine needle aspiration, or needle biopsy prior to operation, conservative treatment may be recommended. But when the patient is having symptoms, or when the risk of rupture is a possibility because the lesion has increased in size significantly under continued observation, surgical resection should be suggested. In fact, the vast majority of patients with PEComas received hepatectomy because of indefinable preoperative diagnosis. In our study, all the five patients received surgical resection.
Several studies have showed that angiomyolipomas in patients with TSC or sporadic lymphangioleiomyomatosis are associated with mutations in tuberous sclerosis genes resulting in constitutive activation of the mammalian mTOR (mammalian target of rapamycin). The drug sirolimus suppresses mTOR signaling. Recently, Bissler et alconducted a 24-month, nonrandomized, open-label trial to determine whether an mTOR suppressor (sirolimus) reduces the angiomyolipoma volume in patients with TSC or sporadic lymphangioleiomyomatosis. The result indicates that treatment with sirolimus decreases the size of angiomyolipomas and improves lung function.32 Positivity of the melanocytic markers S100 and HMB-45 suggests that this tumor is derived from the pleuripotent stem cells of the neural crest, as proposed by Sturtz et al5 and Nguyen et al.4
In conclusion, a correct preoperative diagnosis of PEComa by imaging scans, needle biopsy, and other techniques is difficult, especially in patients with a low fat content in hepatic PEComa. A more specific diagnostic is needed. Despite considerable advances in our recognition of this lesion, more cases with longer clinical follow-up need to be evaluated in a systematic fashion. Ultimately, further research will allow accurate prediction of the behavior of this lesion and establish firm criteria for discrimination between malignant and benign tumors. Finally, further research into the etiology of PEComa may yield new drug targets for treating this distinctive tumor.
References
- 1 A.A. Petrolla, W. Xin; Hepatic angiomyolipoma; Arch Pathol Lab Med, 132 (2008), pp. 1679–1682
- 2 A. Nonomura, Y. Enomoto, M. Takeda, et al.; Invasive growth of hepatic angiomyolipoma; a hitherto unreported ominous histological feature; Histopathology, 48 (2006), pp. 831–835
- 3 A. Nonomura, H. Minato, H. Kurumaya; Angiomyolipoma predominantly composed of smooth muscle cells: problems in histological diagnosis; Histopathology, 33 (1998), pp. 20–27
- 4 T.T. Nguyen, B. Gorman, D. Shields, Z. Goodman; Malignant hepatic angiomyolipoma: report of a case and review of literature; Am J Surg Pathol, 32 (2008), pp. 793–798
- 5 C.L. Sturtz, D.J. Dabbs; Angiomyolipomas: the nature and expression of the HMB45 antigen; Mod Pathol, 7 (1994), pp. 842–855
- 6 S. Hirohashi, H.E. Blum, K.G. Ishak; Tumours of the liver and intrahepatic bile ducts; S.R. Hamilton, L.A. Aaltonen (Eds.), Pathology and Genetics. Tumours of the Digestive System. World Health Organisation Classification of Tumours, IARC Press, Lyon (2000), pp. 157–202
- 7 F. Bonetti, M. Pea, G. Martignoni, G. Zamboni; PEC and sugar; Am J Surg Pathol, 16 (1992), pp. 307–308
- 8 G. Zamboni, M. Pea, G. Martignoni, et al.; Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells; Am J Surg Pathol, 20 (1996), pp. 722–730
- 9 J.N. Eble; Angiomyolipoma of kidney; Semin Diagn Pathol, 15 (1998), pp. 21–40
- 10 H. L'Hostis, C. Deminiere, J.M. Ferriere, J.M. Coindre; Renal angiomyolipoma: a clinicopathologic, immunohistochemical, and follow-up study of 46 cases; Am J Surg Pathol, 23 (1999), pp. 1011–1020
- 11 W.M. Tsui, R. Colombari, B.C. Portmann, et al.; Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants; Am J Surg Pathol, 23 (1999), pp. 34–48
- 12 M.J. Gaffey, S.E. Mills, F.B. Askin, et al.; Clear cell tumor of the lung. A clinicopathologic, immunohistochemical, and ultrastructural study of eight cases; Am J Surg Pathol, 14 (1990), pp. 248–259
- 13 A.A. Gal, M.N. Koss, L. Hochholzer, G. Chejfec; An immunohistochemical study of benign clear cell (‘sugar') tumor of the lung; Arch Pathol Lab Med, 115 (1991), pp. 1034–1038
- 14 K. Matsui, A. Tatsuguchi, J. Valencia, et al.; Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases; Hum Pathol, 31 (2000), pp. 1242–1248
- 15 E.J. Sullivan; Lymphangioleiomyomatosis: a review; Chest, 114 (1998), pp. 1689–1703
- 16 S.R. Prasad, H. Wang, H. Rosas, et al.; Fat-containing lesions of the liver: radiologic–pathologic correlation; Radiographics, 25 (2005), pp. 321–331
- 17 M. van Slegtenhorst, R. de Hoogt, C. Hermans, et al.; Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34; Science, 277 (1997), pp. 805–808
- 18 The European Chromosome 16 Tuberous Sclerosis Consortium; Identification and characterization of the tuberous sclerosis gene on chromosome 16; Cell, 75 (1993), pp. 1305–1315
- 19 A. Nonomura, Y. Mizukami, M. Kadoya, N. Takayanagi, T. Hirono; Multiple angiomyolipoma of the liver; J Clin Gastroenterol, 20 (1995), pp. 248–251
- 20 L.H. Tang, P. Hui, G. Garcia-Tsao, R.R. Salem, D. Jain; Multiple angiomyolipomata of the liver: a case report; Mod Pathol, 15 (2002), pp. 167–171
- 21 M. Pea, F. Bonetti, G. Zamboni, et al.; Melanocyte-marker HMB-45 is regularly expressed in angiomyolipoma of the kidney; Pathology, 23 (1991), pp. 185–188
- 22 D.A. Weeks, R.L. Malott, M. Arnesen, C. Zuppan, D. Aitken, G. Mierau; Hepatic angiomyolipoma with striated granules and positivity with melanoma—specific antibody (HMB-45): a report of two cases; Ultrastruct Pathol, 15 (1991), pp. 563–571
- 23 J.K. Chan, W.Y. Tsang, M.Y. Pau, M.C. Tang, S.W. Pang, C.D. Fletcher; Lymphangiomyomatosis and angiomyolipoma: closely related entities characterised by hamartomatous proliferation of HMB-45-positive smooth muscle; Histopathology, 22 (1993), pp. 445–455
- 24 R. Ashfaq, A.G. Weinberg, J. Albores-Saavedra; Renal angiomyolipomas and HMB-45 reactivity; Cancer, 71 (1993), pp. 3091–3097
- 25 U. Sundram, J.D. Harvell, R.V. Rouse, Y. Natkunam; Expression of the B-cell proliferation marker MUM1 by melanocytic lesions and comparison with S100, gpl00 (HMB45), and melanA; Mod Pathol, 16 (2003), pp. 802–810
- 26 T. Li, L. Wang, H.H. Yu, et al.; Hepatic angiomyolipoma: a retrospective study of 25 cases; Surg Today, 38 (2008), pp. 529–535
- 27 C.E. Paiva, F.A. Moraes Neto, A. Agaimy, M.A. Custodio Domingues, S.R. Rogatto; Perivascular epithelioid cell tumor of the liver coexisting with a gastrointestinal stromal tumor; World J Gastroenterol, 14 (2008), pp. 800–802
- 28 H.R. Makhlouf, H.E. Remotti, K.G. Ishak; Expression of KIT(CD117) in angiomyolipoma; Am J Surg Pathol, 26 (2002), pp. 493–497
- 29 I. Dalle, R. Sciot, R. de Vos, et al.; Malignant angiomyolipoma of the liver: a hitherto unreported variant; Histopathology, 36 (2000), pp. 44–50
- 30 J.R. Parfitt, A.J. Bella, J.I. Izawa, B.M. Wehrli; Malignant neoplasm of perivascular epithelioid cells of the liver; Arch Pathol Lab Med, 130 (2006), pp. 1219–1222
- 31 A. Nonomura, Y. Mizukami, M. Kadoya, O. Matsui, K. Shimizu, R. Izumi; Angiomyolipoma of the liver: its clinical and pathological diversity; J Hepatobiliary Pancreat Surg, 3 (1996), pp. 122–132
- 32 J.J. Bissler, F.X. McCormack, L.R. Young, et al.; Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis; N Engl J Med, 358 (2008), pp. 140–151
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?