中医五行生克的治疗方法准确地描述了信号分子的传输通路
Authors: Yang LIU*
Affiliations:
The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong.
- To whom correspondence should be addressed: mmyliu@polyu.edu.hk
在我们的前一篇文章,我们建立了中医五行阳阴气与信号分子的直接映射关系。其中心“行”包括IGF, Ang, 和Mg; 脾“行”包括ANP, aldosterone, retinoic acid 和ghrelin; 肺“行”包括FGF7, VEGF, ascorbic acid, 和HIF; 肾“行”包括calcitonin, PTHrP, Wnt, 和NO; 肝“行”包括EPO, HGF, SOD, renin, AKR, 和GSH。中医认为五行按照心-脾-肺-肾-肝-心的顺序具有相生的作用,而按照心-肺-肝-脾-肾-心的顺序具有相克的作用,即五行生克理论。根据五行生克理论发展出了八种著名的治疗方法,即滋水涵木法、益火补土法、培土生金法、金水相生法、抑木扶土法、培土制水法、佐金平木法和泻南补北法。本文检验了各个“行”的信号分子对心脾肺肾肝各个器官的作用。发现只有按照“心-脾-肺-肾-肝-心”和“心-肺-肝-脾-肾-心”的顺序,各个“行”的信号分子对下一个器官有正面作用,否则可能会有相反作用。我们将中医八种著名治疗方法与信号分子传输通路进行比较,发现精准一致。这进一步证明了中医和西医用不同的术语描述同样的生理过程。
词汇表
| AKR | aldo-keto reductase, Liver Yin in TCM |
| Ang | Angiotensin, Heart Yin in TCM |
| ANP | atrial natriuretic peptide, Spleen Yang in TCM |
| EPO | Erythropoietin, Liver Yang in TCM |
| FGF7 | fibroblast growth factor-7, Lung Yang in TCM |
| Five Phases | TCM term, includes heart phase, spleen phase, lung phase, kidney phase, liver phase |
| GSH | Glutathione, Liver Qi in TCM |
| Heart phase | TCM term, includes heart organ, Heart Yang, Heart Yin, and Heart Qi |
| HGF | hepatocyte growth factor, Liver Yang in TCM |
| HIF | hypoxia inducible factor, Lung Qi in TCM |
| IGF | insulin-like growth factor, Heart Yang in TCM |
| Kidney phase | TCM term, includes kidney organ, Kidney Yang, Kidney Yin, and Kidney Qi |
| Liver phase | TCM term, includes liver organ, Liver Yang, Liver Yin, and Liver Qi |
| Lung phase | TCM term, includes lung organ, Lung Yang, Lung Yin, and Lung Qi |
| Mg | Magnesium, Heart Qi in TCM |
| NO | nitric oxide, Kidney Qi in TCM |
| PTHrP | parathyroid hormone-related protein, Kidney Yin in TCM |
| Qi | substance in TCM, has function of activation and regulation |
| SOD | superoxide dismutase, Liver Yin in TCM |
| Spleen phase | TCM term, includes spleen organ, Spleen Yang, Spleen Yin, and Spleen Qi |
| TCM | traditional Chinese medicine |
| VEGF | vascular endothelial growth factor, Lung Yang in TCM |
| Yang | Key substance in TCM, has function of warming/developing/proliferation |
| YE-81 | Yellow Emperor’s Classic on 81 Medical Problems |
| YEIC | Yellow Emperor’s Inner Canon |
| Yin | Key substance in TCM, has function of cooling and astringent |
1. 简介
中医使用阳阴气和五行生克的理论来制定治疗策略。 在前一篇文章,我们建立了中医五行中的阳阴气和信号分子之间的直接映射关系[1,2], 并证明中医和现代医学用不同的语言定义描述了相同的病理生理过程。如表一和图一所示,心的阳-阴-气包括IGF, Ang, 和 Mg; 脾的阳-阴-气包括ANP, aldosterone/retinoic acid,和ghrelin; 肺的阳-阴-气包括FGF7/VEGF, ascorbic acid (vitamin C), 和 HIF; 肾的阳-阴-气包括calcitonin/PTHrP, Wnt, 和NO; 以及肝的阳-阴-气包括EPO/HGF, SOD/renin/AKR, 和GSH. 中医理论认为心生脾,脾生肺,肺生肾,肾生肝,肝生心,即按照心-脾-肺-肾-肝-心的顺序具有相生的作用;而心为肺之主,肺为肝之主,肝为脾之主,脾为肾之主,肾为心之主,即按照心-肺-肝-脾-肾-心的顺序具有相克的作用。根据这些五行相生相克的关系,中医发展出了八种行之有效的治疗方法,即滋水涵木法、益火补土法、培土生金法、金水相生法、抑木扶土法、培土制水法、佐金平木法和泻南补北法[3].
2. 结果
为了进一步阐述中医五行与信号分子的一致性,在本文中我们研究每一“行”的信号分子对其他脏器的影响,并检查其是否与相生相克的理论相符合。我们又进一步研究信号分子的传输通路是否与中医五行生克的八种治疗方法有一致性。结果表明,中医五行生克理论和八种治疗方法精准地描述了信号分子的传输通路,这不但进一步证明了我们前文[1,2]的正确,而且更重要的是证实了中医理论具有坚实的科学基础,其和现代医学一样都能正确的描述生理发展过程,只是使用了不同的术语而已。
2.1 每一“行”的信号分子与其他脏器的关系
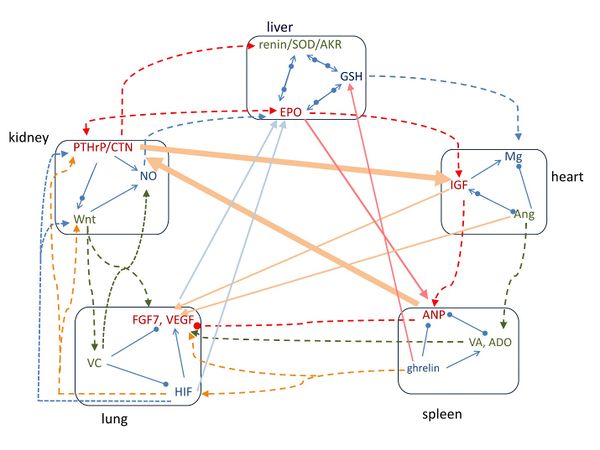
如图 1 所示,中医认为沿外圆周,即按照心-脾-肺-肾-肝-心的顺序具有相生的作用;而沿着对角线方向,即按照心-肺-肝-脾-肾-心的顺序具有相克的作用 [3]。以下我们按照相生和相克的顺序分别讨论。
2.1.1 心-脾-肺-肾-肝-心的相生作用
心的阳阴气对应的信号分子对脾脏具有辅助促进作用。例如,镁分子缺乏会导致脾脏形态的和免疫的改变[4];本地化的IGF-1分泌能增强小鼠胚胎的脾脏中红细胞生成[5], 较低的IGF-1水平与比较高的脾脏纵径相关联[6], IGF-1 对骨髓祖细胞的生存和转入肾脏是至关重要的[7]; Ang II对在脾中建立有效的 T 细胞反应起到一定的作用[8].
脾的阳阴气对应的信号分子对肺脏具有辅助促进作用。例如, ANP抑制脂多糖诱导的肺血管渗漏[9]; Retinoic acid 视黄酸调节肺脏的形态发育[10]; aldosterone有助于肺部水肿的清除[11]; ghrelin由胎儿肺脏产生并促进肺脏生长[12].
肺的阳阴气对应的信号分子对肾脏具有辅助促进作用。例如,HIF参与了多种与调节肾功能有关的在生理和病理条件下的生物过程,包括葡萄糖和能量代谢、 血管生成、红细胞生成和铁稳态、 细胞迁移、细胞与细胞以及细胞与基质的相互作用[13]; 在肾脏的生长过程中,FGF-7 是控制收集系统大小和肾单位数目的信号通路的一部分[14]; VEGF是肾小球和肾小管周围血管内皮细胞生长和增殖所必需的[15]; 以及维生素C缺乏症在肾衰竭患者中很常见[16]。
肾的阳阴气对应的信号分子对肝脏具有辅助促进作用。例如, PTHrP激活肝星状细胞[17]; calcitonin 增加胆汁酸与氨基酸牛磺酸和甘氨酸在肝细胞和泪小管的分泌,以增强胆汁的溶解力、保持胆固醇在溶质状态和防止胆道结石的形成[18]; 短时间低剂量的NO有利于肝脏,但长时间大剂量的NO对肝脏有害[19]; Wnt 信号调节各种基本的细胞活动,包括分化、 增殖、 生存、 氧化应激和形态发生,对肝的生理和病理有辅助促进作用[20].
肝的阳阴气对应的信号分子对心脏具有辅助促进作用。例如, 低水平的GSH有心血管疾病的高风险[21]; 通过 EPO 治疗,可以发现症状及心功能明显改善[22]; HGF具有治疗心肌梗死和心衰的潜力 [23]; 胞外SOD保护心肌对抗心肌梗死后产生的抗氧化应激和肥厚[24]; 在缺血性心脏病中AKR会被激活[25]; renin-angiotensin-aldosterone (肾素 - 血管紧张素 - 醛固酮)系统在调节血容量和全身血管阻力方面发挥重要作用,共同影响心脏输出量和动脉压 [26]。
2.1.2 心-肺-肝-脾-肾-心的相克作用
心的阳阴气对应的信号分子对肺脏具有调节作用。例如, IGF-1诱导肺成纤维细胞活化,对小鼠肺纤维化模型封锁 IGF 通路能改善结果并减少纤维化[27]; Ang II可以调解对肺损伤的反应[28];镁缺乏会导致肺部并发症[29].
肺的阳阴气对应的信号分子对肝脏具有调节作用。例如, FGF7 促进肝再生[30]; 通过由窦内皮细胞增殖而重建肝血窦,VEGF 促进肝细胞的增殖[31]; 缺乏vitamin C 会加重脂肪性肝病的发展[32] 而且vitamin C 加上vitamin E是治疗脂肪肝病患者的安全和有效的选择[33]; HIF对肝脏病理生理学有广泛作用 [34].
肝阳对应的信号分子对脾脏具有调节作用。例如,EPO 对脾脏中红细胞集落的发展非常必要[35]; 大鼠烧伤引起HGF在脾脏的产生 [36].
脾的阳阴气对应的信号分子对肾脏具有调节作用。例如, ANP 可直接或间接(通过抑制aldosterone的生物合成)作用于肾脏以改变钠输运和调节细胞外的液体分布[37]; Aldosterone对肾脏里的钠保存至关重要 [38];肾脏发育需要retinoic acid[39]; 肾脏会降解ghrelin,在慢性肾脏疾病中总ghrelin水平的增加主要是由于ghrelin降解的减少[40].
肾的阳阴气对应的信号分子对心脏具有调节作用。例如, NO调节心脏功能[41]; 充血性心力衰竭与循环降钙素基因相关肽 calcitonin gene-related peptide (CGRP) 的增加相关,但 CGRP 减轻心肌梗死的发展[42]; PTHrP 对心脏有保护作用[43]; Wnt 控制心脏发育但在成人心脏重塑过程中也被调制[44].
2.2 五行中信号分子间的相互关系
图二表示了五行分子之间的信号传输通路。沿着外圆周的心-脾-肺-肾-肝-心的顺序,信号分子按顺序传输而形成了一个顺时针的闭环。对于肝“行”和心“行”,使用EPO能增加IGF-1水平 [45]; 在体内和体外实验中,GSH增加了细胞内的 Mg水平[46]. 对于心“行”和脾“行”, IGF-II增加蛋白的ANP [47], Ang 引起肾上腺的aldosterone释放[26]。 对于脾“行”和肺“行”,使用ghrelin 可显著提高HIF-1α 和VEGF mRNAs [48], aldosterone 可增加人嗜中性粒细胞中VEGF-A的产生 [49], 以及 retinoic acid增加HIF-1α [50] 和显著刺激FGF7的表达[51]; 然而,在体外实验中 ANP阻止VEGF信号的产生[52]。 对于肺“行”和肾“行”, HIF 调节 Wnt/β-catenin 信号 [53], 诱导 PTHrP [54], 并受NO调节 [55]; 在表皮的分层Wnt 激活 FGF7 [56], 调节vitamin C 的生物合成 [57]; vitamin C 增加一氧化氮合酶活性[58]。 对于肾“行”和肝“行”, calcitonin gene-related peptide (CGRP) 和 PTHrP 激发 renin 的分泌 [59,60], PTHrP 和 EPO 互相正相关[61], PTHrP 上调 AKR 1C3[62], NO 激发 EPO[63]。
在图二中沿着对角线方向的心-肺-肝-脾-肾-心顺序,信号分子会加强下一“行”的信号分子表达。例如, 对于心“行”和肺“行”, IGF-1 诱导肺成纤维细胞活化[27] 及促进VEGF [64]; Ang II 增加FGF7 mRNA 水平 [65] 及VEGF合成 [66]。 对于肺“行”和肝“行”, VEGF 是 EPO 诱导改善心脏功能的关键 [67]; vitamin C 维生素 C 提升健康成人血红细胞中的GSH [68]; HIF 促进EPO 产生[69] HIF-1随着GSH的氧化还原比例而变化[70]。 对于肝“行”和脾“行”, EPO 促进 ANP的分泌[71], 用 ghrelin治疗可增加GSH水平[72]和SOD活性 [73]。 对于脾“行”和肾“行”, ANP 增加人体循环的calcitonin gene-related peptide (CGRP) [74]。 对于肾“行”和心“行”, calcitonin 增加IGF的浓度 [75], IGF信号对抗 Wnt 通路[76].
2.3 信号分子传输通路和中医五行生克八种治疗方法的高度一致性
根据五行相生相克的关系,中医发展出了八种著名的治疗方法,即滋水涵木法、益火补土法、培土生金法、金水相生法、抑木扶土法、培土制水法、佐金平木法和泻南补北法[3]。我们将每一种方法与信号分子的传输通路进行比较,如图三所示。
滋水涵木法,是滋肾阴以养肝阴的治法,又称滋肾养肝法、滋补肝肾法。适用于肾阴亏损而肝阴不足[3]。 在现代生物医学中, Wnt/β-catenin信号上调伴随着SOD水平的恢复 [77], Wnt/β-catenin 是控制多个renin-angiotensin系统基因的主调节者[78], SOD1 过度表达可以恢复受损的 Wnt 信号[79], 这些表明Wnt (肾阴分子) 和renin/SOD (肝阴分子)互相扶助促进。 因此,滋水涵木法准确地描述了分子信号的相互关系。
益火补土法,是温肾阳以补脾阳的治法,又称温肾健脾法、温补脾肾法。适用于肾阳衰微而致脾阳不振之证[3]。 在现代生物医学中,, calcitonin gene-related peptide (CGRP) 激发 ANP的分泌[80], 表明增加CGRP (肾阳分子)会促进ANP信号 (脾阳分子).
培土生金法,是健脾生气以补益肺气的治法。主要用于脾气虚衰,生气无源,以致肺气虚弱之证,若肺气虚衰,兼见脾运不健者,亦可应用[3]。 在现代生物医学中, 用ghrelin 治疗能极大的提高HIF-1α 和VEGF mRNAs [48], retinoic acid 增加HIF-1α [50]. 因此,增加ghrelin (脾气分子)和retinoic acid (脾阴) 可以增强HIF (肺气分子).
金水相生法,是滋养肺肾之阴的治法,亦称滋养肺肾法。主要用于肺阴亏虚,不能滋养肾阴,或肾阴亏虚,不能滋养肺阴的肺肾阴虚证[3]。 在现代生物医学中, Wnt 调节 vitamin C 合成 [57]; 用ascorbic acid治疗能提高 Wnt信号的效果[81], 表明Wnt (肾阴分子) 和 ascorbic acid (肺阴分子) 互相扶助促进。
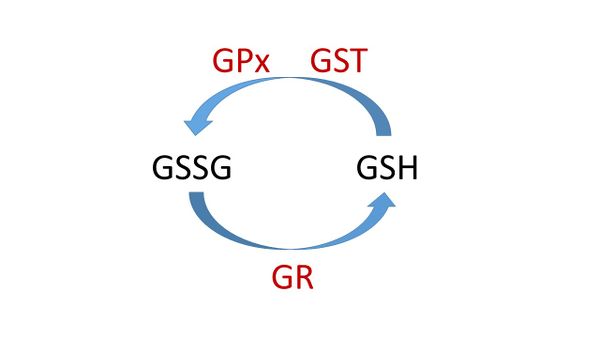
抑木扶土法,是疏肝健脾以治疗肝脾不和病证的治法,又称疏肝健脾法、调理肝脾法[3]。 肝气分子是 谷胱甘肽glutathione,其以还原态GSH和氧化态GSSG(Glutathione disulfide二硫化谷胱甘肽)的形式存在。如图四所示,在GSH 氧化还原循环中, GSH 被glutathione peroxide (GPx)催化转变成GSSG; GSSG被glutathione reductase (GR)催化再生成 GSH。 线粒体谷胱甘肽-S-转移酶Glutathione-S-transferases (GSTs) 同时具有GPx和 GST活性,其通过GSH共轭或GSH介导的过氧化物减少而解毒有害的副产品[82]. 在现代生物医学中,用ghrelin治疗可以降低缺氧大鼠血浆的EPO和 EPO基因表达 [83], 以及增加SOD, GPx, GST, GR 和 GSH [84,85], 表明 ghrelin (脾气分子)能增强GSH 氧化还原循环,即“疏通肝”。
培土制水法,是健脾利水以治疗水湿停聚病证的治法,又称为敦土利水法。适用于脾虚不运,水湿泛滥而致水肿胀满之证[3]。 在现代生物医学中, ghrelin促进利尿[86,87], 用ghrelin治疗有助于减少急性全身缺氧所致的脑水肿[88], aldosterone水平提高能够维持钠平衡及避免水肿状态[89], 9-cis retinoic acid (alitretinoin) 能有效地防止术后淋巴水肿[90]。表明ghrelin (脾气分子), 和retinoic acid and aldosterone (脾阴分子)有“利水”功效。
佐金平木法,是滋肺阴清肝火以治疗肝火犯肺病证的治法,也可称为“滋肺清肝法”。适用于肺阴不足,右降不及的肝火犯肺证[3]。 在现代生物医学中,GST 和 GSH结合有解毒作用;GST提高血清ascorbic acid 对vitamin C摄入量的反应[91];当膳食vitamin C 不足时,GST提供保护免受血清ascorbic acid缺乏的影响 [92]. 这些表明,“解毒”(清肝)提高了血清的ascorbic acid反应(肺阴分子)。
泻南补北法,是泻心火补肾水以治疗心肾不交病证的治法,又称为泻火补水法、滋阴降火法。适用于肾阴不足,心火偏旺,水火不济,心肾不交之证[3]。在现代生物医学中,IGF信号对抗 Wnt 信号通路[76]。 即当IGF(心阳分子)太强时,会抑制Wnt 信号通路(肾阴分子);而降低IGF,有助于提高Wnt 信号通路。
从以上讨论可以看到,中医的基本治疗原则和信号分子通路高度一致。
3. 讨论
如图一所示,中医理论认为按照心-脾-肺-肾-肝-心的顺序是相生的关系,而按照心-肺-肝-脾-肾-心的顺序是相克的关系。由于我们已经建立了五行阳阴气与信号分子之间的直接映射关系,因此我们能够检验各个“行”的信号分子对各个脏器的作用,结果发现中医的五行生克理论精准的描述了信号分子对脏器的作用。沿着图一外圆周,按照心-脾-肺-肾-肝-心的顺序,各个“行”的信号分子对下一个脏器具有辅助促进作用。然而,当按照相反的顺序时,即肝-肾-肺-脾-心-肝的顺序时,各个“行”的“阳”信号分子对下一个脏器却代表着负面的影响。例如,对于肝阳信号分子,多囊肾病患者有更高水平的 EPO[93];对于肾阳信号分子,肺小细胞癌常伴随异常升高的血浆calcitonin [94], 肺癌与高水平的PTHrP相关联[95]; VEGF(肺阳信号分子)增加了造血干细胞和循环内皮前体细胞在脾脏的募集和运动从而导致脾脏肿大[96]; 循环ANP (脾阳信号分子)在充血性心力衰竭时大大增加 [97]。。但是,心阳信号分子IGF对肝脏有正面作用, 例如肝脏功能下降的慢性肝脏疾病患者的IGF浓度较低[98]。
而沿着对角线,和中医五行生克理论所述一样,按照心-肺-肝-脾-肾-心的顺序,各个“行”的信号分子对下一个脏器具有正面调节作用。当沿着相反的顺序,则“阳”信号分子可能对下一个脏器有负面影响。例如,对于心阳信号分子,高水平的血液IGF-1 与慢性肾脏疾病正相关[99]; 对于肾阳信号分子,降钙素基因相关肽抑制脾 T 淋巴细胞增殖 [100] 以及内毒素诱导脾间质细胞的PTHrP基因表达[101]; 对于肝阳信号分子,血清EPO 和肺功能指标负相关[102]。但是脾阳信号分子对肝脏有正面作用, 因为ANP 保护肝脏免受缺氧损伤[103]; 肺阳信号分子对心脏也有正面作用,例如VEGF和其受体在心血管的发展,包括干细胞分化为心肌细胞,干细胞迁移和生存,以及心脏发育等许多方面发挥作用[104], 用VEGF 和 FGF7治疗能帮助优化心外膜的发展 [105].
各个“行”的信号分子之间的关系也符合中医心-脾-肺-肾-肝-心的相生关系。如图二所示,心“行”的信号分子对脾“行”的信号分子有激发作用,脾“行”信号分子对肺“行”信号分子有调节作用,肺“行”的信号分子对肾“行”的信号分子有调节作用,肾“行”的信号分子对肝“行”的信号分子有激发作用,肝“行”的信号分子对心“行”的信号分子有激发作用。
并且,各个行的“阳”信号分子符合中医心-肺-肝-脾-肾-心的相克关系。如图二所示,心“行”的阳信号分子激发肺“行”的阳信号分子,肺“行”的阳信号分子激发肝“行”的阳信号分子,肝“行”的阳信号分子激发脾“行”的阳信号分子(但是脾气信号分子ghrelin激发肝气信号分子GSH和肝阴信号分子SOD),脾“行”的阳信号分子激发肾“行”的阳信号分子,肾“行”的阳信号分子激发心 “行”的阳信号分子。
通过比较信号分子传输通路和中医五行生克八种治疗方法,我们可以明白中医使用了精准的语言描述着信号分子传输通路。例如,疏肝健脾法(抑木扶土法或)中的“疏肝”生动地描述了脾气信号分子ghrelin对GSH氧化还原循环的作用;滋肺清肝法(佐金平木法)中的“清肝”准确地描述了GST的解毒功能可以提高血清ascorbic acid反应。
总之,用中医五行阳阴气和信号分子的直接映射,我们证明中医五行生克治疗方法准确地描述了信号分子的传输通路,中医理论实质上是基于人体信号分子水平的传输通路从而具有坚实的科学基础。中医和现代医学描述着同样的生理过程只是用了不同的术语而已。
References
1. Y., L. Traditional chinese medicine describes the pathways of signaling molecules. Traditional Chinese Medicine and Molecular Signaling 2017.
2. Yang, L. 中医阴阳五行描述了信号分子的传输通路. Traditional Chinese Medicine and Molecular Signaling 2017.
3. Sun, G.R. Fundamental theory of tcm. China Press of TCM: Beijing, 2002.
4. Malpuech-Brugere, C.; Kuryszko, J.; Nowacki, W.; Rock, E.; Rayssiguier, Y.; Mazur, A. Early morphological and immunological alterations in the spleen during magnesium deficiency in the rat. Magnesium research 1998, 11, 161-169.
5. Tan, K.S.; Inoue, T.; Kulkeaw, K.; Tanaka, Y.; Lai, M.I.; Sugiyama, D. Localized scf and igf-1 secretion enhances erythropoiesis in the spleen of murine embryos. Biology open 2015, 4, 596-607.
6. Savastano, S.; Di Somma, C.; Pizza, G.; De Rosa, A.; Nedi, V.; Rossi, A.; Orio, F.; Lombardi, G.; Colao, A.; Tarantino, G. Liver-spleen axis, insulin-like growth factor-(igf)-i axis and fat mass in overweight/obese females. Journal of translational medicine 2011, 9, 136.
7. Welniak, L.A.; Karas, M.; Yakar, S.; Anver, M.R.; Murphy, W.J.; LeRoith, D. Effects of organ-specific loss of insulin-like growth factor-i production on murine hematopoiesis. Biology of Blood and Marrow Transplantation 2004, 10, 32-39.
8. Silva-Filho, J.L.; Souza, M.C.; Ferreira-DaSilva, C.T.; Silva, L.S.; Costa, M.F.S.; Padua, T.A.; das Graças Henriques, M.; Morrot, A.; Savino, W.; Caruso-Neves, C. Angiotensin ii is a new component involved in splenic t lymphocyte responses during plasmodium berghei anka infection. PloS one 2013, 8, e62999.
9. Birukova, A.A.; Xing, J.; Fu, P.; Yakubov, B.; Dubrovskyi, O.; Fortune, J.A.; Klibanov, A.M.; Birukov, K.G. Atrial natriuretic peptide attenuates lps-induced lung vascular leak: Role of pak1. American Journal of Physiology-Lung Cellular and Molecular Physiology 2010, 299, L652-L663.
10. Malpel, S.; Mendelsohn, C.; Cardoso, W.V. Regulation of retinoic acid signaling during lung morphogenesis. DEVELOPMENT-CAMBRIDGE- 2000, 127, 3057-3067.
11. Olivera, W.G.; Ciccolella, D.E.; Barquin, N.; Ridge, K.M.; Rutschman, D.H.; Yeates, D.B.; Sznajder, J.I. Aldosterone regulates na, k-atpase and increases lung edema clearance in rats. American journal of respiratory and critical care medicine 2000, 161, 567-573.
12. Santos, M.; Bastos, P.; Gonzaga, S.; Roriz, J.-M.; Baptista, M.J.; Nogueira-Silva, C.; Melo-Rocha, G.; Henriques-Coelho, T.; Roncon-Albuquerque, R.; Leite-Moreira, A.F. Ghrelin expression in human and rat fetal lungs and the effect of ghrelin administration in nitrofen-induced congenital diaphragmatic hernia. Pediatric research 2006, 59, 531-537.
13. Haase, V.H. Hypoxia-inducible factors in the kidney. American Journal of Physiology-Renal Physiology 2006, 291, F271-F281.
14. Qiao, J.; Uzzo, R.; Obara-Ishihara, T.; Degenstein, L.; Fuchs, E.; Herzlinger, D. Fgf-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 1999, 126, 547-554.
15. Schrijvers, B.F.; Flyvbjerg, A.; De Vriese, A.S. The role of vascular endothelial growth factor (vegf) in renal pathophysiology. Kidney international 2004, 65, 2003-2017.
16. Singer, R.F. Vitamin c supplementation in kidney failure: Effect on uraemic symptoms. Nephrology Dialysis Transplantation 2011, 26, 614-620.
17. Liang, F.-F.; Liu, C.-P.; Li, L.-X.; Xue, M.-M.; Xie, F.; Guo, Y.; Bai, L. Activated effects of parathyroid hormone-related protein on human hepatic stellate cells. PloS one 2013, 8, e76517.
18. Gorenko, Z.A.; Karbovska, L.S.; Vascheka, I.P.; Veselsky, S.P. The influence of calcitonin on the liver bile formation function in rats. International Journal of Physiology and Pathophysiology 2012, 3.
19. Hon, W.M.; Lee, K.H.; Khoo, H.E. Nitric oxide in liver diseases. Annals of the New York Academy of Sciences 2002, 962, 275-295.
20. Thompson, M.D.; Monga, S.P. Wnt/β‐catenin signaling in liver health and disease. Hepatology 2007, 45, 1298-1305.
21. Shimizu, H.; Kiyohara, Y.; Kato, I.; Kitazono, T.; Tanizaki, Y.; Kubo, M.; Ueno, H.; Ibayashi, S.; Fujishima, M.; Iida, M. Relationship between plasma glutathione levels and cardiovascular disease in a defined population. Stroke 2004, 35, 2072-2077.
22. van der Meer, P.; Voors, A.A.; Lipsic, E.; van Gilst, W.H.; van Veldhuisen, D.J. Erythropoietin in cardiovascular diseases. European Heart Journal 2004, 25, 285-291.
23. Jin, H.; Wyss, J.M.; Yang, R.; Schwall, R. The therapeutic potential of hepatocyte growth factor for myocardial infarction and heart failure. Current pharmaceutical design 2004, 10, 2525-2533.
24. van Deel, E.D.; Lu, Z.; Xu, X.; Zhu, G.; Hu, X.; Oury, T.D.; Bache, R.J.; Duncker, D.J.; Chen, Y. Extracellular superoxide dismutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radical Biology and Medicine 2008, 44, 1305-1313.
25. Kaiserova, K.; Srivastava, S.; Hoetker, J.D.; Awe, S.O.; Tang, X.-L.; Cai, J.; Bhatnagar, A. Redox activation of aldose reductase in the ischemic heart. Journal of Biological Chemistry 2006, 281, 15110-15120.
26. Klabunde, R.E. Cardiovascular physiology concepts. 2nd ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, 2012; p xi, 243 p.
27. Hung, C.F.; Rohani, M.G.; Lee, S.S.; Chen, P.; Schnapp, L.M. Role of igf-1 pathway in lung fibroblast activation. Respiratory Research 2013, 14.
28. Marshall, R.P. The pulmonary renin-angiotensin system. Current pharmaceutical design 2003, 9, 715-722.
29. Landon, R.A.; Young, E.A. Role of magnesium in regulation of lung function. J Am Diet Assoc 1993, 93, 674-677.
30. Takase, H.M.; Itoh, T.; Ino, S.; Wang, T.; Koji, T.; Akira, S.; Takikawa, Y.; Miyajima, A. Fgf7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes & Development 2013, 27, 169-181.
31. Taniguchi, E.; Sakisaka, S.; Matsuo, K.; Tanikawa, K.; Sata, M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. Journal of Histochemistry & Cytochemistry 2001, 49, 121-129.
32. Ipsen, D.H.; Tveden-Nyborg, P.; Lykkesfeldt, J. Does vitamin c deficiency promote fatty liver disease development? Nutrients 2014, 6, 5473-5499.
33. Ersoz, G.; Gunsar, F.; Karasu, Z.; Akay, S.; Batur, Y.; Akarca, U.S. Management of fatty liver disease with vitamin e and c compared to ursodeoxycholic acid treatment. Turk J Gastroenterol 2005, 16, 124-128.
34. Nath, B.; Szabo, G. Hypoxia and hypoxia inducible factors: Diverse roles in liver diseases. Hepatology 2012, 55, 622-633.
35. Schooley, J.C. The effect of erythropoietin on the growth and development of spleen colony‐forming cells. Journal of Cellular Physiology 1966, 68, 249-262.
36. Yamashita, Y.; Jeschke, M.G.; Wolf, S.E. Differential expression of hepatocyte growth factor in liver, kidney, lung, and spleen following burn in rats. Cytokine 2000, 12, 1293-1298.
37. Blaine, E.H. Atrial natriuretic factor plays a significant role in body fluid homeostasis. Hypertension 1990, 15, 2-8.
38. Arai, K.; Chrousos, G.P. Aldosterone deficiency and resistance. 2016.
39. Mallipattu, S.K.; He, J.C. The beneficial role of retinoids in glomerular disease. Frontiers in medicine 2015, 2.
40. Yoshimoto, A.; Mori, K.; Sugawara, A.; Mukoyama, M.; Yahata, K.; Suganami, T.; Takaya, K.; Hosoda, H.; Kojima, M.; Kangawa, K. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. Journal of the American Society of Nephrology 2002, 13, 2748-2752.
41. Massion, P.; Feron, O.; Dessy, C.; Balligand, J.-L. Nitric oxide and cardiac function. Circulation research 2003, 93, 388-398.
42. Franco-Cereceda, A.; Liska, J. Potential of calcitonin gene-related peptide in coronary heart disease. Pharmacology 2000, 60, 1-8.
43. Ross, G.; Schlüter, K.-D. Cardiac-specific effects of parathyroid hormone-related peptide: Modification by aging and hypertension. Cardiovascular research 2005, 66, 334-344.
44. Bergmann, M.W. Wnt signaling in adult cardiac hypertrophy and remodeling. Circulation research 2010, 107, 1198-1208.
45. Sohmiya, M.; Sohmiya, Y. Effects of recombinant human erythropoietin (rhuepo) treatment on plasma insulin-like growth factor-i (igf-i) and hemoglobin concentra-tions in patients with type 2 diabetes mellitus associated with neph-ropathy and anemia of chronic renal failure. Biomedical Research 2010, 21.
46. Barbagallo, M.; Dominguez, L.J.; Tagliamonte, M.R.; Resnick, L.M.; Paolisso, G. Effects of glutathione on red blood cell intracellular magnesium - relation to glucose metabolism. Hypertension 1999, 34, 76-82.
47. Chu, C.H.; Tzang, B.S.; Chen, L.M.; Kuo, C.H.; Cheng, Y.C.; Chen, L.Y.; Tsai, F.J.; Tsai, C.H.; Kuo, W.W.; Huang, C.Y. Igf-ii/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/bnp expression via g alpha q interaction and protein kinase c-alpha/camkii activation in h9c2 cardiomyoblast cells. Journal of Endocrinology 2008, 197, 381-390.
48. Konturek, P.C.; Brzozowski, T.; Walter, B.; Burnat, G.; Hess, T.; Hahn, E.G.; Konturek, S.J. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. European Journal of Pharmacology 2006, 536, 171-181.
49. Walczak, C.; Gaignier, F.; Gilet, A.; Zou, F.; Thornton, S.N.; Ropars, A. Aldosterone increases vegf-a production in human neutrophils through pi3k, erk1/2 and p38 pathways. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2011, 1813, 2125-2132.
50. Fernandez-Martinez, A.B.; Jimenez, M.I.A.; Cazana, F.J.L. Retinoic acid increases hypoxia-inducible factor-1 alpha through intracrine prostaglandin e-2 signaling in human renal proximal tubular cells hk-2. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids 2012, 1821, 672-683.
51. Mackenzie, I.C.; Gao, Z.R. Keratinocyte growth factor expression in human gingival fibroblasts and stimulation of in vitro gene expression by retinoic acid. Journal of Periodontology 2001, 72, 445-453.
52. Lara-Castillo, N.; Zandi, S.; Nakao, S.; Ito, Y.; Noda, K.; She, H.; Ahmed, M.; Frimmel, S.; Ablonczy, Z.; Hafezi-Moghadam, A. Atrial natriuretic peptide reduces vascular leakage and choroidal neovascularization. The American journal of pathology 2009, 175, 2343-2350.
53. Mazumdar, J.; O'Brien, W.T.; Johnson, R.S.; LaManna, J.C.; Chavez, J.C.; Klein, P.S.; Simon, M.C. O-2 regulates stem cells through wnt/beta-catenin signalling. Nature Cell Biology 2010, 12, 1007-1013.
54. Pelosi, M.; Lazzarano, S.; Thoms, B.L.; Murphy, C.L. Parathyroid hormone-related protein is induced by hypoxia and promotes expression of the differentiated phenotype of human articular chondrocytes. Clinical Science 2013, 125, 461-470.
55. Agani, F.H.; Puchowicz, M.; Chavez, J.C.; Pichiule, P.; LaManna, J. Role of nitric oxide in the regulation of hif-1 alpha expression during hypoxia. American Journal of Physiology-Cell Physiology 2002, 283, C178-C186.
56. Zhu, X.J.; Liu, Y.; Dai, Z.M.; Zhang, X.; Yang, X.; Li, Y.; Qiu, M.; Fu, J.; Hsu, W.; Chen, Y., et al. Bmp-fgf signaling axis mediates wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet 2014, 10, e1004687.
57. Nejak-Bowen, K.N.; Zeng, G.; Tan, X.P.; Cieply, B.; Monga, S.P. Beta-catenin regulates vitamin c biosynthesis and cell survival in murine liver. Journal of Biological Chemistry 2009, 284, 28115-28127.
58. d'Uscio, L.V.; Milstien, S.; Richardson, D.; Smith, L.; Katusic, Z.S. Long-term vitamin c treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circulation Research 2003, 92, 88-95.
59. Kurtz, A.; Muff, R.; Born, W.; Lundberg, J.; Millberg, B.; Gnädinger, M.; Uehlinger, D.; Weidmann, P.; Hökfelt, T.; Fischer, J. Calcitonin gene-related peptide is a stimulator of renin secretion. Journal of Clinical Investigation 1988, 82, 538.
60. Atchison, D.K.; Westrick, E.; Szandzik, D.L.; Gordish, K.L.; Beierwaltes, W.H. Parathyroid hormone-related protein stimulates plasma renin activity via its anorexic effects on sodium chloride intake. American Journal of Physiology-Endocrinology and Metabolism 2012, 303, E457-E463.
61. Feng, C.-c.; Ding, G.-x.; Song, N.-h.; Li, X.; Wu, Z.; Jiang, H.-w.; Ding, Q. Paraneoplastic hormones: Parathyroid hormone-related protein (pthrp) and erythropoietin (epo) are related to vascular endothelial growth factor (vegf) expression in clear cell renal cell carcinoma. Tumor Biology 2013, 34, 3471-3476.
62. Downs, T.M.; Burton, D.W.; Araiza, F.L.; Hastings, R.H.; Deftos, L.J. Pthrp stimulates prostate cancer cell growth and upregulates aldo–keto reductase 1c3. Cancer letters 2011, 306, 52-59.
63. Cokic, B.B.B.; Cokic, V.P.; Suresh, S.; Wirt, S.; Noguchi, C.T. Nitric oxide and hypoxia stimulate erythropoietin receptor via mapk kinase in endothelial cells. Microvascular research 2014, 92, 34-40.
64. Slomiany, M.G.; Rosenzweig, S.A. Igf-1-induced vegf and igfbp-3 secretion correlates with increased hif-1 alpha expression and activity in retinal pigment epithelial cell line d407. Investigative Ophthalmology & Visual Science 2004, 45, 2838-2847.
65. Stirling, D.; Magness, R.R.; Stone, R.; Waterman, M.R.; Simpson, E.R. Angiotensin-ii inhibits luteinizing hormone-stimulated cholesterol side-chain cleavage expression and stimulates basic fibroblast growth-factor expression in bovine luteal cells in primary culture. Journal of Biological Chemistry 1990, 265, 5-8.
66. Pupilli, C.; Lasagni, L.; Romagnani, P.; Bellini, F.; Mannelli, M.R.; Misciglia, N.; Mavilia, C.; Vellei, U.; Villari, D.; Serio, T. Angiotensin ii stimulates the synthesis and secretion of vascular permeability factor vascular endothelial growth factor in human mesangial cells. Journal of the American Society of Nephrology 1999, 10, 245-255.
67. Westenbrink, B.D.; Ruifrok, W.P.T.; Voors, A.A.; Tilton, R.G.; van Veldhuisen, D.J.; Schoemaker, R.G.; van Gilst, W.H.; de Boer, R.A. Vascular endothelial growth factor is crucial for erythropoietin-induced improvement of cardiac function in heart failure. Cardiovascular Research 2010, 87, 30-39.
68. Johnston, C.S.; Meyer, C.G.; Srilakshmi, J.C. Vitamin-c elevates red-blood-cell glutathione in healthy-adults. American Journal of Clinical Nutrition 1993, 58, 103-105.
69. Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor-1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular o-2 tension. Proceedings of the National Academy of Sciences of the United States of America 1995, 92, 5510-5514.
70. Tajima, M.; Kurashima, Y.; Sugiyama, K.; Ogura, T.; Sakagami, H. The redox state of glutathione regulates the hypoxic induction of hif-1. European Journal of Pharmacology 2009, 606, 45-49.
71. Porat, O.; Neumann, D.; Zamir, O.; Nachshon, S.; Feigin, E.; Cohen, J.; Zamir, N. Erythropoietin stimulates atrial natriuretic peptide secretion from adult rat cardiac atrium. J Pharmacol Exp Ther 1996, 276, 1162-1168.
72. Cao, Y.K.; Tang, J.; Yang, T.; Ma, H.; Yi, D.H.; Gu, C.H.; Yu, S.Q. Cardioprotective effect of ghrelin in cardiopulmonary bypass involves a reduction in inflammatory response. Plos One 2013, 8.
73. Zwirska-Korczala, K.; Adamczyk-Sowa, M.; Sowa, P.; Pilc, K.; Suchanek, R.; Pierzchala, K.; Namyslowski, G.; Misiolek, M.; Sodowski, K.; Kato, I., et al. Role of leptin, ghrelin, angiotensin ii and orexins in 3t3 l1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol 2007, 58 Suppl 1, 53-64.
74. Vesely, D.L.; Overton, R.M.; McCormick, M.T.; Schocken, D.D. Atrial natriuretic peptides increase calcitonin gene-related peptide within human circulation. Metabolism-Clinical and Experimental 1997, 46, 818-825.
75. Farley, J.; Dimai, H.P.; Stilt-Coffing, B.; Farley, P.; Pham, T.; Mohan, S. Calcitonin increases the concentration of insulin-like growth factors in serum-free cultures of human osteoblast-line cells. Calcified Tissue International 2000, 67, 247-254.
76. Schlupf, J.; Steinbeisser, H. Igf antagonizes the wnt/beta-catenin pathway and promotes differentiation of extra-embryonic endoderm. Differentiation 2014, 87, 209-219.
77. Yang, Y.Y.; Lee, P.C.; Huang, Y.T.; Lee, W.P.; Kuo, Y.J.; Lee, K.C.; Hsieh, Y.C.; Lee, T.Y.; Lin, H.C. Involvement of the hif-1 alpha and wnt/beta-catenin pathways in the protective effects of losartan on fatty liver graft with ischaemia/reperfusion injury. Clinical Science 2014, 126, 163-174.
78. Zhou, L.; Liu, Y. Wnt/β-catenin signaling and renin–angiotensin system in chronic kidney disease. Current opinion in nephrology and hypertension 2016, 25, 100-106.
79. Wang, F.; Fisher, S.A.; Zhong, J.; Wu, Y.; Yang, P. Superoxide dismutase 1 in vivo ameliorates maternal diabetes-induced apoptosis and heart defects through restoration of impaired wnt signaling. Circulation: Cardiovascular Genetics 2015, CIRCGENETICS. 115.001138.
80. Yamamoto, A.; Kimura, S.; Hasui, K.; Fujisawa, Y.; Tamaki, T.; Fukui, K.; Iwao, H.; Abe, Y. Calcitonin gene-related peptide (cgrp) stimulates the release of atrial natriuretic peptide(anp) from isolated rat atria. Biochemical and Biophysical Research Communications 1988, 155, 1452-1458.
81. Ivanyuk, D.; Budash, G.; Zheng, Y.; Gaspar, J.A.; Chaudhari, U.; Fatima, A.; Bahmanpour, S.; Grin, V.K.; Popandopulo, A.G.; Sachinidis, A. Ascorbic acid-induced cardiac differentiation of murine pluripotent stem cells: Transcriptional profiling and effect of a small molecule synergist of wnt/β-catenin signaling pathway. Cellular Physiology and Biochemistry 2015, 36, 810-830.
82. Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Glutathione and mitochondria. 2014.
83. Feizi, H.; Rajaee, K.; Keyhanmanesh, R.; Aliparasti, M.; Almasi, S.; Alipour, M. Effect of ghrelin on renal erythropoietin production in chronic hypoxic rats. Endocrine regulations 2014, 48, 3-8.
84. Dobutovic, B.; Sudar, E.; Tepavcevic, S.; Djordjevic, J.; Djordjevic, A.; Radojcic, M.; Isenovic, E.R. Effects of ghrelin on protein expression of antioxidative enzymes and inos in the rat liver. Arch Med Sci 2014, 10, 806-816.
85. Karatug, A.; Sacan, O.; Coskun, Z.M.; Bolkent, S.; Yanardag, R.; Turk, N.; Bolkent, S. Regulation of gene expression and biochemical changes in small intestine of newborn diabetic rats by exogenous ghrelin. Peptides 2012, 33, 101-108.
86. Mao, Y.; Tokudome, T.; Kishimoto, I. Ghrelin and blood pressure regulation. Current hypertension reports 2016, 18, 1-6.
87. Aoki, H.; Nakata, M.; Dezaki, K.; Lu, M.; Gantulga, D.; Yamamoto, K.; Shimada, K.; Kario, K.; Yada, T. Ghrelin counteracts salt-induced hypertension via promoting diuresis and renal nitric oxide production in dahl rats. Endocrine journal 2013, 60, 571-581.
88. Hossienzadeh, F.; Babri, S.; Alipour, M.R.; Ebrahimi, H.; Mohaddes, G. Effect of ghrelin on brain edema induced by acute and chronic systemic hypoxia. Neuroscience letters 2013, 534, 47-51.
89. Prakash, E.S. “Aldosterone escape” or refractory hyperaldosteronism? Medscape General Medicine 2005, 7, 25.
90. Bramos, A.; Perrault, D.; Yang, S.; Jung, E.; Hong, Y.K.; Wong, A.K. Prevention of postsurgical lymphedema by 9-cis retinoic acid. Annals of surgery 2016, 264, 353-361.
91. Cahill, L.; Fontaine-Bisson, B.; El-Sohemy, A. Glutathione s-transferase gene polymorphisms and vitamin c. The FASEB Journal 2009, 23, 725.721-725.721.
92. Cahill, L.E.; Fontaine-Bisson, B.; El-Sohemy, A. Functional genetic variants of glutathione s-transferase protect against serum ascorbic acid deficiency. The American journal of clinical nutrition 2009, 90, 1411-1417.
93. Chandra, M.; Miller, M.; Mossey, R.; McVicar, M. Serum immunoreactive erythropoietin levels in patients with polycystic kidney disease as compared with other hemodialysis patients. Nephron 1985, 39, 26-29.
94. Gropp, C.; Havemann, K.; Scheuer, A. Ectopic hormones in lung cancer patients at diagnosis and during therapy. Cancer 1980, 46, 347-354.
95. Furlhata, M.; Sonobe, H.; Iwata, J.; Ido, E.; Ohtsukl, Y.; Asahi, Y.; Kubonishl, I.; Miyoshi, I. Lung squamous cell carcinoma producing both parathyroid hormone‐related peptide and granulocyte colony stimulating factor. Pathology international 1996, 46, 376-379.
96. Hattori, K.; Dias, S.; Heissig, B.; Hackett, N.R.; Lyden, D.; Tateno, M.; Hicklin, D.J.; Zhu, Z.; Witte, L.; Crystal, R.G. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. The Journal of experimental medicine 2001, 193, 1005-1014.
97. Brandt, R.R.; Wright, R.S.; Redfield, M.M.; Burnett, J.C. Atrial natriuretic peptide in heart failure. Journal of the American College of Cardiology 1993, 22, A86-A92.
98. Møller, S.; Becker, U. Insulin-like growth factor 1 and growth hormone in chronic liver disease. Digestive diseases 1992, 10, 239-248.
99. Teppala, S.; Shankar, A.; Sabanayagam, C. Association between igf-1 and chronic kidney disease among us adults. Clinical and experimental nephrology 2010, 14, 440-444.
100. Haberstock-Debic, H.; Weyns, A.; Marotti, T.; De Potter, W. Calcitonin gene-related peptide receptors in pig spleen and the involvement of the cgrp 1 receptor in the splenocyte function. Neuropeptides 1999, 33, 47-53.
101. Funk, J.L.; Lausier, J.; Moser, A.H.; Shigenaga, J.K.; Huling, S.; Nissenson, R.A.; Strewler, G.J.; Grunfeld, C.; Feingold, K.R. Endotoxin induces parathyroid hormone-related protein gene expression in splenic stromal and smooth muscle cells, not in splenic lymphocytes. Endocrinology 1995, 136, 3412-3421.
102. Graudal, N.; Galløe, A.; Nielsen, O. Erythropoietin in chronic obstructive pulmonary disease. Respiration 1991, 58, 141-144.
103. Carini, R.; De Cesaris, M.G.; Splendore, R.; Domenicotti, C.; Nitti, M.P.; Pronzato, M.A.; Albano, E. Mechanisms of hepatocyte protection against hypoxic injury by atrial natriuretic peptide. Hepatology 2003, 37, 277-285.
104. Madonna, R.; De Caterina, R. Vegf receptor switching in heart development and disease. Cardiovascular research 2009, 84, 4-6.
105. Smart, N.; Risebro, C.A.; Melville, A.A.; Moses, K.; Schwartz, R.J.; Chien, K.R.; Riley, P.R. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007, 445, 177-182.
Table 1 Corresponding signaling molecules of each organ phase
| Yang | Yin | Qi | |
| Heart phase | IGF | Ang | Mg |
| Spleen phase | ANP | aldosterone,
retinoic acid |
ghrelin |
| Lung phase | FGF7, VEGF | ascorbic acid | HIF |
| Kidney phase | calcitonin, PTHrP | Wnt | NO |
| Liver phase | EPO, HGF | renin, SOD, AKR | GSH |
Figure Legends
Figure 1. The interrelationships of signaling molecules with organs. The small square indicates the closed-loop pathways of signaling molecules within the same phase. Along the outer circle, the thick red arrow shows that the molecules of each phase have assisting effect on the following organ, and it follows heart-spleen-lung-kidney-liver-heart arrangement. In the diagonal direction, the thick green arrow shows that the molecules of each phase have regulating effect on the following organ, and it follows heart-lung-liver-spleen-kidney-heart arrangement. Arrow means assisting/increasing, solid circle means inhibiting/decreasing, and arrow and circle together indicate regulating.
Figure 2. The pathways of signaling molecules among the five groups of molecules. Along the outer circle, the pathways follow the order of heart-spleen-lung-kidney-liver-heart groups. In the diagonal arrangement, the pathways follow the order of heart-lung-liver-spleen-kidney-heart groups. Arrow means increasing, solid circle means decreasing, and arrow and circle together indicate regulating.
Figure 3. The consistence between TCM classic therapeutic strategies and molecular signaling.
Figure 4. The glutathione redox cycle. GSH is the reduced state and GSSG (glutathione disulfide) is the oxidized state. GSH is converted by glutathione peroxide (GPx) to GSSG; GSH can be regenerated from GSSG by the enzyme glutathione reductase (GR). Glutathione-S-transferase (GST) GST can catalyze the conjugation of GSH for detoxification.
Figure 1. The interrelationships of signaling molecules with organs. The small square indicates the closed-loop pathways of signaling molecules within the same phase. Along the outer circle, the thick red arrow shows that the molecules of each phase have assisting effect on the following organ, and it follows heart-spleen-lung-kidney-liver-heart arrangement. In the diagonal direction, the thick green arrow shows that the molecules of each phase have regulating effect on the following organ, and it follows heart-lung-liver-spleen-kidney-heart arrangement. Arrow means assisting/increasing, solid circle means inhibiting/decreasing, and arrow and circle together indicate regulating.
Figure 2. The pathways of signaling molecules among the five groups of molecules. Along the outer circle, the pathways follow the order of heart-spleen-lung-kidney-liver-heart groups. In the diagonal arrangement, the pathways follow the order of heart-lung-liver-spleen-kidney-heart groups. Arrow means increasing, solid circle means decreasing, and arrow and circle together indicate regulating.
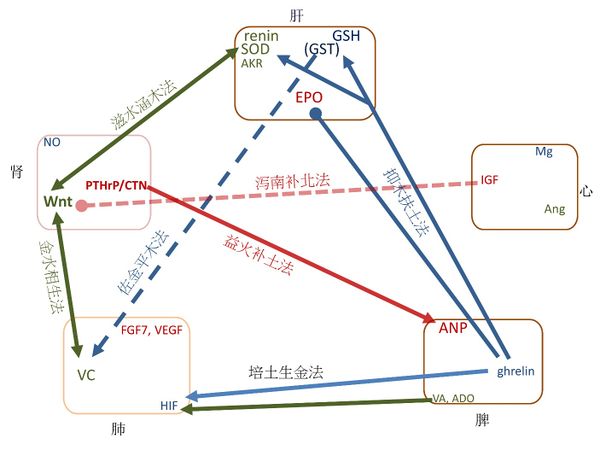 Figure 3. The consistence between TCM classic therapeutic strategies and molecular signaling.
Figure 3. The consistence between TCM classic therapeutic strategies and molecular signaling.
Figure 4. The glutathione redox cycle. GSH is the reduced state and GSSG (glutathione disulfide) is the oxidized state. GSH is converted by glutathione peroxide (GPx) to GSSG; GSH can be regenerated from GSSG by the enzyme glutathione reductase (GR). Glutathione-S-transferase (GST) GST can catalyze the conjugation of GSH for detoxification.
Document information
Published on 01/01/2017
Licence: CC BY-NC-SA license