Abstract
DNA double-strand breaks (DSBs), which arise following exposure to a number of endogenous and exogenous agents, can be repaired by either the homologous recombination (HR) or non-homologous end-joining (NHEJ) pathways in eukaryotic cells. A vital step in HR repair is DNA end resection , which generates a long 3′ single-stranded DNA (ssDNA) tail that can invade the homologous DNA strand. The generation of 3′ ssDNA is not only essential for HR repair, but also promotes activation of the ataxia telangiectasia and Rad3-related protein (ATR). Multiple factors, including the MRN/X complex, C-terminal-binding protein interacting protein (CtIP)/Sae2, exonuclease 1 (EXO1), Bloom syndrome protein (BLM)/Sgs1, DNA2 nuclease/helicase, and several chromatin remodelers, cooperate to complete the process of end resection. Here we review the basic machinery involved in DNA end resection in eukaryotic cells.
Keywords
DNA end resection ; Homologous recombination ; DNA double-strand breaks ; Chromatin remodeling factors ; Genome stability
Introduction
Double-strand breaks (DSBs) are one of the most dangerous types of DNA damage because they disrupt the continuity of chromosomes [1] and [2] . Failure to eliminate DSBs leads to genome instability and tumorigenesis [1] and [3] . DSBs are predominantly repaired by either the non-homologous end-joining (NHEJ) pathway or the homologous recombination (HR) pathway [4] and [5] . NHEJ directly ligates the broken DNA ends, whereas HR uses a homologous sequence from sister chromatid as a repair template [4] and [6] .
Using an identical or similar sequence as a template, HR is commonly considered to be an error-free mechanism for the repair of DSBs [7] and [8] . When DSBs occur, a process termed DNA end resection is activated, which catalyzes the nucleolytic degradation of the broken ends in the 5′ to the 3′ direction [9] and [10] . The resulting 3′ single-stranded DNA (ssDNA) then provides a platform for the recruitment of proteins that participate in HR repair [9] , [10] and [11] . Interestingly, DNA end resection inhibits NHEJ and triggers homology-directed DSB repair [11] . Multiple proteins or protein complexes have been shown to be involved in this process. These include the MRN complex (MRX complex in budding yeast), C-terminal-binding protein interacting protein (CtIP; Sae2 in budding yeast), exonuclease 1 (EXO1), Bloom syndrome protein (BLM; Sgs1 in budding yeast), DNA2 nuclease/helicase, and several chromatin remodeling factors [12] . Here, we discuss the pivotal proteins and their mechanisms during DNA end resection.
DNA end resection and the repair pathway choice
Although DSBs can occur at any phase of the cell cycle, DNA end resection only happens in the S and G2 phases [9] and [13] . During other cell cycle phases, DNA end resection is inhibited by Ku70/80 heterodimers and other proteins; therefore, only the NHEJ pathway can be initiated [11] . NHEJ promotes direct ligation of the DNA ends; subsequent processing of the broken DNA ends is unnecessary [11] . This phenomenon is also consistent with the finding that sister chromatids only exist in the S and G2 phases. However, the repair pathway choice also depends on substrate complexity and other factors besides the cell cycle [13] and [14] .
The MRN/X complex
The MRN complex, which comprises MRE11, RAD50, and nibrin (NBS1), plays key roles in DNA end resection and HR repair in mammalian cells [15] and [16] . The counterpart of the MRN complex in budding yeast is the MRX complex, which consisting of Mre11, Rad50, and Xrs2 [17] . The MRN/X complex not only functions in DNA end resection, but also plays critical roles in the DNA damage checkpoint response [18] .
The MRN complex binds DNA through its globular domain, in which MRE11 and NBS1 associate with the Walker A and Walker B motifs of RAD50 [19] . Previous studies suggest that the DNA binding activity requires primarily MRE11 and RAD50 [19] , [20] and [21] . The extended coiled-coil tail of RAD50 forms another structural domain in the whole MRN complex, which is important for the DNA-binding and -tethering activities of the complex [22] , [23] , [24] , [25] and [26] .
MRE11 is the core component of the MRN complex and exhibits a variety of enzymatic activities, including 3′ to 5′ exonuclease activity on dsDNA, endonuclease activity on ssDNA, and DNA-annealing and -unwinding activities [20] , [21] , [27] and [28] . In vitro experiments revealed that the five phosphoesterase motifs within the N-terminal region of MRE11 are essential for its biochemical activities [12] and [29] . Paradoxically, generation of the 3′ overhang requires the activity of 5′ to 3′ exonuclease, which is opposite to the observed exonuclease activity of MRE11 [30] and [31] . A two-step mechanism of MRE11 has thus been proposed, that is, MRE11 makes the initial ssDNA nick via its ssDNA endonuclease activity at first and then digests toward the DSB end through its 3′ to 5′ exonuclease activity to produce 3′ ssDNA tails [31] , [32] , [33] and [34] .
Human NBS1 contains two BRCA1 C terminus (BRCT) domains and a forkhead-associated (FHA) domain [35] . Mutations within the NBS1 gene are responsible for the Nijmegen breakage syndrome, a rare autosomal recessive disease that increases the predisposition to develop malignancies [36] , [37] and [38] . Cells derived from NBS patients exhibit defects in DSB repair and cell cycle checkpoint [36] . Although lacking enzymatic activities, NBS1 is considered to be an important regulator in the MRN complex, since NBS1 influences both DNA binding and nuclease activity of MRE11 [39] , [40] and [41] .
CtIP/Sae2
CtIP was first identified as a cofactor for the transcriptional repressor C-terminal-binding protein (CtBP) [12] and [42] . Further studies reveal that CtIP functions in many other cellular processes, including cell cycle regulation and tumorigenesis [43] . Interestingly, CtIP is now better known as an interacting partner of the MRN complex, for its involvement in DNA end resection and DSB repair [44] , [45] , [46] and [47] .
CtIP shows sequence homology to the budding yeast Sae2 at the C terminus [48] . CtIP plays at least two roles in the process of DNA end resection, distinguished by the involvement of its catalytic activity or not [49] . Briefly, the resection of DSBs with clean broken ends produced by restriction enzymes is dependent on the presence of CtIP protein, but independent of its nuclease activity [49] and [50] . By contrast, the repair of more complex DNA lesions created by topoisomerase poisons or ionizing radiation (IR) requires not only the presence of CtIP protein but also its endonuclease activity [51] .
EXO1
EXO1 belongs to the xeroderma pigmentosum complementation group G (XPG) family of nucleases, which contain conserved nuclease motifs in the N-terminal region [52] and [53] . EXO1 exhibits 5′ to 3′ dsDNA exonuclease and 5′ flap endonuclease activities in vitro[12] and [53] . Interestingly, EXO1 prefers dsDNA substrates with a recessed 5′ end, which is produced by the MRN/X complex and CtIP/Sae2 [54] , [55] and [56] . Taken together with the finding that MRE11 lacks the 5′ to 3′ exonuclease activity required to produce long 3′ ssDNA tails necessary for replication protein A (RPA) binding, a two-step model has been suggested for DSB processing [12] . In this model, the MRN/X complex and CtIP/Sae2 remove the first 50–100 nucleotides from the 5′ end of the broken DNA, followed by the generation of long 3′ ssDNA tails catalyzed by EXO1 [12] . This model is also supported by the finding that CtIP is required for the accumulation of EXO1 at DSB sites in vivo[57] .
DNA2–BLM/Sgs1
BLM is a member of the RecQ family of helicases that unwinds DNA in mammals and Sgs1 is its ortholog in Saccharomyces cerevisiae[58] . DNA2, which is related to the bacterial RecB proteins, exhibits both helicase and nuclease activities in vitro[33] . However, the helicase activity of DNA2 is not necessary for DNA end resection, while the nuclease activity of DNA2 is essential to this process [59] , [60] and [61] . Previous reports suggest that EXO1 and DNA2–BLM/Sgs1 function in parallel at the second step of end resection [12] . Interestingly, studies in yeast indicate that Sgs1-Dna2-catalyzed end resection is dependent on RPA [61] . In the absence of RPA, DNA2 cannot be recruited to DSBs [61] . Although it can degrade either 3′- or 5′-terminated ssDNA, DNA2 exhibits 5′ endonuclease activity only in the presence of RPA, which may explain the strand bias in the end resection [61] .
Chromatin remodeling factors
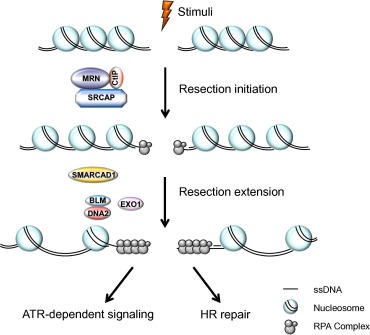
Eukaryotic DNA is normally wrapped around a histone octamer to form nucleosomes [62] . This condensation allows the long genetic molecules to fit into the relatively-small nucleus, but at the same time, forms a barrier for resection enzymes to access [63] . Certain histone modifiers, histone chaperones, and chromatin remodelers modify chromatin structure and hence regulate the dynamics of the chromatin [64] and [65] . For instance, several chromatin remodeling factors, such as remodels the structure of chromatin (RSC), INO80, switch/sucrose non-fermentable (SWI/SNF), SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A containing DEAD/H box 1 (SMARCAD1; Fun30 in yeast), and Snf2-related CREB-binding protein (CREBBP) activator protein (SRCAP; SWR1 in yeast), are involved in the process of overcoming barriers to allow repair proteins to access (Figure 1 ) [66] , [67] , [68] , [69] , [70] and [71] .
|
|
|
Figure 1. DNA-end resection occurs via a two-step process—resection initiation and resection extension Resection initiation is stimulated by SRCAP, CtIP, and the MRN complex. SMARCAD1 cooperates with EXO1 and BLM/DNA2 to promote resection extension. The figure is adapted from [70] . ATR, ataxia telangiectasia and Rad3-related protein; BLM, Bloom syndrome protein; CtIP, C-terminal-binding protein interacting protein; EXO1, exonuclease 1; HR, homologous recombination; MRN, MRE11, RAD50, and nibrin; RPA, replication protein A; SMARCAD1, switch/sucrose non-fermentable (SWI/SNF)-related matrix-associated actin-dependent regulator of chromatin subfamily A containing DEAD/H box 1; SRCAP, Snf2-related CREB-binding protein activator protein; ssDNA, single-stranded DNA. |
Rad9, the yeast 53BP1 ortholog, is a checkpoint mediator protein and is recruited to DSB sites by γ-H2A and K79-methylated histone H3 [72] . Rad9 is known to inhibit DNA end resection [72] . On the other hand, the yeast Fun30, which possesses intrinsic ATP-dependent chromatin remodeling activity, works together with DNA2 and EXO1 to promote extensive DSB end resection [71] , [73] , [74] and [75] . Mechanically, Fun30 overcomes the barrier formed by Rad9-bound chromatin, thus promoting extensive resection process [71] , [73] , [74] and [75] .
SRCAP is a member of the INO80 ATPase family and belongs to the human SRCAP chromatin remodeling complex [76] . SRCAP was first discovered as the binding partner of CREBBP (also known as CBP), and mutations in SRCAP cause a rare genetic disorder known as Floating–Harbor syndrome [76] and [77] . Our recent findings support a new function for SRCAP in promoting DSB resection and HR repair (Figure 1 ). SRCAP-depleted cells exhibit RPA2 hyperphosphorylation and defects in RPA2 focus formation, indicating that SRCAP is involved in DSB end processing [78] . Accordingly, SRCAP depletion only affects IR- and camptothecin (CPT)-induced RPA2 focus formation but not hydroxyurea (HU)-induced RPA2 focus formation (HU stalls replication forks by deprivation of dNTPs) [78] . Moreover, zinc finger HIT-type 1 (ZNHIT1)/p18, another component of the SRCAP complex, also promotes DNA end resection [78] . Mechanistically, SRCAP promotes chromatin relaxation to allow CtIP accumulation at DSB sites, thereby facilitating DSB end processing (Figure 1 ) [78] .
Summary
Correct repair of DSBs is critical for the maintenance of genome stability. HR and NHEJ are the two dominant repair pathways involved in DSB repair [4] and [6] . While NHEJ facilitates the direct ligation of the DSB ends in an error-prone manner, HR allows for precise repair of DSBs due to the employment of homologous chromatids [8] . DNA end resection is a pivotal step in HR repair to produce 3’ overhangs that not only inhibit NHEJ but also provide a platform to recruit proteins involved in HR repair [11] . DNA end resection is completed through a two-step process in which the MRN/X complex and CtIP/Sae2 protein are involved in the initial step, and EXO1 and DNA2-BLM/Sgs1 are involved in the second step [79] . However, precisely how these resection factors are regulated in a coordinated manner is still unclear. Further studies are therefore required to resolve this issue.
Competing interests
The authors declare no competing financial interests.
Acknowledgments
This work was supported in part by the grants from the National Natural Science Foundation of China (Grant Nos. 31071243 and 31171347 ), the Fundamental Research Funds for the Central Universities of China , and the Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20110101120152 ).
References
- [1] D.C. van Gent, J.H. Hoeijmakers, R. Kanaar; Chromosomal stability and the DNA double-stranded break connection; Nat Rev Genet, 2 (2001), pp. 196–206
- [2] Y. Shiloh, A.R. Lehmann; Maintaining integrity; Nat Cell Biol, 6 (2004), pp. 923–928
- [3] M. O’Driscoll; Diseases associated with defective responses to DNA damage; Cold Spring Harb Perspect Biol (2012), p. 4 http://dx.doi.org/10.1101/cshperspect.a012773
- [4] T. Liu, J. Huang; Quality control of homologous recombination; Cell Mol Life Sci, 71 (2014), pp. 3779–3797
- [5] J. San Filippo, P. Sung, H. Klein; Mechanism of eukaryotic homologous recombination; Annu Rev Biochem, 77 (2008), pp. 229–257
- [6] M.R. Lieber; The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway; Annu Rev Biochem, 79 (2010), pp. 181–211
- [7] M. Betermier, P. Bertrand, B.S. Lopez; Is non-homologous end-joining really an inherently error-prone process?; PLoS Genet, 10 (2014), p. e1004086
- [8] E. Sonoda, H. Hochegger, A. Saberi, Y. Taniguchi, S. Takeda; Differential usage of non-homologous end-joining and homologous recombination in double strand break repair; DNA Repair (Amst), 5 (2006), pp. 1021–1029
- [9] M.P. Longhese, D. Bonetti, N. Manfrini, M. Clerici; Mechanisms and regulation of DNA end resection; EMBO J, 29 (2010), pp. 2864–2874
- [10] E.P. Mimitou, L.S. Symington; DNA end resection–unraveling the tail; DNA Repair (Amst), 10 (2011), pp. 344–348
- [11] P. Huertas; DNA resection in eukaryotes: deciding how to fix the break; Nat Struct Mol Biol, 17 (2010), pp. 11–16
- [12] E.P. Mimitou, L.S. Symington; DNA end resection: many nucleases make light work; DNA Repair (Amst), 8 (2009), pp. 983–995
- [13] J.M. Daley, P. Sung; To Cut or Not to Cut: Discovery of a novel regulator of DNA break resection; Mol Cell, 61 (2016), pp. 325–326
- [14] A. Kakarougkas, P.A. Jeggo; DNA DSB repair pathway choice: an orchestrated handover mechanism; Br J Radiol, 87 (2014), p. 20130685
- [15] K. Ohta, A. Nicolas, M. Furuse, A. Nabetani, H. Ogawa, T. Shibata; Mutations in the MRE11 , RAD50, XRS2 , and MRE2 genes alter chromatin configuration at meiotic DNA double-stranded break sites in premeiotic and meiotic cells ; Proc Natl Acad Sci U S A, 95 (1998), pp. 646–651
- [16] D. D’Amours, S.P. Jackson; The Mre11 complex: at the crossroads of dna repair and checkpoint signalling; Nat Rev Mol Cell Biol, 3 (2002), pp. 317–327
- [17] K.M. Trujillo, D.H. Roh, L. Chen, S. Van Komen, A. Tomkinson, P. Sung; Yeast Xrs2 binds DNA and helps target Rad50 and Mre11 to DNA ends; J Biol Chem, 278 (2003), pp. 48957–48964
- [18] C. Lukas, F. Melander, M. Stucki, J. Falck, S. Bekker-Jensen, M. Goldberg, et al.; Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention; EMBO J, 23 (2004), pp. 2674–2683
- [19] K.P. Hopfner, A. Karcher, L. Craig, T.T. Woo, J.P. Carney, J.A. Tainer; Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase; Cell, 105 (2001), pp. 473–485
- [20] H. Tsubouchi, H. Ogawa; A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis ; Mol Cell Biol, 18 (1998), pp. 260–268
- [21] M. Furuse, Y. Nagase, H. Tsubouchi, K. Murakami-Murofushi, T. Shibata, K. Ohta; Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination ; EMBO J, 17 (1998), pp. 6412–6425
- [22] J. van Noort, T. van Der Heijden, M. de Jager, C. Wyman, R. Kanaar, C. Dekker; The coiled-coil of the human Rad50 DNA repair protein contains specific segments of increased flexibility; Proc Natl Acad Sci U S A, 100 (2003), pp. 7581–7586
- [23] M. Lichten; Rad50 connects by hook or by crook; Nat Struct Mol Biol, 12 (2005), pp. 392–393
- [24] T.T. Paull; New glimpses of an old machine; Cell, 107 (2001), pp. 563–565
- [25] M. de Jager, J. van Noort, D.C. van Gent, C. Dekker, R. Kanaar, C. Wyman; Human Rad50/Mre11 is a flexible complex that can tether DNA ends; Mol Cell, 8 (2001), pp. 1129–1135
- [26] K.P. Hopfner, L. Craig, G. Moncalian, R.A. Zinkel, T. Usui, B.A. Owen, et al.; The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair; Nature, 418 (2002), pp. 562–566
- [27] J.H. Lee, T.T. Paull; Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex; Science, 304 (2004), pp. 93–96
- [28] J.E. Haber; The many interfaces of Mre11; Cell, 95 (1998), pp. 583–586
- [29] M.J. Neale, J. Pan, S. Keeney; Endonucleolytic processing of covalent protein-linked DNA double-strand breaks; Nature, 436 (2005), pp. 1053–1057
- [30] T.H. Stracker, J.H. Petrini; The MRE11 complex: starting from the ends; Nat Rev Mol Cell Biol, 12 (2011), pp. 90–103
- [31] E.P. Mimitou, L.S. Symington; Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing; Nature, 455 (2008), pp. 770–774
- [32] A. Shibata, D. Moiani, A.S. Arvai, J. Perry, S.M. Harding, M.M. Genois, et al.; DNA double-strand break repair pathway choice is directed by distinct MRE11 nuclease activities; Mol Cell, 53 (2014), pp. 7–18
- [33] Z. Zhu, W.H. Chung, E.Y. Shim, S.E. Lee, G. Ira; Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends; Cell, 134 (2008), pp. 981–994
- [34] S. Gravel, J.R. Chapman, C. Magill, S.P. Jackson; DNA helicases Sgs1 and BLM promote DNA double-strand break resection; Genes Dev, 22 (2008), pp. 2767–2772
- [35] T.H. Stracker, M. Morales, S.S. Couto, H. Hussein, J.H. Petrini; The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex; Nature, 447 (2007), pp. 218–221
- [36] B.R. Williams, O.K. Mirzoeva, W.F. Morgan, J. Lin, W. Dunnick, J.H. Petrini; A murine model of Nijmegen breakage syndrome; Curr Biol, 12 (2002), pp. 648–653
- [37] J. Zhu, S. Petersen, L. Tessarollo, A. Nussenzweig; Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice; Curr Biol, 11 (2001), pp. 105–109
- [38] H. Tauchi, J. Kobayashi, K. Morishima, D.C. van Gent, T. Shiraishi, N.S. Verkaik, et al.; Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells; Nature, 420 (2002), pp. 93–98
- [39] Z. Dong, Q. Zhong, P.L. Chen; The Nijmegen breakage syndrome protein is essential for Mre11 phosphorylation upon DNA damage; J Biol Chem, 274 (1999), pp. 19513–19516
- [40] T.T. Paull, M. Gellert; Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex; Genes Dev, 13 (1999), pp. 1276–1288
- [41] R.S. Williams, G.E. Dodson, O. Limbo, Y. Yamada, J.S. Williams, G. Guenther, et al.; Nbs1 flexibly tethers Ctp1 and Mre11-Rad50 to coordinate DNA double-strand break processing and repair; Cell, 139 (2009), pp. 87–99
- [42] U. Schaeper, T. Subramanian, L. Lim, J.M. Boyd, G. Chinnadurai; Interaction between a cellular protein that binds to the C-terminal region of adenovirus E1A (CtBP) and a novel cellular protein is disrupted by E1A through a conserved PLDLS motif; J Biol Chem, 273 (1998), pp. 8549–8552
- [43] A.K. Wong, P.A. Ormonde, R. Pero, Y. Chen, L. Lian, G. Salada, et al.; Characterization of a carboxy-terminal BRCA1 interacting protein; Oncogene, 17 (1998), pp. 2279–2285
- [44] Z. You, L.Z. Shi, Q. Zhu, P. Wu, Y.W. Zhang, A. Basilio, et al.; CtIP links DNA double-strand break sensing to resection; Mol Cell, 36 (2009), pp. 954–969
- [45] A.A. Sartori, C. Lukas, J. Coates, M. Mistrik, S. Fu, J. Bartek, et al.; Human CtIP promotes DNA end resection; Nature, 450 (2007), pp. 509–514
- [46] S. Takeda, K. Nakamura, Y. Taniguchi, T.T. Paull; Ctp1/CtIP and the MRN complex collaborate in the initial steps of homologous recombination; Mol Cell, 28 (2007), pp. 351–352
- [47] L. Chen, C.J. Nievera, A.Y. Lee, X. Wu; Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair; J Biol Chem, 283 (2008), pp. 7713–7720
- [48] Y. Akamatsu, Y. Murayama, T. Yamada, T. Nakazaki, Y. Tsutsui, K. Ohta, et al.; Molecular characterization of the role of the Schizosaccharomyces pombe nip1+/ctp1+ gene in DNA double-strand break repair in association with the Mre11-Rad50-Nbs1 complex ; Mol Cell Biol, 28 (2008), pp. 3639–3651
- [49] N. Makharashvili, A.T. Tubbs, S.H. Yang, H. Wang, O. Barton, Y. Zhou, et al.; Catalytic and noncatalytic roles of the CtIP endonuclease in double-strand break end resection; Mol Cell, 54 (2014), pp. 1022–1033
- [50] H. Wang, Y. Li, L.N. Truong, L.Z. Shi, P.Y. Hwang, J. He, et al.; CtIP maintains stability at common fragile sites and inverted repeats by end resection-independent endonuclease activity; Mol Cell, 54 (2014), pp. 1012–1021
- [51] N. Makharashvili, T.T. Paull; CtIP: A DNA damage response protein at the intersection of DNA metabolism; DNA Repair (Amst), 32 (2015), pp. 75–81
- [52] P. Szankasi, G.R. Smith; A DNA exonuclease induced during meiosis of Schizosaccharomyces pombe; J Biol Chem, 267 (1992), pp. 3014–3023
- [53] P. Szankasi, G.R. Smith; A role for exonuclease I from S. pombe in mutation avoidance and mismatch correction ; Science, 267 (1995), pp. 1166–1169
- [54] E.Y. Shim, W.H. Chung, M.L. Nicolette, Y. Zhang, M. Davis, Z. Zhu, et al.; Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks ; EMBO J, 29 (2010), pp. 3370–3380
- [55] D.S. Krasner, J.M. Daley, P. Sung, H. Niu; Interplay between Ku and replication protein A in the restriction of Exo1-mediated DNA break end resection; J Biol Chem, 290 (2015), pp. 18806–18816
- [56] E. Cannavo, P. Cejka, S.C. Kowalczykowski; Relationship of DNA degradation by Saccharomyces cerevisiae exonuclease 1 and its stimulation by RPA and Mre11-Rad50-Xrs2 to DNA end resection ; Proc Natl Acad Sci U S A, 110 (2013), pp. E1661–E1668
- [57] W. Eid, M. Steger, M. El-Shemerly, L.P. Ferretti, J. Pena-Diaz, C. Konig, et al.; DNA end resection by CtIP and exonuclease 1 prevents genomic instability; EMBO Rep, 11 (2010), pp. 962–968
- [58] A.V. Nimonkar, A.Z. Ozsoy, J. Genschel, P. Modrich, S.C. Kowalczykowski; Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair; Proc Natl Acad Sci U S A, 105 (2008), pp. 16906–16911
- [59] P. Cejka, E. Cannavo, P. Polaczek, T. Masuda-Sasa, S. Pokharel, J.L. Campbell, et al.; DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2; Nature, 467 (2010), pp. 112–116
- [60] H. Niu, W.H. Chung, Z. Zhu, Y. Kwon, W. Zhao, P. Chi, et al.; Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae; Nature, 467 (2010), pp. 108–111
- [61] A.V. Nimonkar, J. Genschel, E. Kinoshita, P. Polaczek, J.L. Campbell, C. Wyman, et al.; BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair; Genes Dev, 25 (2011), pp. 350–362
- [62] E. Unal, A. Arbel-Eden, U. Sattler, R. Shroff, M. Lichten, J.E. Haber, et al.; DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain; Mol Cell, 16 (2004), pp. 991–1002
- [63] H. van Attikum, S.M. Gasser; The histone code at DNA breaks: a guide to repair?; Nat Rev Mol Cell Biol, 6 (2005), pp. 757–765
- [64] L.L. Cao, C. Shen, W.G. Zhu; Histone modifications in DNA damage response; Sci China Life Sci, 59 (2016), pp. 257–270
- [65] E.P. Rogakou, D.R. Pilch, A.H. Orr, V.S. Ivanova, W.M. Bonner; DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139; J Biol Chem, 273 (1998), pp. 5858–5868
- [66] H. van Attikum, O. Fritsch, B. Hohn, S.M. Gasser; Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair; Cell, 119 (2004), pp. 777–788
- [67] A.J. Morrison, J. Highland, N.J. Krogan, A. Arbel-Eden, J.F. Greenblatt, J.E. Haber, et al.; INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair; Cell, 119 (2004), pp. 767–775
- [68] E.Y. Shim, J.L. Ma, J.H. Oum, Y. Yanez, S.E. Lee; The yeast chromatin remodeler RSC complex facilitates end joining repair of DNA double-strand breaks; Mol Cell Biol, 25 (2005), pp. 3934–3944
- [69] E.Y. Shim, S.J. Hong, J.H. Oum, Y. Yanez, Y. Zhang, S.E. Lee; RSC mobilizes nucleosomes to improve accessibility of repair machinery to the damaged chromatin; Mol Cell Biol, 27 (2007), pp. 1602–1613
- [70] A.L. Chambers, J.A. Downs; The RSC and INO80 chromatin-remodeling complexes in DNA double-strand break repair; Prog Mol Biol Transl Sci, 110 (2012), pp. 229–261
- [71] T. Costelloe, R. Louge, N. Tomimatsu, B. Mukherjee, E. Martini, B. Khadaroo, et al.; The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection; Nature, 489 (2012), pp. 581–584
- [72] F. Lazzaro, V. Sapountzi, M. Granata, A. Pellicioli, M. Vaze, J.E. Haber, et al.; Histone methyltransferase Dot1 and Rad9 inhibit single-stranded DNA accumulation at DSBs and uncapped telomeres; EMBO J, 27 (2008), pp. 1502–1512
- [73] X. Chen, D. Cui, A. Papusha, X. Zhang, C.D. Chu, J. Tang, et al.; The Fun30 nucleosome remodeller promotes resection of DNA double-strand break ends; Nature, 489 (2012), pp. 576–580
- [74] M. Durand-Dubief, W.R. Will, E. Petrini, D. Theodorou, R.R. Harris, M.R. Crawford, et al.; SWI/SNF-like chromatin remodeling factor Fun30 supports point centromere function in S. cerevisiae; PLoS Genet, 8 (2012), p. e1002974
- [75] V.V. Eapen, N. Sugawara, M. Tsabar, W.H. Wu, J.E. Haber; The Saccharomyces cerevisiae chromatin remodeler Fun30 regulates DNA end resection and checkpoint deactivation ; Mol Cell Biol, 32 (2012), pp. 4727–4740
- [76] H. Johnston, J. Kneer, I. Chackalaparampil, P. Yaciuk, J. Chrivia; Identification of a novel SNF2/SWI2 protein family member, SRCAP, which interacts with CREB-binding protein; J Biol Chem, 274 (1999), pp. 16370–16376
- [77] R.L. Hood, M.A. Lines, S.M. Nikkel, J. Schwartzentruber, C. Beaulieu, M.J. Nowaczyk, et al.; Mutations in SRCAP, encoding SNF2-related CREBBP activator protein, cause Floating-Harbor syndrome; Am J Hum Genet, 90 (2012), pp. 308–313
- [78] S. Dong, J. Han, H. Chen, T. Liu, M.S. Huen, Y. Yang, et al.; The human SRCAP chromatin remodeling complex promotes DNA-end resection; Curr Biol, 24 (2014), pp. 2097–2110
- [79] K.A. Bernstein, R. Rothstein; At loose ends: resecting a double-strand break; Cell, 137 (2009), pp. 807–810
Document information
Published on 20/10/16
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?