中医阴阳五行描述了信号分子的传输通路
刘阳
香港理工大学
摘要
信号分子在生命过程中起着重要作用,它的传输通路对调节生理功能和病理的发展具有决定性作用。但是我们对这些通路的认识仍不全面。中医用阴阳五行来描述人体内的生理过程,但至今还没找到科学依据支持中医理论。黄帝内经认为心肝脾肺肾(五行)各有其阳阴气,并指出了每个“行”的生理功能和五行之间的相互关系。我们用迭代筛选法,将信号分子与黄帝内经所列各“行”的生理功能和相互关系一一比较,找出了五行中阳阴气与信号分子之间的直接映射,而满足这些关系的概率大概为十万分之二到百万分之一。这使我们发现阴阳五行实际上描述了人体中信号分子的传输通路,中草药的功用是调节各个信号分子。通过这一映射关系,可以清楚地看到现代医学和中医对一些疾病,如血尿、 便秘、 痔病、 遗尿症、 尿频、 胆结石、 急性胆囊炎和高血压,所认定的发病机理和治疗策略是一致的,只是使用了不同的术语。
关键词: 信号分子,中医,黄帝内经,信号分子通路,阳阴气
词汇表
| AKR | aldo-keto reductase, AKR 醛-酮還原酶,肝阴 |
| Aldosterone | 醛固酮, 脾阴 |
| Ang | Angiotensin, 血管紧张素, 心阴 |
| ANP | atrial natriuretic peptide, 心房利鈉肽,脾阳 |
| ascorbic acid | 抗坏血酸,维他命C,肺阴 |
| calcitonin | 降钙素,肾阳 |
| EPO | Erythropoietin, 红细胞生成素, 肝阳 |
| FGF7 | fibroblast growth factor-7, 成纤维细胞生长因子-7, 肺阳 |
| Ghrelin | 生长素释放肽, 脾气 |
| GSH | Glutathione, 谷胱甘肽, 肝气 |
| HGF | hepatocyte growth factor, 肝細胞生長因子,肝阳 |
| HIF | hypoxia inducible factor, 缺氧诱导因子,肺气 |
| IGF | insulin-like growth factor, 胰岛素样生长因子,心阳 |
| Mg | Magnesium, 镁,心气 |
| NO | nitric oxide, 氧化氮,肾气 |
| PTHrP | parathyroid hormone-related protein, 甲状旁腺激素相关蛋白,肾阳 |
| renin | 肾素,肝阴 |
| retinoic acid | 维甲酸,维他命A, 脾阴 |
| SOD | superoxide dismutase, 超氧化物歧化酶,肝阴 |
| VEGF | vascular endothelial growth factor, 血管内皮生长因子,肺阳 |
| Wnt | 肾阴 |
1.前言
了解信号分子的传输通路对理解信号分子调节生理功能的过程、预测疾病的发展、和采取正确的治疗策略等至关重要。然而,我们对信号分子的传输通路仍然缺乏透彻了解。
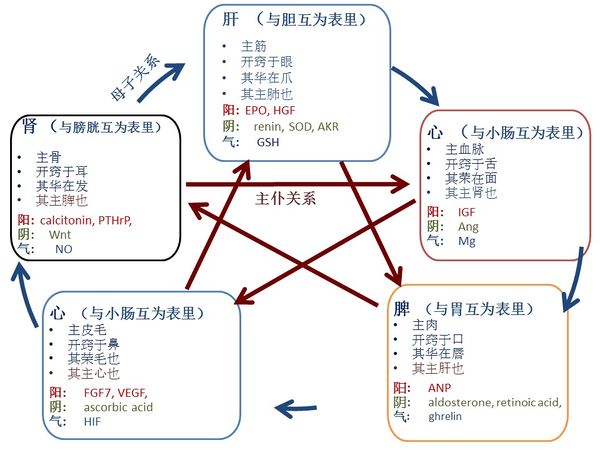
古代中医医家认为人体内存在关键物质用来调节生理过程,并将它们命名为阳与阴,其中阳有温暖生发功能而阴有寒冷收涩功能,阴阳功能相反,在人体内相互依存。根据这些关键物质的生理功能,古代中医医家将这些阳与阴分成五组:心、 脾、 肺、 肾和肝,即五行。除了阳与阴,中医医家还发现存在另一种物质,其功能是驱动调节,他们把它命名为气。因此,每个“行”包括器官本身,和属于该器官的阳、 阴和气。根据"黄帝内经" [1],每个“行”有特殊的生理功能,五行之间遵行 "母子" 或 "主仆" 关系 (图一)。中医医家基于这些辩证的相互关系而发展出来中医诊断理论和治疗策略。
然而,中医理论和现代医学之间有一个障碍,就是阳、 阴和气不能用现代科学方法来定义。由于近年来对信号分子的广泛研究,使我们有前所未有的机会去探讨中医理论与生命科学之间的联系。我们用迭代筛选法,找出了五行中阳阴气和信号分子之间的直接映射(如表一所示),并有三个主要发现:第一,各个器官的阳、阴和气对应着一种信号分子;第二,各个信号分子的传输通路与中医五行关系和治疗策略高度一致;第三,现代医学和中医对一些疾病,如血尿、 便秘、 痔病、 遗尿症、 尿频、 胆结石、 急性胆囊炎和高血压,所认定的发病机理和治疗策略是一致的,只是使用了不同的术语。
2. 迭代筛选法
用中医的心脾肺肾肝的功能作为约束条件,应用下面的迭代选择方法,以确定信号分子。假设所有信号分子为下列一群,
X = {x: 信号分子}, 心脾肺肾肝分别记为A, B, C, D, E。
第 1 步︰ 标识属于每个器官的信号分子
各个器官有四个约束条件,
φA(u1, u2, u3, u4) 是心的约束条件,其中
u1: 该分子应对心有重要影响
u2: 该分子对血管心脏功能有影响,
u3: 该分子在舌头上有表现,
u4: 该分子能影响皮肤形态.
φB(u1, u2, u3, u4) 是脾的约束条件,其中
u1: 该分子对脾有重要影响,
u2: 该分子对肉有重要影响,
u3: 该分子在口里有表现,
u4: 该分子能影响嘴唇形态
φC(u1, u2, u3, u4) 是肺的约束条件,其中
u1: 该分子对肺有重要影响,,
u2: 该分子对皮肤头发有重要影响,
u3: 该分子在鼻子里有表现,,,
u4: 该分子能影响毛发形态..
φD(u1, u2, u3, u4) 是肾的约束条件,其中u1: 该分子对肾有重要影响,,
u2: 该分子对骨有重要影响,
u3: 该分子在耳朵里有表现,
u4: 该分子能影响头发形态..
φE(u1, u2, u3, u4) 是肝的约束条件,其中
u1: 该分子对肝有重要影响,,
u2: 该分子对筋有重要影响,
u3: 该分子在眼睛里有表现,
u4: 该分子能影响指甲手指的形态.
然后将X 和 φ进行比较, 得到Y 群,
Y = {x: φj(u1, u2, u3, u4)}, 代表分别属于心脾肺肾肝的信号分子。
第 2 步︰ 确定每个器官的阳、阴和气
阳有生发温暖效应,阴有阴涩冷却效果,而气具激活调节功能。将这些限制条件设为Φ1, Φ2, 和 Φ3。然后将Y群和Φ1, Φ2, 及 Φ3 分别比较,得到各个器官的阳、阴和气:
A1 = y: Φ1, A2 = y: Φ2, A3 = y: Φ3;
B1 = y: Φ1, B2 = y: Φ2, B3 = y: Φ3;
C1 = y: Φ1, C2 = y: Φ2, C3 = y: Φ3;
D1 = y: Φ1, D2 = y: Φ2, D3 = y: Φ3;
E1 = y: Φ1, E2 = y: Φ2, E3 = y: Φ3.
第 3 步︰ 用五行中的"母子关系"进一步优化
因为阳具有生发功能,对应“阳“的信号分子应由“母”器官生成/分泌。而“阴”的信号分子也应在“母”器官有所存在,用这一条件优化第二部中的信号分子。
第 4 步︰ 用五行中的"主仆关系"进一步优化
能同时满足第一步至第四步约束条件的“阳阴”信号分子的概率大约是百万分之一量级,而“气”信号分子的概率大概是十万分之二。
第 5 步: 用中草药验证
用现有的临床和实验数据,比较中草药的调节阴阳气功能和对信号分子的影响,从而进一步验证筛选结果。
3.结果
3.1 阴阳气和信号分子的直接映射
我们筛选出与阳阴气对应的信号分子如表一所示。
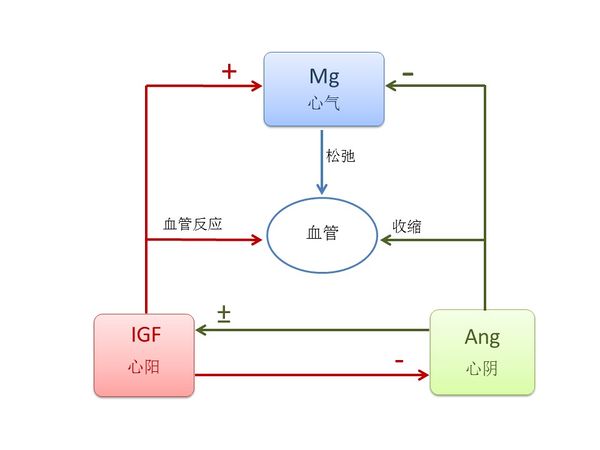
3.1.1 心的关键元素是IGF, Ang 和Mg. 按照《黄帝内经》,这些元素对心脏有关键作用,生于肝,主血脉,开窍于舌,其荣在面[1]。
现代医学发现,IGF由肝脏分泌,对心脏功能至关重要。IGF-1调节心脏的代谢、收缩力、衰老、细胞自噬、肥厚,和细胞凋亡;它可以激活心脏中的规范和非规范信号通路;IGF-1缺乏会导致心血管疾病,而当地IGF-1治疗可以预防实验模型的心脏损伤[2]。 IGF对血管循环系统有生理影响,并对多种血管疾病的病理过程有作用,包括高血压、糖尿病血管病变、动脉粥样硬化、血管生成和血管狭窄[3]。长期使用IGF-1会显著增加血管反应性 [4]。 IGF 也影响到舌头的大小,增加IGF 水平会导致舌头重量的显著增加和组织病理的变化;当IGF水平恢复至正常时,舌头的大小也恢复正常[5]。IGF水平也能在皮肤上反映出来,较高的IGF水平往往会减少皮肤皱纹[6]。由于IGF-1有温暖的效果[7], 我们将IGF认定为心阳。
Ang 由肝脏分泌,Ang II调节心脏和血管收缩,并参与心脏的生长、 重塑,和细胞凋亡[8]。Angiotensin-converting enzyme inhibitor(血管紧张素转换酶抑制剂)可诱发舌头血管性水肿,意味着Ang对舌头有重要作用 [9]。完整的renin-angiotensin(肾素-血管紧张素)系统存在于人体皮肤并对皮肤的动态平衡起重要作用[10]. 由于Ang II 可引致低温症[11], 我们认定Ang为心阴。
Mg对心脏的正常节律很重要,是超过 300 个酶系统中的辅助因子,调节体内生化反应,包括血压调节及血糖控制[12]. Mg 舒张血管[13];前舌灼口综合征患者往往在唾液和红细胞中表现出Mg缺乏[14]. 而且, Mg 会影响皮肤, 已经发现富含Mg的死海泥可以增强皮肤水化,提高皮肤屏障功能,并降低特应性皮肤干燥的炎症[15]。 由于Mg具有很强的调节功能,我们认定Mg为心气。
中医认为阴阳相互调节,图 二 显示了IGF, Ang 和 Mg之间的相互关系。已经发现给大鼠Ang II 输液会降低循环和骨骼肌IGF-1水平[16], Ang II 刺激心脏的IGF-1水平 [17]和IGF-1受体在血管平滑肌细胞中的转录[18], 而且 IGF-1降低Ang II [19]。 对血小板用Ang II处理会显著降低细胞内的Mg浓度[20], Mg水平和IGF-1 密切相关[21].
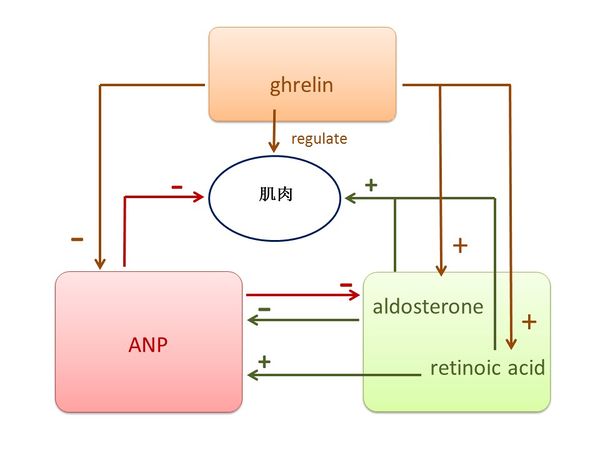
3.1.2 脾的关键元素是ANP, aldosterone, retinoic acid, 和 ghrelin. 按照《黄帝内经》,这些元素对脾脏有关键作用,生于心,主肉,开窍于口,其荣在唇[1]。
现代医学发现,ANP是由心脏肌肉细胞分泌的,它的功能是干预短期和长期的血压控制及水和电解质平衡[22]。 脾脏是 ANP 诱导液体外渗到全身淋巴系统的一个重要地点。ANP 通过提高脾内微血管压力并通过增加过滤面积,而强化等渗流体从脾血管的外渗[23]。 ANP,通过产生cGMP ,抑制血管平滑肌细胞和内皮细胞的增殖和肥大[24] ,ANP还对唾液分泌的调节起了生理的作用[25]. 已经知道皮脂和女士嘴唇颜色是依赖雌激素[26] 而男人嘴唇的特证依赖于睾丸激素[27], 研究发现雌激素受体会增加ANP [28]而ANP 会诱发睾丸激素的生成[29], 这意味着嘴唇反映了ANP水平。 由于肾阳的著名功能是“温暖”,而维持身体温度是ANP的重要功能[30], 因此我们认定ANP为肾阳。
Aldosterone(醛固酮)保持人体内所需的盐分,并帮助控制血压、 血液、 电解质的平衡和液体在体内的分布。最近发现在大鼠的心腔内产生了Aldosterone [31]。 Aldosterone增加脾脏大小和重量[32], 和刺激血管平滑肌细胞增殖[33]。 低水平Aldosterone会导致口干[34] 而且 aldosterone 使口腔清新[35]。 此外,estrogen receptor-β ligands(雌激素受体 β 配体)调节aldosterone合成[36] 以及短期的服用testosterone(睾酮)会减少男人的aldosterone水平[37], 意味着嘴唇的型态可以反映aldosterone的水平。 Retinoic acid (维他命 A) 控制脾脏细胞的动态平衡[38] 并可在心外膜生成[39]. 它也诱导分化成人骨骼肌原卫星细胞和肌细胞系[40], 调节口腔上皮的分化[41], 并作为治疗口腔黏膜白斑的有效方法[42]. 此外,过剩的外源性Retinoic acid可诱导腭裂[43], 意味着它能影响嘴唇的外观。由于 aldosterone和 retinoic acid 都抑制体内热的生成[44] [45], 因此我们认定其为脾阴。
Ghrelin是一种胃肽,负责能量调节的分布和消耗,抑制脾 T 细胞增殖[46] 和调节心肌、光滑肌和骨骼肌功能[47]. Ghrelin在口腔中对炎症感染的先天免疫反应起调节作用 [48]. 此外,雌激素替代疗法增加活性血浆ghrelin水平[49] 睾酮与男性ghrelin水平呈正相关关系[50], 表明嘴唇的型态也能反映ghrelin水平。.由于在中医里脾气有传递和转化食物能量的功能,而ghrelin可有效调节食物摄入量和能量平衡[51], 我们认定ghrelin为脾气。
图三显示了ANP 和 aldosterone/retinoic acid之间相互抑制又支持的关系。ANP 和aldosterone具有完全相反的功能, ANP 降低aldosterone,反之亦然[52]。 Retinoic acid信号明显刺激利钠肽受体 A 基因的表达[53]. ANP抑制血管平滑肌细胞增殖[24], aldosterone刺激平滑肌细胞增殖[33] 以及retinoic acid诱导肌肉分化[40]. 此外,模型大鼠经过ghrelin治疗后ANP 水平显著降低[54]; ghrelin 提升 aldosterone水平 [55]; 将ghrelin用于正常的肺会增加retinoic acid受体 α/γ 表达[56].
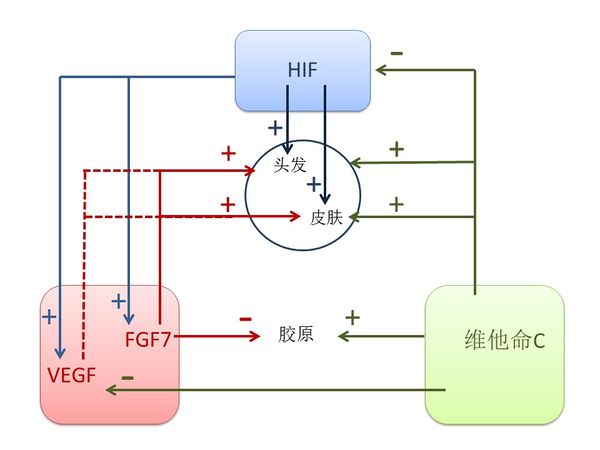
3.1.3 肺脏的关键元素是FGF7, VEGF, ascorbic acid 和 HIF. 按照《黄帝内经》,这些元素对肺脏有关键作用,生于脾,主皮毛,开窍于鼻,其荣在毛[1]。
FGF7是由脾分泌的[57]强效的有丝分裂原,其增强在各种器官,包括肺、 皮肤、 肠、 乳和肝的细胞增殖。它控制肺形态发育、呼吸道上皮细胞分化和增殖[58]. FGF7 治疗过的皮肤明显强于控制载体转染的皮肤,表明 FGF7 可以增加上皮厚度和强度[59]。 FGF7 表达在毛囊并对头发生长周期有多重功能[60]。 FGF7对鼻上皮细胞增殖起重要作用,在鼻息肉间质中过量的FGF7合成可能有助于慢性鼻窦炎伴鼻息肉患者鼻黏膜肥厚[61]。由于它的增殖效果,我们认定FGF7为肺阳。
VEGF对肺的维护与发展至关重要,也与多种急性和慢性肺疾病相关[62]. 已发现脾脏 T 细胞能产生 VEGF [63]。VEGF 是毛囊生长和循环的主要调节者,并促进头发的生长、增加毛囊和增粗毛发[64]。 VEGF也是主要的皮肤血管生成因子[65]。 此外, VEGF促进鼻腔上皮细胞的生长并抑制细胞凋亡[66]。富含血小板血浆注射已被用于治疗雄激素源性脱发,已经发现通过提高 FGF7 水平和增加 VEGF 水平而活化的富血小板血浆可以延长生长期的头发生长周期[67], 表明FGF7和VEGF能影响头发的型态。 由于棕色脂肪组织的功能是生成人体热量而 VEGF 对棕色脂肪组织发展和维护非常重要, [68], 因此我们认定VEGF为肺阳。
Ascorbic acid (Vitamin C) 在1900 年代被发现,当时主要是要研究治疗与肺炎相关的坏血病的方法。维生素 C 是一种生理的抗氧化剂保护宿主细胞对抗感染引起的氧化应激,能起预防和治疗肺炎[69] 和其他肺部疾病的作用[70]。 已经发现在脾脏中的维生素 C 含量远远高于其他器官[71]并在肺里存在胞外维生素 C池即使在坏血病情况下也是如此[72]。 二氢睾酮 (DHT)-依赖性导致脱发,已经发现 L-抗坏血酸 2-磷酸(一种稳定的维生素 C)可以抑制人毛囊真皮乳头细胞里由二氢睾酮诱导的 DKK-1 表达[73]。 维生素 C 是产生胶原蛋白的一个重要元素,是有效的抗氧化剂,可以帮助修复老年人及光损害的皮肤[74]。 维生素 C 也表现在鼻子,它是用于治疗常年性过敏性鼻炎的安全和自然疗法[75]。 此外,维他命C的不足会导致上臂毛囊的扩大和角化[76], 显示身体毛发反映维他命C水平。 中医认为肺阴具有"滋润肺"的效果,因此我们确定维他命C为肺阴。
HIF是高度保守的转录因子, 存在于几乎所有的细胞类型,受到氧含量的严格调制,并调节数百个基因的表达,对肺脏发育起决定性作用[77]。 头发矩阵以上的前皮层含有分化干细胞,HIF-2α 和 HIF-1β 在这里有高表达; 而HIF-2α / HIF-1β 调节毛囊分化[78]。 此外,HIF-1α在皮肤稳态及应激反应中起着关键作用[79] 并调节鼻息肉的形成[80]。 由于HIF具有很强的调节功能,我们认定它为肺气。
图四显示了HIF, FGF7, VEGF 和vitamin C之间的辩证调节关系。 HIF-1激活缺氧细胞中的 VEGF 转录[81]。 HIF促进 FGF7 mRNA 水平的表达[82]。维生素 C 可防止内皮细胞 VEGF 和 VEGFR-2 的过分表达 [83] 并抑制 NO 诱导的HIF-1α稳定和积累[84]。 维生素 C刺激胶原蛋白合成,但 FGF 抑制胶原凝胶的合成 [85]。
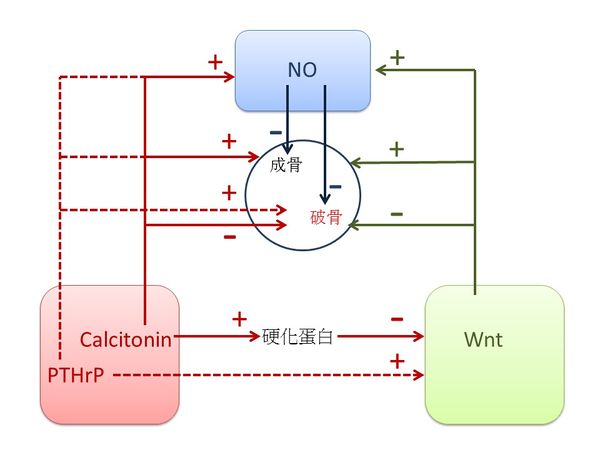
3.1.4 肾脏的关键元素是calcitonin, PTHrP, Wnt 和 NO. 按照《黄帝内经》,这些元素对肾脏有关键作用,生于肺,主骨,开窍于耳,其华在发[1]。
Calcitonin 和 PTHrP 都是从肺分泌的[86] [87]. Calcitonin增加肾对钙的再吸收能力[88], 提高成骨细胞骨形成和抑制骨吸收破骨细胞的活动[89]. Calcitonin治疗可以稳定听力损失或提高Paget's (佩吉特)病人的听力[90]。 此外,calcitonin gene-related peptide (CGRP) (降钙素基因相关肽)治疗减少了毛囊色素沉着[91], 意味著头发的颜色可反映CGRP 水平。PTHrP是强效的肾调控因子及促各种类型肾细胞的有丝分裂,并在肾脏发育中起作用 [92]。 它促进成骨细胞的补充和生存因而在成骨过程中起中心作用,它还可能通过增加破骨细胞进而调节骨吸收[93]。此外,在耳泡囊中检测到PTHrP mRNA[94]。 PTHrP可能诱发头发生长从退行期到休止期的过早过渡[95], 表明PTHrP 可能会影响头发的观感。 中医认为肾阳的重要功能是“温暖”,由于calcitonin 和 PTHrP 都具有温暖的效果 [96] [97], 我们因此认定calcitonin 和PTHrP 为肾阳。
Wnt 信号在发育和成人组织稳态期调节细胞间的相互作用,与肾发育和肾疾病相关联[98]。 多个 Wnt基因在肺里有表达[99]。在生理和病理条件下,Wnt对骨重塑具有潜在作用,它作用于成骨细胞前体细胞并促进其分化为成熟的成骨细胞,它通过各种信号通路可以抑制骨吸收或增强破骨细胞形成[100]。 Wnt信号在背侧区域耳小囊处非常活跃,它调节前庭形态所需的发生基因 (Dlx5/6 and Gbx2)的表达[101]. Wnt信号是黑色毛发再生的关键途径[102], 表明头发的外表反映了Wnt水平。 由于Wnt 信号组织体内生热[103],我们认定Wnt为肾阴。
NO是第二信使以及是心血管系统、 免疫和神经系统的一个主要调节器。NO在肾脏拥有众多的重要功能包括肾血流动力学的调节、髓质血流灌注的维护、促钠排泄压力的调解、钝化管球反馈、 抑制肾小管钠再吸收,和调节肾交感神经的神经活动[104]。破骨细胞和成骨细胞都有inducible NO synthase (iNOS)并且产生NO,而NO抑制破骨细胞的增殖和降低成骨细胞的增殖[105]. NO 在内耳中起重要作用以调节耳蜗和前庭部分的生理反应[106]。此外,NO调节毛囊活动[107], 表明NO可影响头发的观感. 由于NO具有很强的调节功能,我们认定NO为肾气。
图五表示了calcitonin, PTHrP, Wnt 和 NO之间的关系。 Calcitonin刺激硬化蛋白(sclerostin)的表达[108]而硬化蛋白抑制Wnt, 使用PTHrP能增强Wnt信号[109]。 此外,calcitonin增加血浆 NO 水平[110], PTHrP 激活 NO的释放 [111], 以及Wnt-5a增加NO 的产出[112]。 Calcitonin 促进成骨细胞骨形成和抑制破骨细胞的骨吸收活动[89]。 PTHrP 促进成骨细胞的补充和生存并且增加破骨细胞的形成[93]。 Wnt 信号刺激成骨细胞生成[113] 和减少破骨细胞形成[114]。
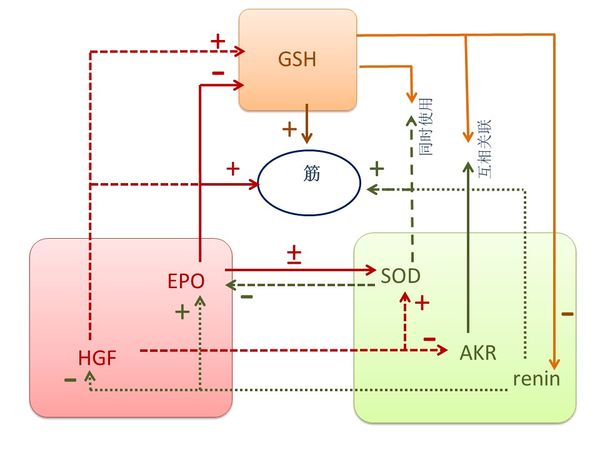
3.1.5 肝脏的关键元素是EPO, HGF, renin, SOD, AKR, 和GSH. 按照《黄帝内经》,这些元素对肝脏有关键作用,生于肾,主筋,开窍于耳、眼,其华在爪[1]。
EPO是一种糖蛋白,促进红细胞前体的增殖和分化。据报道,高剂量 EPO 通过影响生化、形态和组织病理学的参数而增加肝切除术后的肝再生[115]。 EPO在胎儿妊娠期主要由肝生成,而成人后主要在肾生成 [116]. 在非造血组织中它提供保护和再生的作用,并促进肌腱愈合[117]。 在糖尿病人和老鼠的眼睛中观察到EPO是有力的血管生成因子[118]。 此外,杵状指的定义是指甲根部的结构发生了变化,已被证明其与 EPO 水平的增加相关[119];使用红细胞生成刺激剂的患者有较低的甲真菌病发病率[120], 表明指甲和手指的状态反映EPO水平。 由于EPO 增加棕色脂肪基因表达[121] 和有增殖作用,因此我们认定EPO为肝阳。 黄帝内经说“肾主骨,骨生髓”以及“肾生肝“ [1]. HGF由间充质细胞生成,而骨髓干包含间充质干细胞,它还促进肝的再生和功能[122]. 富血小板血浆含有HGF已被广泛应用于骨科/运动医学屈肌腱损伤的修复[123],局部使用重组HGH能促进腱-骨交界的愈合过程[124]。 HGF is also enlightened in eyes. 越来越多的证据表明,HGH由自分泌环路合成,该环路包括眼部细胞如角膜内皮细胞、 晶状体上皮细胞、 视网膜色素上皮细胞等等; 此外,HGH是泪水、房液和玻璃体液的重要成分[125]。此外。过量的HGF会加重杵状指[126], 表明手指的状态反映了HGF水平。 由于HGF对脂肪组织发育有重要作用[127]并且有增殖作用,我们因此认定HGF为肝阳。
Renin(肾素)产生于肾并显著影响肝脏。肝脏疾病患者的肾素-底物浓度比正常人低得多[128]。荷载诱导的牙周膜组织动态平衡是通过renin–angiotensin system(肾素-血管紧张素系统)来调节的[129]。 人的眼睛里也含有Renin [130]。 此外,银屑病通常会影响手指甲和脚趾甲,引起点蚀、 增厚和不规则轮廓; 研究发现,银屑病患者的血浆肾素活性增高[131], 表明renin 会影响指甲的外表。 由于抑制renin-angiotensin system(肾素-血管紧张素系统)能促进肝再生 [132], 我们认定renin为肝阴。
SOD 是一种重要的作用于几乎所有细胞的抗氧化剂,它密集地表达在肾脏中 [133]。 SOD模拟物能提高肝移植大鼠术后的肝功能、发育和存活[134] 而且 Cu/Zn SOD 在肝中广泛分布[135]. Mn-SOD过度表达在体外培养的大鼠髌腱的成纤维细胞[136]。 SOD也影响到眼睛,视网膜含有CuZn-SOD活动,角膜和巩膜载有比晶状体多几倍的 SOD 活动[137]。 此外,银屑病患者的MnSOD水平降低了[138], 表明SOD会影响指甲的外表。暴露于低温能激活棕色脂肪组织产热,但降低了SOD表达[139], 表明SOD有“寒冷“效果,因此我们认定SOD为肝阴。
AKR在肾上有表达[140]。 AKR 家族包括几种催化氧化还原转换的酶参与中间代谢、 解毒和生物合成[141]。 AKR-7A保护肝细胞和组织免受对乙酰氨基酚诱导的氧化应激和肝毒性 [142]。 肌腱黄色瘤患者的AKR含量明显增多,表明AKR 对筋有影响[143]。 AKR1B1和AKR1B10也表达在角膜、虹膜、睫状体、晶状体和视网膜中 [144]。 由于低温可以显著抑制AKR水平[145], 表明AKR有“寒冷”功效,因此我们认定 AKR为肝阴。
GSH(谷胱甘肽)是由肝脏自然产生的一种物质,能有效地清除自由基和其他活性氧类 [146]。 GSH在肝脏的排毒反应中起着关键作用,并与肌腱和眼睛有密切关系。在后损伤鸡模型中GSH水平显著下降,增加GSH可减少愈合肌腱的粘连程度 [147] 。 GSH和相关的酶属于防御系统保护眼睛不受化学与氧化应激; 老化、白内障、糖尿病、辐照和一些药物可能导致在晶状体、角膜、视网膜和其他眼组织中GSH及相关的酶活性的变化[148]. 此外,指甲色素沉着与GSH的水平下降相关[149], 表明指甲的表观反映GSH的水平。 在中医,肝气具有"净化疏泄"功能; 由于GSH具有很强的解毒作用,我们确定GSH为肝气。
图六表示了EPO, HGF, renin, SOD, AKR 和 GSH之间的关系。HGF提高GSH水平以及SOD1表达 [150] [151],下调属于AKR家族的醛糖还原酶[152] [153]。 当用 EPO 治疗后,在大鼠肾脏中的GSH 和 SOD水平显著下降[154]。 胞外SOD是低氧诱导 EPO 基因表达的主要的阻遏物[155], 但EPO 治疗可增加 SOD1 在血管的表达[156]。 SOD 和 GSH共同使用可防止或修复活性氧物所造成的损害。SODs将超氧化物自由基转换为过氧化氢和分子氧,谷胱甘肽过氧化物酶 (GPx) 将过氧化氢转换成水,而GSH有助于 GPx 的产生[157]。 GSH和 AKR 也相互关联,因为在AKR 缺乏的菌株中GSH水平下降[158]。 此外,renin-angiotensin system(肾素-血管紧张素系统)刺激 EPO的 分泌[159], 阻断肾素-血管紧张素系统能增强肝的HGF生产[160], 以及抑制GSH增加血浆的renin(肾素)活性[161]。
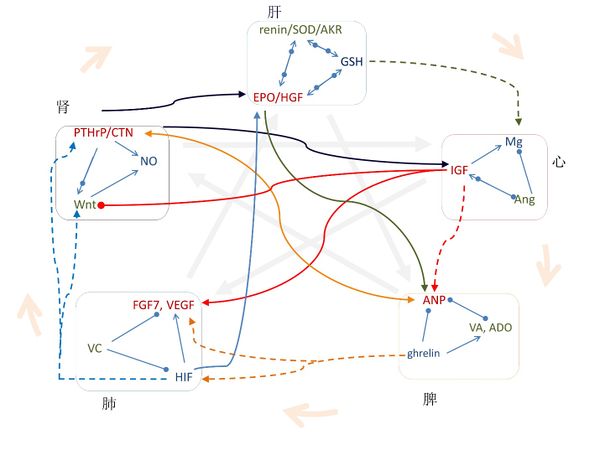
3.2 信号分子的传输通路与中医五行的相互关系高度一致
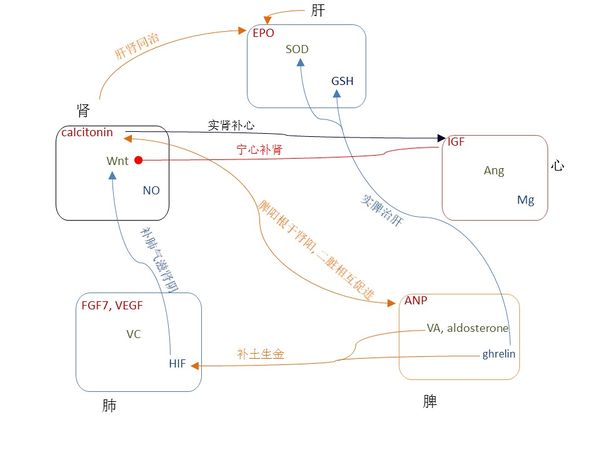
图七表示,在不同“行”之间的信号分子之间的关系与中医五行高度一致。有些中医五行的关系已被广泛应用了几百年,图八表示这些关系与信号分子的传输通路高度一致。
在图一,中间对角的关系表明“主仆关系”,我们可以证明每一个“主仆关系”都和信号分子的传输通路一致。《黄帝内经》说“脾,其主肝也”,《黄帝八十一难经》说“所谓治未病者,见肝之病,则知肝当传之与脾,故先实其脾气,无令得受肝之邪,故曰治未病焉”[162];在现代医学中, circulating retinoic acid(循环视黄酸)在非酒精性脂肪性肝病患者身上浓度较低并与肝脏脂质代谢相关 [163],用ghrelin治疗可以增加GSH的水平[164]和SOD的活性[165]。此外,EPO 刺激 ANP 的分泌[166]。
《黄帝内经》说“肾,其主脾也”,张景岳认为脾阳根于肾阳, 二脏相互促进[167];在现代医学中, ANP 提高了人体循环系统的calcitonin gene-related peptide (CGRP) (降钙素基因相关肽)水平 [168],而CGRP刺激了ANP的分泌[169]。
《黄帝内经》说“心,其主肾也”,周慎斋说“欲补心者须实肾,使肾得升,欲补肾者须宁心,使心得降[170]”。在现代医学中,calcitonin 增加IGF浓度 [171], 而IGF抑制 Wnt 的传输通路[172].
《黄帝内经》说“肺,其主心也”。在现代医学中,Mg缺乏与肺部并发症有关[173]; IGF-1 促使肺成纤维细胞活化[174]; IGF-1 诱导VEGF分泌 [175], 外源性施用 FGF7 cDNA 引起IGF-1的增加 [176], 将IGF-1 和 FGF7 cDNA共同使用可促进真皮和表皮再生[177]; Ang II增加FGF7 mRNA的水平 [178] 并刺激VEGF合成 [179]。
《黄帝内经》说“肝,其主肺也”。在现代医学中,FGF7促进肝的再生[180]; VEGF 通过肝血窦内皮细胞的增殖重建肝血窦进而促进肝细胞的增殖[181]; 缺乏维他命C会加速脂肪肝疾病的发展[182],而且维他命C和维他命E合用是治疗脂肪肝疾病的有效安全方法 [183]; HIF在肝脏病理生理学中有很多作用[184]。此外,VEGF在EPO诱导的心脏功能改善过程中起到决定性作用[185]; 维他命C提高健康成年人红细胞的GSH含量[186]; HIF 诱导EPO的生成[187]并且使用HIF-1可以改变GSSG/GSH比例[188]; FGF-7刺激HGF mRNA 蛋白在卵巢表面上皮细胞的表达[189].
图一中外圆上的箭头表示“母子关系”,对应于阳的信号分子都是在其“母”器官上分泌或生成的。此外,李中梓说“乙癸同源,肾肝同治”[190]; 在现代医学中, EPO 在胎儿孕期主要在肝脏生成而出生后就在肾脏生成,在贫血状态下EPO在肾脏和肝脏的基因转录非常显著[191].
对于肺脾关系,李中梓说“肺金虚者,必甘缓以培土之基,即补土生金”[190], 也就是补脾益肺气。在现代医学中,使用ghrelin 可显著增加HIF-1α 和 VEGF mRNAs [192]; retinoic acid 增加 HIF-1α [193] 并刺激FGF7表达[194].
对于肺和肾,周慎斋说“理宜补养肺金,使金水相生”,补肺气可以滋肾阴[170]。 在现代医学中,HIF 调节Wnt/β-catenin信号[195], 诱发PTHrP [196], 而且NO调节HIF [197]; 在表皮分层中,Wnt 激活 FGF7[198]并调节维他命C的合成[199]; 而且维他命C增加氧化氮合酶(NO synthase)的活性[200]。
对于肝和心,中医认为肝提升心;在现代医学中,在体内和内外实验中GSH 可增加细胞内的Mg[201]。对于心和脾,IGF-II 增加了ANP的蛋白水平 [202] 。
3.3 中草药和中成药的功效是调节相应的信号分子
中草药被分类用以调节各个器官的阳阴气。我们来讨论是否中草药能调节相应的信号分子以验证我们的研究结果。表二 列出一些代表性的草药和他们对相应的信号分子的作用。
人参是著名的中草药,归脾、肺。特别用于补脾气和肺气[203]。 在现代药物实验中,人参增加ANP水平[204] 以及增强全all-trans retinoic acid (ATRA) (反式维甲酸)诱导白血病细胞终末分化的能力[205]; 人参皂甙 Rb1 显著刺激ghrelin内分泌以抑制血管内皮损伤[206]。 人参皂苷 Rg1 可以有效的缓解aldosterone(醛固酮)诱导的氧化应激,从而间接地抑制醛固酮诱导的足细胞自噬[207]。在角质形成细胞培养物中,人参皂甙 Rb1 增强 VEGF 生产并显著增加HIF-1α的表达[208]。
黄芪是常用的中草药用于补气升阳,入脾肺二经[203]. 在现代药物实验中,黄芪总黄酮显著增加HIF-1α在mRNA和蛋白的表达[209] 并增加VEGF mRNA表达[210]。此外,黄芪通过显著改善肾对ANP的反应而诱导促钠排泄[211]。
鹿茸是著名中药用于壮肾阳益精血,入肾肝二经[203]。 在现代药物实验中, 鹿茸中含有大量calcitonin 和 PTHrP,calcitonin含量在快速鹿茸生长时期显著增加[212], PTHrP及其受体在生长期的鹿茸骨軟骨结构和底层的绒表皮表达强烈[213]. 此外,鹿茸能增加血清的EPO[214], SOD 活性[215], 和谷胱甘肽过氧化物酶(glutathione peroxidase) [216]。
锁阳具有暖肾阳和滋肝阴的功能[217]。 在现代药物实验中,, 锁阳能显著增加calcitonin [218],CuZn-SOD和 谷胱甘肽过氧化物酶 (glutathione peroxidase)的水平 [219]。
当归以增强造血功能闻名,入肝心二经[203]。 在现代药物实验中, 当归促进 EPO以增强造血功能[220] [221], 提升HGF [222], 增加GSH [223], 增强SOD活性 [224], 及调节renin活动[225]。 此外,当归多糖刺激IGF-1的表达[226]但是抑制Ang II [225]。
冬虫夏草以补肺闻名[203]。在现代药物实验中,冬虫夏草上调HIF-1α 的表达[227], 使人的肺上皮细胞保持较高的HIF-1水平[228], 富含维他命C [229], 降低肺中的VEGF水平[230]。
百合以滋润肺阴闻名 [203]. 而百合富含维他命C [231].
枸杞以滋补肾阴和肝阴闻名[203]。 在现代药物实验中, 枸杞植物化学物质激活 Wnt 信号[232], 增加血液中的SOD 和GPx[233]。
中成药被广泛用于调节各个器官的阳阴气。表 S2 列出一些代表性的中成药,并显示这些中成药调节相应的信号分子。六君子汤用于补脾阴[234], 它广泛用于治疗功能性消化不良、胃食管反流病、消化道手术患者的消化不良、和癌症患者化疗所致的消化不良,口服六君子汤增强ghrelin 促进食欲的功能[235]。
一贯煎用于滋肝阴疏肝气[234]。 在现代药物实验中,一贯煎大幅提高Cu/Zn SOD、谷胱甘肽 S-转移酶 (GST) 和 AKR 家庭 7的蛋白表达,而值得注意的是减少谷胱甘肽合成酶 (GSS) 的表达 (GSS) [236]。
六味地黄丸用于补肾阴[234]。 在现代药物实验中,六味地黄丸上调典型Wnt/β-catenin 信号[237]。
左归丸用于补肾阴[238], 而左归丸 在Wnt 信号转导通路中增加 Wnt1 [239]。
肾气丸主治肾气不足[234],而药物实验表明,肾气丸能增加NO 的生成 [240]。
唐宗海主张调治脾胃应补脾阴[241],并建议用张景岳[167]的麦门冬汤补脾阴。麦门冬汤包含麦冬和粳米,其中麦冬含有维他命A [203]。 我国的粳米生长在温带地区,而温带粳稻在所有类型的水稻中具有最高的类胡萝卜素含量[242], 而类胡萝卜素作为维生素 A 的唯一前体对人类饮食异常重要[243].
4.讨论
用阳阴气与信号分子的直接映射关系,我们可以证明中医和现代医学实际上认定同样的发病机制和采用相同的治疗策略。中医认为心和小肠互为表里,即小肠的病症会在心上表现出来。巢元方说“心主受于血,与小肠合。若心家有热,结受于小肠,故小便血也”[244], 中医的方法是降心火(心阳)。在现代医学中,出现血尿的紫癜患者的IGF-1水平显著高于对比组[245], angiotensin-converting enzyme inhibitor(血管紧张素转换酶抑制剂)能有效地治疗血尿[246]. 由于angiotensin-converting enzyme inhibitor 治疗可以显著地降低循环IGF-1 [247], 因此现代医学的治疗策略和中医是一样的。
中医认为脾和胃互为表里,即胃的病症会在脾上表现出来。李东垣说“升阳益胃”[248]。 在现代医学中,内源的ANP 刺激胃窦的somatostatin(生长抑素)分泌,伴随着抑制胃窦的gastrin(胃泌素)分泌[249];而且 ghrelin刺激了胃蠕动 [250]。
中医认为肺和大肠互为表里,即大肠的病症会在肺上表现出来。唐容川在《血证论》中说“肺移热于大肠则使结,肺津不润则便结”[241],李时珍在《本草纲目》中建议用无花果治疗[251]。在现代医学中,已经发现在痔组织中VEGF的表达非常高 [252], 而且便秘儿童的维他命C的日摄取量往往很低[253]。而无花果能显著降低VEGF水平[254]并且富含维他命C[255].
中医认为肾和膀胱互为表里,即膀胱的病症会在肾上表现出来。巢元方《诸病源候论》中说“膀胱气虚,失于约束,可见小便频数,遗尿。温肾固气” [244]。 在现代医学中,NO synthase (氧化氮合酶)起着控制排尿的功能性作用[256], neuronal NO synthase gene polymorphism (nNOS基因多态性) 可能和儿童的原发性夜间遗尿有关[257], 而 Nicorandil,一种NO供体, 可以治疗尿频[258]。这和中医的描述是一样的。
中医认为肝和胆互为表里,即胆的病症会在肝上表现出来。《中医肝胆病良方》中说“急性胆结石的一个原因是肝气郁,胆囊炎的一个原因是肝气滞”[259]。现代医学发现,胆结石患者的GSH水平明显低于对比组 [260]; 急性胆囊炎患者血液中的GSH较低,而红细胞中GSH代谢酶的活性受到抑制[261].
以高血压作为另外一个例子。现代中医专家认为高血压的发病机制之一是肝阳上亢[262]。 现代医学发现,长期使用EPO 会导致高血压[263], 高血压患者血清中的HGF浓度显著高于正常人[264]。 现代中医专家认为另外一个高血压的发病机制是肝肾阴虚[262]。 现代医学发现,高血压患者血浆里的renin活性很低或几乎检测不到[265];肺动脉胞外SOD的本地化损失会加重慢性缺氧性肺动脉高血压的症状[266]; 在自发性高血压大鼠的髓质中,AKR活性显著的低 [267];而且高血压与典型 Wnt的信号通路的下降相关[268]。 所有这些例子表明,中医与现代医学是用不同的术语描述着同样的高血压病理!
5.结论
用迭代筛选法,我们建立了中医五行阳阴气和信号分子的一一映射关系。这将使我们能借用中医阴阳平衡的理念进一步了解信号分子之间的平衡对于人类健康的意义。中医五行是一个闭环信号系统,中西医在信号分子水平的结合将使我们发展新的治疗策略和方法。
表一 阳阴气与信号分子映射关系
| 阳 | 阴 | 气 | |
| 心 | IGF | Ang | Mg |
| 脾 | ANP | aldosterone,
retinoic acid |
ghrelin |
| 肺 | FGF7, VEGF | ascorbic acid | HIF |
| 肾 | calcitonin, PTHrP | Wnt | NO |
| 肝 | EPO, HGF | renin, SOD, AKR | GSH |
表二 中草药调节相应的信号分子
| 中草药 | 中医功效 | 对信号分子的作用 |
| 人参 | 入脾经[203]
|
ANP[204], ATRA[205],
ghrelin[206], aldosterone[207] |
| 入肺经[203] | VEGF, HIF [208] | |
| 黄芪 | 肺气,
肺阳[203] |
HIF [209]
VEGF [210] |
| 脾阳[203] | ANP [211] | |
| 鹿茸 | 肾阳[203] | 富含 calcitonin & PTHrP [212, 213] |
| 益血,
入肝经[203] |
EPO[214], SOD[215],
GSH px[216] | |
| 锁阳 | 温肾阳[217] | calcitonin [218] |
| 补肝阴[217] | SOD, GSH px [219] | |
| 当归 | 造血,
入肝经 |
EPO[220, 221], HGF[222], GSH[223], SOD[224], renin[225] |
| 入心经[203] | IGF[226], Ang II [225] | |
| 冬虫夏草
|
入肺经[203] |
HIF[227, 228], ascorbic acid[229], VEGF[230] |
百合 |
润肺阴[203] |
富含 ascorbic acid[231] |
枸杞 |
补肾阴肝阴[203] |
Wnt[232],SOD, GSH px[233] |
表三 中成药调节相应的信号分子
中成药 |
中医功效 |
对信号分子的作用 |
六君子汤 |
脾气[234] |
ghrelin [235] |
一贯煎 |
肝阴 |
SOD, AKR [236] |
| 疏肝气[234] | GST, GSS [236] | |
| 六味地黄丸 | 肾阴[234] | Wnt [237] |
| 左归丸 | 肾阴[238] | Wnt [239] |
| 肾气丸 | 肾气[234] | NO [240] |
| 麦门冬汤 | 脾阴[241] | vitamin A [203, 242, 243] |
图片说明
图一 中医五行的功能和之间的关系。
图二 心阳(IGF)、心阴(Ang)和心气(Mg)之间的关系。
图三 脾阳(ANP)、脾阴(Aldosterone,retinoic acid)和脾气(ghrelin)之间的关系。
图四 肺阳(FGF7, VEGF)、肺阴(Ascorbic acid)和肺气(HIF)之间的关系。
图五 肾阳(Calcitonin,PTHrP)、肾阴(Wnt)和肾气(NO)之间的关系。
图六 肝阳(EPO,HGF)、肝阴(renin, SOD, AKR)和肝气(GSH)之间的关系。
图七 信号分子的传输通路和中医五行之间的关系一致。图中,CTN为 calcitonin,VC为维他命C, VA为retinoic acid,ADO为aldosterone。箭头表明增加的关系,实心圆点表明减小的关系,箭头和实心圆点一起表明调节的关系。
图八 著名常用的中医治疗策略和信号分子的传输通路一致。箭头表明增加的关系,实心圆点表明减小的关系,箭头和实心圆点一起表明调节的关系。
References
1. 黄帝, 黄帝内经素问. 2012, 北京: 人民卫生出版社.
2. Troncoso, R., et al., New insights into IGF-1 signaling in the heart. Trends in Endocrinology and Metabolism, 2014. 25(3): p. 128-137.
3. Delafontaine, P., Y.H. Song, and Y.X. Li, Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arteriosclerosis Thrombosis and Vascular Biology, 2004. 24(3): p. 435-444.
4. Xu, Z.P., et al., Effects of insulin-like growth factor-1 on the relaxation responses of the cavernous smooth muscle from aged rats. Scandinavian Journal of Urology, 2015. 49(3): p. 260-266.
5. Kobayashi, A., et al., Morphological and histopathological changes in tongues of experimentally developed acromegaly-like rats. Hormone and Metabolic Research, 2006. 38(3): p. 146-151.
6. Noordam, R., et al., Serum insulin-like growth factor 1 and facial ageing: high levels associate with reduced skin wrinkling in a cross-sectional study. British Journal of Dermatology, 2013. 168(3): p. 533-538.
7. Sanchez-Alavez, M., et al., Insulin-like Growth Factor 1-mediated Hyperthermia Involves Anterior Hypothalamic Insulin Receptors. Journal of Biological Chemistry, 2011. 286(17): p. 14983-14990.
8. De Mello, W.C. and A.H.J. Danser, Angiotensin II and the heart - On the intracrine renin-angiotensin system. Hypertension, 2000. 35(6): p. 1183-1188.
9. Mlynarek, A., A. Hagr, and K. Kost, Angiotensin-converting enzyme inhibitor-induced unilateral tongue angioedema. Otolaryngology-Head and Neck Surgery, 2003. 129(5): p. 593-595.
10. Steckelings, U.M., et al., Human skin: source of and target organ for angiotensin II. Experimental Dermatology, 2004. 13(3): p. 148-154.
11. Wilson, K.M. and M.J. Fregly, Angiotensin Ii-Induced Hypothermia in Rats. Journal of Applied Physiology, 1985. 58(2): p. 534-543.
12. NIH-library-Mg. Magnesium. Available from: [https:// https://]www.nlm.nih.gov/medlineplus/druginfo/natural/998.html.
13. Dean, C., The magnesium miracle : discover the essential nutrient that will lower therisk of heart disease, prevent stroke and obesity, treat diabetes, and improve mood and memory. Ballantine books trade pbk. ed. 2007, New York: Ballantine Books. xxii, 309 p.
14. Henkin, R.I., V. Gouliouk, and A. Fordyce, Distinguishing patients with glossopyrosis from those with oropyrosis based upon clinical differences and differences in saliva and erythrocyte magnesium. Archives of Oral Biology, 2012. 57(2): p. 205-210.
15. Proksch, E., et al., Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. International Journal of Dermatology, 2005. 44(2): p. 151-157.
16. Song, Y.H., et al., Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. Journal of Clinical Investigation, 2005. 115(2): p. 451-458.
17. Brink, M., et al., Angiotensin II stimulates gene expression of cardiac insulin-like growth factor I and its receptor through effects on blood pressure and food intake. Hypertension, 1999. 34(5): p. 1053-1059.
18. Ma, Y.W., et al., Angiotensin II stimulates transcription of insulin-like growth factor I receptor in vascular smooth muscle cells: Role of nuclear factor-kappa B. Endocrinology, 2006. 147(3): p. 1256-1263.
19. Kajstura, J., et al., IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes, 2001. 50(6): p. 1414-1424.
20. Touyz, R.M. and E.L. Schiffrin, The Effect of Angiotensin-Ii on Platelet Intracellular Free Magnesium and Calcium Ionic Concentrations in Essential-Hypertension. Journal of Hypertension, 1993. 11(5): p. 551-558.
21. Maggio, M., et al., Magnesium and anabolic hormones in older men. International Journal of Andrology, 2011. 34(6): p. E594-E600.
22. Debold, A.J., Atrial Natriuretic Factor - a Hormone Produced by the Heart. Science, 1985. 230(4727): p. 767-770.
23. Sultanian, R., Y.M. Deng, and S. Kaufman, Atrial natriuretic factor increases splenic microvascular pressure and fluid extravasation in the rat. Journal of Physiology-London, 2001. 533(1): p. 273-280.
24. Abell, T.J., et al., Atrial Natriuretic Factor Inhibits Proliferation of Vascular Smooth-Muscle Cells Stimulated by Platelet-Derived Growth-Factor. Biochemical and Biophysical Research Communications, 1989. 160(3): p. 1392-1396.
25. Bianciotti, L.G., et al., Atrial-Natriuretic-Factor Enhances Induced Salivary Secretion in the Rat. Regulatory Peptides, 1994. 49(3): p. 195-202.
26. Caisey, L., et al., Influence of age and hormone replacement therapy on the functional properties of the lips. Skin Research and Technology, 2008. 14(2): p. 220-225.
27. Mitteroecker, P., et al., The Morphometrics of "Masculinity" in Human Faces. Plos One, 2015. 10(2).
28. Jankowski, M., et al., Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proceedings of the National Academy of Sciences, 2001. 98(20): p. 11765-11770.
29. Henesy, M.B., et al., Calcineurin regulates homologous desensitization of natriuretic peptide receptor-A and inhibits ANP-induced testosterone production in MA-10 cells. PloS one, 2012. 7(8): p. e41711.
30. Palmer, B.F. and D.J. Clegg, An Emerging Role of Natriuretic Peptides: Igniting the Fat Furnace to Fuel and Warm the Heart. Mayo Clinic Proceedings, 2015. 90(12): p. 1666-1678.
31. Delcayre, C., et al., Cardiac aldosterone production and ventricular remodeling. Kidney International, 2000. 57(4): p. 1346-1351.
32. McGraw, A.P., et al., Aldosterone Increases Early Atherosclerosis and Promotes Plaque Inflammation Through a Placental Growth Factor-Dependent Mechanism. Journal of the American Heart Association, 2013. 2(1).
33. Ishizawa, K., et al., Aldosterone stimulates vascular smooth muscle cell proliferation via big mitogen-activated protein kinase 1 activation. Hypertension, 2005. 46(4): p. 1046-1052.
34. NIH-library. Spironolactone. Available from: [https:// https://]www.nlm.nih.gov/medlineplus/druginfo/meds/a682627.html.
35. Kamei, H. and K. Arakawa, A Case of Pseudo-Aldosteronism Due to Addiction of Jintan, a Mouth Refresher Popular among Japanese. Japanese Heart Journal, 1982. 23(4): p. 651-659.
36. Caroccia, B., et al., GPER-1 and estrogen receptor-β ligands modulate aldosterone synthesis. Endocrinology, 2014. 155(11): p. 4296-4304.
37. Goncharov, N., et al., Effects of short-term testosterone administration on variables of the metabolic syndrome, in particular aldosterone. Hormone molecular biology and clinical investigation, 2012. 12(2): p. 401-406.
38. Klebanoff, C.A., et al., Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. Journal of Experimental Medicine, 2013. 210(10): p. 1961-1976.
39. Chen, T.H.P., et al., Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Developmental Biology, 2002. 250(1): p. 198-207.
40. Halevy, O. and O. Lerman, Retinoic Acid Induces Adult Muscle-Cell Differentiation Mediated by the Retinoic Acid Receptor-Alpha. Journal of Cellular Physiology, 1993. 154(3): p. 566-572.
41. Kautsky, M.B., P. Fleckman, and B.A. Dale, Retinoic acid regulates oral epithelial differentiation by two mechanisms. J Invest Dermatol, 1995. 104(4): p. 546-53.
42. Hong, W.K., et al., 13-Cis-Retinoic Acid in the Treatment of Oral Leukoplakia. New England Journal of Medicine, 1986. 315(24): p. 1501-1505.
43. Abbott, B.D., M.W. Harris, and L.S. Birnbaum, Etiology of Retinoic Acid-Induced Cleft-Palate Varies with the Embryonic Stage. Teratology, 1989. 40(6): p. 533-553.
44. Marzolla, V., et al., The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Molecular and Cellular Endocrinology, 2012. 350(2): p. 281-288.
45. Murholm, M., et al., Retinoic acid has different effects on UCP1 expression in mouse and human adipocytes. Bmc Cell Biology, 2013. 14.
46. Xia, Q., et al., Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regulatory Peptides, 2004. 122(3): p. 173-178.
47. Leite-Moreira, A.F., A. Rocha-Sousa, and T. Henriques-Coelho, Cardiac, skeletal, and smooth muscle regulation by ghrelin. Vitamins and Hormones, Ghrelin, 2008. 77: p. 207-+.
48. Ohta, K., et al., Expression and Possible Immune-regulatory Function of Ghrelin in Oral Epithelium. Journal of Dental Research, 2011. 90(11): p. 1286-1292.
49. Kellokoski, E., et al., Estrogen replacement therapy increases plasma ghrelin levels. The Journal of Clinical Endocrinology & Metabolism, 2005. 90(5): p. 2954-2963.
50. Greenman, Y., et al., Testosterone is a strong correlate of ghrelin levels in men and postmenopausal women. Neuroendocrinology, 2008. 89(1): p. 79-85.
51. Ueno, H., et al., Ghrelin: a gastric peptide that regulates food intake and energy homeostasis. Regulatory Peptides, 2005. 126(1-2): p. 11-19.
52. Klabunde, R.E., Cardiovascular physiology concepts. 2nd ed. 2012, Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. xi, 243 p.
53. Kumar, P., et al., All-Trans Retinoic Acid and Sodium Butyrate Enhance Natriuretic Peptide Receptor A Gene Transcription: Role of Histone Modification. Molecular Pharmacology, 2014. 85(6): p. 946-957.
54. Torsello, A., et al., Ghrelin plays a minor role in the physiological control of cardiac function in the rat. Endocrinology, 2003. 144(5): p. 1787-1792.
55. Milosevic, V.L., et al., Central effects of ghrelin on the adrenal cortex: a morphological and hormonal study. General Physiology and Biophysics, 2010. 29(2): p. 194-202.
56. Pereira-Terra, P., et al., Neuroendocrine factors regulate retinoic acid receptors in normal and hypoplastic lung development. Journal of Physiology-London, 2015. 593(15): p. 3301-3311.
57. Suzuki, M., et al., Spleen-Derived Growth-Factor, Sdgf-3, Is Identified as Keratinocyte Growth-Factor (Kgf). Febs Letters, 1993. 328(1-2): p. 17-20.
58. Tichelaar, J.W., W. Lu, and J.A. Whitsett, Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem, 2000. 275(16): p. 11858-64.
59. Dou, C.Q., et al., Strengthening the Skin with Topical Delivery of Keratinocyte Growth Factor-1 Using a Novel DNA Plasmid. Molecular Therapy, 2014. 22(4): p. 752-761.
60. Rosenquist, T.A. and G.R. Martin, Fibroblast growth factor signalling in the hair growth cycle: Expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Developmental Dynamics, 1996. 205(4): p. 379-386.
61. Ishibashi, T., et al., Keratinocyte growth factor and its receptor messenger RNA expression in nasal mucosa and nasal polyps. Annals of Otology Rhinology and Laryngology, 1998. 107(10): p. 885-890.
62. Voelkel, N.F., R.W. Vandivier, and R.M. Tuder, Vascular endothelial growth factor in the lung. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2006. 290(2): p. L209-L221.
63. Owen, J.L., et al., Up-regulation of matrix metalloproteinase-9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J Immunol, 2003. 171(8): p. 4340-51.
64. Yano, K., L.F. Brown, and M. Detmar, Control of hair growth and follicle size by VEGF-mediated angiogenesis. Journal of Clinical Investigation, 2001. 107(4): p. 409-417.
65. Detmar, M., The role of VEGF and thrombospondins in skin angiogenesis. Journal of Dermatological Science, 2000. 24: p. S78-S84.
66. Lee, H.S., A. Myers, and J. Kim, Vascular endothelial growth factor drives autocrine epithelial cell proliferation and survival in chronic rhinosinusitis with nasal polyposis. Am J Respir Crit Care Med, 2009. 180(11): p. 1056-67.
67. Gkini, M.A., et al., Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg, 2014. 7(4): p. 213-9.
68. Bagchi, M., et al., Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. Faseb Journal, 2013. 27(8): p. 3257-3271.
69. Hemila, H. and P. Louhiala, Vitamin C may affect lung infections. Journal of the Royal Society of Medicine, 2007. 100(11): p. 495-498.
70. Fisher, B.J., et al., Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Critical Care Medicine, 2011. 39(6): p. 1454-1460.
71. Tsao, C.S., P.Y. Leung, and M. Young, Effect of Dietary Ascorbic-Acid Intake on Tissue Vitamin-C in Mice. Journal of Nutrition, 1987. 117(2): p. 291-297.
72. Willis, R.J. and C.C. Kratzing, Extracellular Ascorbic-Acid in Lung. Biochimica Et Biophysica Acta, 1976. 444(1): p. 108-117.
73. Kwack, M.H., et al., L-ascorbic acid 2-phosphate represses the dihydrotestosterone-induced dickkopf-1 expression in human balding dermal papilla cells. Exp Dermatol, 2010. 19(12): p. 1110-2.
74. Farris, P.K., Topical vitamin C: A useful agent for treating photoaging and other dermatologic conditions. Dermatologic Surgery, 2005. 31(7): p. 814-818.
75. Thornhill, S.M. and A.M. Kelly, Natural treatment of perennial allergic rhinitis. Altern Med Rev, 2000. 5(5): p. 448-54.
76. Ryan, A.S. and L.A. Goldsmith, Nutrition and the skin. Clinics in dermatology, 1996. 14(4): p. 389-406.
77. Shimoda, L.A. and G.L. Semenza, HIF and the Lung Role of Hypoxia-inducible Factors in Pulmonary Development and Disease. American Journal of Respiratory and Critical Care Medicine, 2011. 183(2): p. 152-156.
78. Imamura, Y., et al., HIF-2 alpha/ARNT complex regulates hair development via induction of p21(Waf1/Cip1) and p27(Kip1). Faseb Journal, 2014. 28(6): p. 2517-2524.
79. Nys, K., et al., Uncovering the role of hypoxia inducible factor-1 alpha in skin carcinogenesis. Biochimica Et Biophysica Acta-Reviews on Cancer, 2011. 1816(1): p. 1-12.
80. Shin, H.W., et al., Hypoxia-inducible Factor 1 Mediates Nasal Polypogenesis by Inducing Epithelial-to-Mesenchymal Transition. American Journal of Respiratory and Critical Care Medicine, 2012. 185(9): p. 944-954.
81. Forsythe, J.A., et al., Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and Cellular Biology, 1996. 16(9): p. 4604-4613.
82. Tsuji, K., S. Kitamura, and H. Makino, Hypoxia-inducible factor 1 alpha regulates branching morphogenesis during kidney development. Biochemical and Biophysical Research Communications, 2014. 447(1): p. 108-114.
83. Rodriguez, J.A., et al., Vitamins C and E prevent endothelial VEGF and VEGFR-2 overexpression induced by porcine hypercholesterolemic LDL. Cardiovascular Research, 2005. 65(3): p. 665-673.
84. Muellner, M.K., et al., Vitamin C inhibits NO-induced stabilization of HIF-1 alpha in HUVECs. Free Radical Research, 2010. 44(7): p. 783-791.
85. Geesin, J.C., et al., Regulation of Collagen-Synthesis in Human Dermal Fibroblasts in Contracted Collagen Gels by Ascorbic-Acid, Growth-Factors, and Inhibitors of Lipid-Peroxidation. Experimental Cell Research, 1993. 206(2): p. 283-290.
86. Gomez-Roman, J.J., et al., Hormone expression and opioid receptors in fetal and adult lung. Archivos De Bronconeumologia, 2002. 38(8): p. 362-366.
87. Hastings, R.H., et al., Alveolar Epithelial-Cells Express and Secrete Parathyroid Hormone-Related Protein. American Journal of Respiratory Cell and Molecular Biology, 1994. 11(6): p. 701-706.
88. Hsu, Y.J., et al., Calcitonin-stimulated renal Ca2+ reabsorption occurs independently of TRPV5. Nephrology Dialysis Transplantation, 2010. 25(5): p. 1428-1435.
89. Wallach, S., et al., Effects of Calcitonin on Bone Quality and Osteoblastic Function. Calcified Tissue International, 1993. 52(5): p. 335-339.
90. Lando, M., L.A. Hoover, and G. Finerman, Stabilization of hearing loss in Paget's disease with calcitonin and etidronate. Arch Otolaryngol Head Neck Surg, 1988. 114(8): p. 891-4.
91. Samuelov, L., et al., Neural controls of human hair growth: calcitonin gene-related peptide (CGRP) induces catagen. Journal of dermatological science, 2012. 67(2): p. 153-155.
92. Esbrit, P. and J. Egido, The emerging role of parathyroid hormone-related protein as a renal regulating factor. Nephrology Dialysis Transplantation, 2000. 15(8): p. 1109-1111.
93. Martin, T.J., Osteoblast-derived PTHrP is a physiological regulator of bone formation. Journal of Clinical Investigation, 2005. 115(9): p. 2322-2324.
94. Karperien, M., et al., Parathyroid hormone related peptide mRNA expression during murine postimplantation development: Evidence for involvement in multiple differentiation processes. International Journal of Developmental Biology, 1996. 40(3): p. 599-608.
95. Skrok, A., et al., The Effect of Parathyroid Hormones on Hair Follicle Physiology: Implications for Treatment of Chemotherapy-Induced Alopecia. Skin pharmacology and physiology, 2015. 28(4): p. 213-225.
96. Fargeas, M.J., J. Fioramonti, and L. Bueno, Central Actions of Calcitonin on Body-Temperature and Intestinal Motility in Rats - Evidence for Different Mediations. Regulatory Peptides, 1985. 11(2): p. 95-103.
97. Kir, S., et al., Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature, 2014. 513(7516): p. 100-+.
98. Pulkkinen, K., S. Murugan, and S. Vainio, Wnt signaling in kidney development and disease. Organogenesis, 2008. 4(2): p. 55-9.
99. Dean, C.H., et al., Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Developmental Biology, 2005. 286(1): p. 270-286.
100. Maeda, K., N. Takahashi, and Y. Kobayashi, Roles of Wnt signals in bone resorption during physiological and pathological states. Journal of Molecular Medicine-Jmm, 2013. 91(1): p. 15-23.
101. Riccomagno, M.M., S. Takada, and D.J. Epstein, Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes & Development, 2005. 19(13): p. 1612-1623.
102. Rabbani, P., et al., Coordinated Activation of Wnt in Epithelial and Melanocyte Stem Cells Initiates Pigmented Hair Regeneration. Cell, 2011. 145(6): p. 941-955.
103. Kang, S., et al., Effects of wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1 alpha. Molecular and Cellular Biology, 2005. 25(4): p. 1272-1282.
104. Mount, P.F. and D.A. Power, Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf), 2006. 187(4): p. 433-46.
105. Danziger, R.S., B.S. Zuckerbraun, and J.M. Pensler, Role of nitric oxide in the regulation of osteoblast metabolism. Plastic and Reconstructive Surgery, 1997. 100(3): p. 670-673.
106. Takumida, M. and M. Anniko, Functional significance of nitric oxide in the inner ear. In Vivo, 2004. 18(3): p. 345-350.
107. Wolf, R., et al., Nitric oxide in the human hair follicle: constitutive and dihydrotestosterone-induced nitric oxide synthase expression and NO production in dermal papilla cells. Journal of molecular medicine, 2003. 81(2): p. 110-117.
108. Gooi, J.H., et al., Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone, 2010. 46(6): p. 1486-1497.
109. Lopez-Herradon, A., et al., Inhibition of the canonical Wnt pathway by high glucose can be reversed by parathyroid hormone-related protein in osteoblastic cells. Journal of Cellular Biochemistry, 2013. 114(8): p. 1908-1916.
110. Tas, N., et al., The effect of calcitonin treatment on plasma nitric oxide levels in post-menopausal osteoporotic patients. Cell Biochemistry and Function, 2002. 20(2): p. 103-105.
111. Kalinowski, L., L.W. Dobrucki, and T. Malinski, Nitric oxide as a second messenger in parathyroid hormone-related protein signaling. Journal of Endocrinology, 2001. 170(2): p. 433-440.
112. Munoz, F.J., et al., Wnt-5 alpha increases NO and modulates NMDA receptor in rat hippocampal neurons. Biochemical and Biophysical Research Communications, 2014. 444(2): p. 189-194.
113. Yavropoulou, M.P. and J.G. Yovos, The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones (Athens), 2007. 6(4): p. 279-94.
114. Glass, D.A., et al., Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Developmental Cell, 2005. 8(5): p. 751-764.
115. Gul, M., et al., Effect of erythropoietin on liver regeneration in an experimental model of partial hepatectomy. International Journal of Surgery, 2013. 11(1): p. 59-63.
116. Zanjani, E.D., et al., Studies on the liver to kidney switch of erythropoietin production. J Clin Invest, 1981. 67(4): p. 1183-8.
117. Uslu, M., et al., Erythropoietin stimulates patellar tendon healing in rats. Knee, 2015. 22(6): p. 461-468.
118. Tong, Z.Z., et al., Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proceedings of the National Academy of Sciences of the United States of America, 2008. 105(19): p. 6998-7003.
119. Kapsoritakis, A.N., et al., Finger clubbing and erythropoietin serum levels in active IBD. Inflammatory bowel diseases, 2006. 12(6): p. 535-536.
120. Gursu, M., et al., Skin disorders in peritoneal dialysis patients: An underdiagnosed subject. World Journal of Nephrology, 2016. 5(4): p. 372.
121. Wang, L., et al., PPAR alpha and Sirt1 Mediate Erythropoietin Action in Increasing Metabolic Activity and Browning of White Adipocytes to Protect Against Obesity and Metabolic Disorders. Diabetes, 2013. 62(12): p. 4122-4131.
122. Ishii, T., et al., Hepatocyte Growth-Factor Stimulates Liver-Regeneration and Elevates Blood Protein Level in Normal and Partially Hepatectomized Rats. Journal of Biochemistry, 1995. 117(5): p. 1105-1112.
123. Zhang, J.Y., et al., HGF Mediates the Anti-inflammatory Effects of PRP on Injured Tendons. Plos One, 2013. 8(6).
124. Nakase, J., et al., Facilitated Tendon-Bone Healing by Local Delivery of Recombinant Hepatocyte Growth Factor in Rabbits. Arthroscopy-the Journal of Arthroscopic and Related Surgery, 2010. 26(1): p. 84-90.
125. Grierson, I., et al., Hepatocyte growth factor/scatter factor in the eye. Progress in Retinal and Eye Research, 2000. 19(6): p. 779-802.
126. Karnath, B., Digital clubbing: a sign of underlying disease. Hospital Physician, 2003. 26: p. 25-27.
127. Bell, L.N., et al., A central role for hepatocyte growth factor in adipose tissue angiogenesis. American Journal of Physiology-Endocrinology and Metabolism, 2008. 294(2): p. E336-E344.
128. Ayers, C.R., Plasma Renin Activity and Renin-Substrate Concentration in Patients with Liver Disease. Circulation Research, 1967. 20(6): p. 594-&.
129. Monnouchi, S., et al., The Roles of Angiotensin II in Stretched Periodontal Ligament Cells. Journal of Dental Research, 2011. 90(2): p. 181-185.
130. VanHaeringen, N.J., The renin-angiotensin system in the human eye. British Journal of Ophthalmology, 1996. 80(2): p. 99-100.
131. Ena, P., et al., High prevalence of cardiovascular diseases and enhanced activity of the renin-angiotensin system in psoriatic patients. Acta cardiologica, 1984. 40(2): p. 199-205.
132. Koh, S.L., et al., Blockade of the renin-angiotensin system improves the early stages of liver regeneration and liver function. Journal of Surgical Research, 2013. 179(1): p. 66-71.
133. Suh, J.G., et al., Sequence analysis, tissue expression and chromosomal localization of a mouse secreted superoxide dismutase gene. Molecules and Cells, 1997. 7(2): p. 204-207.
134. Cui, Y.Y., et al., SOD Mimetic Improves the Function, Growth, and Survival of Small-Size Liver Grafts After Transplantation in Rats. Transplantation, 2012. 94(7): p. 687-694.
135. Okado-Matsumoto, A. and I. Fridovich, Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem, 2001. 276(42): p. 38388-93.
136. Kitamura, N., et al., Stress deprivation enhances manganese superoxide dismutase expression in the rat patellar tendon. International Journal of Molecular Medicine, 2004. 14(4): p. 537-543.
137. Behndig, A., et al., Superoxide dismutase isoenzymes in the human eye. Investigative Ophthalmology & Visual Science, 1998. 39(3): p. 471-475.
138. Li, C. and H.-M. Zhou, The role of manganese superoxide dismutase in inflammation defense. Enzyme research, 2011. 2011.
139. Petrovic, V., et al., Antioxidative defense and mitochondrial thermogenic response in brown adipose tissue. Genes and Nutrition, 2010. 5(3): p. 225-235.
140. MacLeod, A.K., et al., Expression and Localization of Rat Aldo-Keto Reductases and Induction of the 1B13 and 1D2 Isoforms by Phenolic Antioxidants. Drug Metabolism and Disposition, 2010. 38(2): p. 341-346.
141. Barski, O.A., S.M. Tipparaju, and A. Bhatnagar, The Aldo-Keto Reductase Superfamily and Its Role in Drug Metabolism and Detoxification. Drug Metabolism Reviews, 2008. 40(4): p. 553-624.
142. Ahmed, M.M.E., et al., Aldo-Keto Reductase-7A Protects Liver Cells and Tissues From Acetaminophen-Induced Oxidative Stress and Hepatotoxicity. Hepatology, 2011. 54(4): p. 1322-1332.
143. Artieda, M., et al., Tendon xanthomas in familial hypercholesterolemia are associated with a differential inflammatory response of macrophages to oxidized LDL. Febs Letters, 2005. 579(20): p. 4503-4512.
144. Huang, S.P., et al., Aldo-Keto Reductases in the Eye. Journal of Ophthalmology, 2010.
145. Shore, A.M., et al., Cold-Induced Changes in Gene Expression in Brown Adipose Tissue, White Adipose Tissue and Liver. Plos One, 2013. 8(7).
146. Wu, G.Y., et al., Glutathione metabolism and its implications for health. Journal of Nutrition, 2004. 134(3): p. 489-492.
147. Hung, L.K., et al., Local vitamin-C injection reduced tendon adhesion in a chicken model of flexor digitorum profundus tendon injury. J Bone Joint Surg Am, 2013. 95(7): p. e41.
148. Ganea, E. and J.J. Harding, Glutathione-related enzymes and the eye. Current Eye Research, 2006. 31(1): p. 1-11.
149. Niiyama, S. and H. Mukai, Reversible cutaneous hyperpigmentation and nails with white hair due to vitamin B-12 deficiency. European Journal of Dermatology, 2007. 17(6): p. 551-552.
150. Yang, H., et al., Effects of hepatocyte growth factor on glutathione synthesis, growth, and apoptosis is cell density-dependent. Experimental Cell Research, 2008. 314(2): p. 398-412.
151. Valdes-Arzate, A., et al., Hepatocyte growth factor protects hepatocytes against oxidative injury induced by ethanol metabolism. Free Radical Biology and Medicine, 2009. 47(4): p. 424-430.
152. Li, H., et al., HGF protects rat mesangial cells from high-glucose-mediated oxidative stress. American Journal of Nephrology, 2006. 26(5): p. 519-530.
153. Petrash, J.M., All in the family: aldose reductase and closely related aldo-keto reductases. Cellular and Molecular Life Sciences, 2004. 61(7-8): p. 737-749.
154. Hussein, A.M., et al., Effects of combined erythropoietin and epidermal growth factor on renal ischaemia/reperfusion injury: a randomized experimental controlled study. Bju International, 2011. 107(2): p. 323-328.
155. Zelko, I.N. and R.J. Folz, Extracellular superoxide dismutase functions as a major repressor of hypoxia-induced erythropoietin gene expression. Endocrinology, 2005. 146(1): p. 332-340.
156. d'Uscio, L.V., L.A. Smith, and Z.S. Katusic, Erythropoietin Increases Expression and Function of Vascular Copper- and Zinc-Containing Superoxide Dismutase. Hypertension, 2010. 55(4): p. 998-1004.
157. Weydert, C.J. and J.J. Cullen, Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nature Protocols, 2010. 5(1): p. 51-66.
158. Chang, Q. and J.M. Petrash, Disruption of aldo-keto reductase genes leads to elevated markers of oxidative stress and inositol auxotrophy in Saccharomyces cerevisiae. Biochimica Et Biophysica Acta-Molecular Cell Research, 2008. 1783(2): p. 237-245.
159. Vlahakos, D.V., et al., Renin-Angiotensin System Stimulates Erythropoietin Secretion in Chronic-Hemodialysis Patients. Clinical Nephrology, 1995. 43(1): p. 53-59.
160. Yayama, K., et al., Angiotensin-converting enzyme inhibitor enhances liver regeneration following partial hepatectomy: Involvement of bradykinin B-2 and angiotensin AT(1) receptors. Biological & Pharmaceutical Bulletin, 2007. 30(3): p. 591-594.
161. Murakami, E., et al., Blood-Pressure Elevation Caused by Inhibition of Brain Glutathione-Reductase. Journal of Hypertension, Vol 7, Suppl 6, 1989: p. S24-S25.
162. 黄帝难经, 黄帝八十一难经. 1996: 辽宁教育出版社.
163. Liu, Y., et al., Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am J Clin Nutr, 2015. 102(1): p. 130-7.
164. Cao, Y.K., et al., Cardioprotective Effect of Ghrelin in Cardiopulmonary Bypass Involves a Reduction in Inflammatory Response. Plos One, 2013. 8(1).
165. Zwirska-Korczala, K., et al., Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol, 2007. 58 Suppl 1: p. 53-64.
166. Porat, O., et al., Erythropoietin stimulates atrial natriuretic peptide secretion from adult rat cardiac atrium. J Pharmacol Exp Ther, 1996. 276(3): p. 1162-8.
167. 張景岳, 張景岳医学全书. 1999: 中国中医药出版社.
168. Vesely, D.L., et al., Atrial natriuretic peptides increase calcitonin gene-related peptide within human circulation. Metabolism-Clinical and Experimental, 1997. 46(7): p. 818-825.
169. Yamamoto, A., et al., Calcitonin Gene-Related Peptide (Cgrp) Stimulates the Release of Atrial Natriuretic Peptide(Anp) from Isolated Rat Atria. Biochemical and Biophysical Research Communications, 1988. 155(3): p. 1452-1458.
170. 周慎斋, 周慎斋医学全书, 2010: 海南出版社.
171. Farley, J., et al., Calcitonin increases the concentration of insulin-like growth factors in serum-free cultures of human osteoblast-line cells. Calcified Tissue International, 2000. 67(3): p. 247-254.
172. Schlupf, J. and H. Steinbeisser, IGF antagonizes the Wnt/beta-Catenin pathway and promotes differentiation of extra-embryonic endoderm. Differentiation, 2014. 87(5): p. 209-219.
173. Landon, R.A. and E.A. Young, Role of magnesium in regulation of lung function. J Am Diet Assoc, 1993. 93(6): p. 674-7.
174. Hung, C.F., et al., Role of IGF-1 pathway in lung fibroblast activation. Respiratory Research, 2013. 14.
175. Slomiany, M.G. and S.A. Rosenzweig, IGF-1-induced VEGF and IGFBP-3 secretion correlates with increased HIF-1 alpha expression and activity in retinal pigment epithelial cell line D407. Investigative Ophthalmology & Visual Science, 2004. 45(8): p. 2838-2847.
176. Pereira, C.T., et al., Liposomal gene transfer of keratinocyte growth factor improves wound healing by altering growth factor and collagen expression. Journal of Surgical Research, 2007. 139(2): p. 222-228.
177. Jeschke, M.G. and D.N. Herndon, The combination of IGF-I and KGF cDNA improves dermal and epidermal regeneration by increased VEGF expression and neovascularization. Gene Therapy, 2007. 14(16): p. 1235-1242.
178. Stirling, D., et al., Angiotensin-Ii Inhibits Luteinizing Hormone-Stimulated Cholesterol Side-Chain Cleavage Expression and Stimulates Basic Fibroblast Growth-Factor Expression in Bovine Luteal Cells in Primary Culture. Journal of Biological Chemistry, 1990. 265(1): p. 5-8.
179. Pupilli, C., et al., Angiotensin II stimulates the synthesis and secretion of vascular permeability factor vascular endothelial growth factor in human mesangial cells. Journal of the American Society of Nephrology, 1999. 10(2): p. 245-255.
180. Takase, H.M., et al., FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes & Development, 2013. 27(2): p. 169-181.
181. Taniguchi, E., et al., Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. Journal of Histochemistry & Cytochemistry, 2001. 49(1): p. 121-129.
182. Ipsen, D.H., P. Tveden-Nyborg, and J. Lykkesfeldt, Does Vitamin C Deficiency Promote Fatty Liver Disease Development? Nutrients, 2014. 6(12): p. 5473-5499.
183. Ersoz, G., et al., Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol, 2005. 16(3): p. 124-8.
184. Nath, B. and G. Szabo, Hypoxia and hypoxia inducible factors: Diverse roles in liver diseases. Hepatology, 2012. 55(2): p. 622-633.
185. Westenbrink, B.D., et al., Vascular endothelial growth factor is crucial for erythropoietin-induced improvement of cardiac function in heart failure. Cardiovascular Research, 2010. 87(1): p. 30-39.
186. Johnston, C.S., C.G. Meyer, and J.C. Srilakshmi, Vitamin-C Elevates Red-Blood-Cell Glutathione in Healthy-Adults. American Journal of Clinical Nutrition, 1993. 58(1): p. 103-105.
187. Wang, G.L., et al., Hypoxia-Inducible Factor-1 Is a Basic-Helix-Loop-Helix-Pas Heterodimer Regulated by Cellular O-2 Tension. Proceedings of the National Academy of Sciences of the United States of America, 1995. 92(12): p. 5510-5514.
188. Tajima, M., et al., The redox state of glutathione regulates the hypoxic induction of HIF-1. European Journal of Pharmacology, 2009. 606(1-3): p. 45-49.
189. Parrott, J.A., et al., Autocrine interactions of keratinocyte growth factor, hepatocyte growth factor, and kit-ligand in the regulation of normal ovarian surface epithelial cells. Endocrinology, 2000. 141(7): p. 2532-2539.
190. 李中梓, 医宗必读. 2006: 人民卫生出版社
191. Obara, N., et al., Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood, 2008. 111(10): p. 5223-5232.
192. Konturek, P.C., et al., Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. European Journal of Pharmacology, 2006. 536(1-2): p. 171-181.
193. Fernandez-Martinez, A.B., M.I.A. Jimenez, and F.J.L. Cazana, Retinoic acid increases hypoxia-inducible factor-1 alpha through intracrine prostaglandin E-2 signaling in human renal proximal tubular cells HK-2. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids, 2012. 1821(4): p. 672-683.
194. Mackenzie, I.C. and Z.R. Gao, Keratinocyte growth factor expression in human gingival fibroblasts and stimulation of in vitro gene expression by retinoic acid. Journal of Periodontology, 2001. 72(4): p. 445-453.
195. Mazumdar, J., et al., O-2 regulates stem cells through Wnt/beta-catenin signalling. Nature Cell Biology, 2010. 12(10): p. 1007-1013.
196. Pelosi, M., et al., Parathyroid hormone-related protein is induced by hypoxia and promotes expression of the differentiated phenotype of human articular chondrocytes. Clinical Science, 2013. 125(9-10): p. 461-470.
197. Agani, F.H., et al., Role of nitric oxide in the regulation of HIF-1 alpha expression during hypoxia. American Journal of Physiology-Cell Physiology, 2002. 283(1): p. C178-C186.
198. Zhu, X.J., et al., BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet, 2014. 10(10): p. e1004687.
199. Nejak-Bowen, K.N., et al., beta-Catenin Regulates Vitamin C Biosynthesis and Cell Survival in Murine Liver. Journal of Biological Chemistry, 2009. 284(41): p. 28115-28127.
200. d'Uscio, L.V., et al., Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circulation Research, 2003. 92(1): p. 88-95.
201. Barbagallo, M., et al., Effects of glutathione on red blood cell intracellular magnesium - Relation to glucose metabolism. Hypertension, 1999. 34(1): p. 76-82.
202. Chu, C.H., et al., IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via G alpha q interaction and protein kinase C-alpha/CaMKII activation in H9c2 cardiomyoblast cells. Journal of Endocrinology, 2008. 197(2): p. 381-390.
203. 钟赣生, 中药学. 2012: 中国中医药出版社.
204. Hong, M., et al., The Decline of Atrial-Natriuretic-Peptide (Anp) Gene-Expression in Older Rats and the Effects of Ginsenoside on Anp Gene-Expression. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology, 1992. 101(1-2): p. 35-39.
205. Kim, S.H., et al., Enhancement of 1,25-dihydroxyvitamin D3- and all-trans retinoic acid-induced HL-60 leukemia cell differentiation by Panax ginseng. Biosci Biotechnol Biochem, 2009. 73(5): p. 1048-53.
206. Xu, Z.W., et al., The effects of ginsenoside Rb1 on endothelial damage and ghrelin expression induced by hyperhomocysteine. Journal of Vascular Surgery, 2011. 53(1): p. 156-164.
207. Mao, N., et al., Ginsenoside Rg1 protects mouse podocytes from aldosterone-induced injury in vitro. Acta Pharmacologica Sinica, 2014. 35(4): p. 513-522.
208. Kimura, Y., et al., Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. British Journal of Pharmacology, 2006. 148(6): p. 860-870.
209. Zheng, K.Y., et al., Flavonoids from Radix Astragali induce the expression of erythropoietin in cultured cells: a signaling mediated via the accumulation of hypoxia-inducible factor-1alpha. J Agric Food Chem, 2011. 59(5): p. 1697-704.
210. Zhang, Y., et al., Radix Astragali extract promotes angiogenesis involving vascular endothelial growth factor receptor-related phosphatidylinositol 3-kinase/Akt-dependent pathway in human endothelial cells. Phytother Res, 2009. 23(9): p. 1205-13.
211. Ma, J., A. Peng, and S.Y. Lin, Mechanisms of the therapeutic effect of astragalus membranaceus on sodium and water retention in experimental heart failure. Chinese Medical Journal, 1998. 111(1): p. 17-23.
212. Chao, C.C., R.D. Brown, and L.J. Deftos, Seasonal Levels of Serum Parathyroid-Hormone, Calcitonin and Alkaline-Phosphatase in Relation to Antler Cycles in White-Tailed Deer. Acta Endocrinologica, 1984. 106(2): p. 234-240.
213. Barling, P.M., et al., Expression of PTHrP and the PTH/PTHrP receptor in growing red deer antler. Cell Biology International, 2004. 28(10): p. 661-673.
214. Lee, M.-R., et al., Hemopoietic effect of extracts from four parts of deer antler on phenylhydrazine-induced hemolytic anemia in female rats. Journal of the Korean Society of Food Science and Nutrition, 2009. 38(12): p. 1718-1723.
215. 陈晓光, 王., 吴岩, 王丽萍, 李惟, 鹿茸多肽对大鼠心肌缺血损伤的保护作用. 中国中药杂志, 2009. 34(15): p. 1971-1974.
216. 李振华, 赵., 周秋丽, 鹿茸多肽对抗骨关节炎软骨细胞氧化损伤作用的实验研究. 中国骨伤, 2011. 24(3): p. 245-248.
217. Bensky, D., A. Gamble, and T.J. Kaptchuk, Chinese herbal medicine: materia medica. 1993: Eastland Press.
218. Shi P, Z.W., Li XM., Cynomorium Songaricum polysaccharide improves osteoporosis in ovariectomized rats. Journal of Third Military Medical University 2015. 37: p. 2360-2363
219. Yu, F.R., et al., Effects of a Flavonoid Extract from Cynomorium songaricum on the Swimming Endurance of Rats. American Journal of Chinese Medicine, 2010. 38(1): p. 65-73.
220. 华自森等, 当归多糖协同Epo对造血干/祖细胞JAK2/STAT5信号传导通路的影响. 中国中药杂志, 2009. 34(24): p. 3268-3271.
221. Bi, C.W.C., et al., Fo Shou San, an Ancient Herbal Decoction Prepared from Rhizoma Chuanxiong and Radix Angelicae Sinensis, Stimulates the Production of Hemoglobin and Erythropoietin in Cultured Cells. Planta Medica, 2010. 76(14): p. 1525-1529.
222. Wang, Y., et al., Experimental studies on the HGF-inducing effect of L-cells treated with Angelica polysaccharide. Acta Anatomica Sinica, 1995. 27(1): p. 69-74.
223. Chiu, P.Y., et al., Dang-Gui Buxue Tang protects against oxidant injury by enhancing cellular glutathione in H9c2 cells: Role of glutathione synthesis and regeneration. Planta Medica, 2007. 73(2): p. 134-141.
224. Pu, X.Y., et al., Polysaccharides from Angelica and Astragalus exert hepatoprotective effects against carbon-tetrachloride-induced intoxication in mice. Canadian Journal of Physiology and Pharmacology, 2015. 93(1): p. 39-43.
225. Zhou, G.F., et al., Kinetic changes and antihypertensive effect of aqueous extract of Danggui (Angelica sinensis radix) after stir-fry processing. Food Science and Biotechnology, 2013. 22(3): p. 765-771.
226. Wen, Y.X., et al., Angelica Sinensis Polysaccharides Stimulated UDP-Sugar Synthase Genes through Promoting Gene Expression of IGF-1 and IGF1R in Chondrocytes: Promoting Anti-Osteoarthritic Activity. Plos One, 2014. 9(9).
227. Yu, H., et al., [Effect of Cordyceps sinensis on the expression of HIF-1alpha and NGAL in rats with renal ischemia-reperfusion injury]. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2012. 37(1): p. 57-66.
228. Singh, M., et al., Cordyceps sinensis Increases Hypoxia Tolerance by Inducing Heme Oxygenase-1 and Metallothionein via Nrf2 Activation in Human Lung Epithelial Cells. Biomed Research International, 2013.
229. Bhardwaj, A., et al., HPTLC Based Chemometrics of Medicinal Mushrooms. Journal of Liquid Chromatography & Related Technologies, 2015. 38(14): p. 1392-1406.
230. Yang, J.Y., et al., Effects of exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis fungus on c-Myc, c-Fos, and VEGF expression in B16 melanoma-bearing mice. Pathology Research and Practice, 2005. 201(11): p. 745-750.
231. Shi, S.G., et al., Overexpression of l-galactono-1, 4-lactone dehydrogenase (GLDH) in Lanzhou lily (Lilium davidii var. unicolor) via particle bombardment-mediated transformation. In Vitro Cellular & Developmental Biology-Plant, 2012. 48(1): p. 1-6.
232. Jiang, Y., et al., Wolfberry Water Soluble Phytochemicals Down-Regulate ER Stress Biomarkers and Modulate Multiple Signaling Pathways Leading To Inhibition of Proliferation and Induction of Apoptosis in Jurkat Cells. J Nutr Food Sci, 2011. S2.
233. Yi, R., X.M. Liu, and Q. Dong, A Study of Lycium Barbarum Polysaccharides (Lbp) Extraction Technology and Its Anti-Aging Effect. African Journal of Traditional Complementary and Alternative Medicines, 2013. 10(4): p. 171-174.
234. Li QY, L.Z., Jiang YN., Formulas of Traditional Chinese Medicine. . 1998, Beijing: Academic Press
235. Takeda, H., et al., Rikkunshito and Ghrelin Secretion. Current Pharmaceutical Design, 2012. 18(31): p. 4827-4838.
236. 申定珠等, 基于差异蛋白质组学解析一贯煎对大鼠肝硬化形成的影响. 中西医结合学报, 2010. 8(2): p. 158-167.
237. Xia, B.J., et al., The effects of Liuwei Dihuang on canonical Wnt/beta-catenin signaling pathway in osteoporosis. Journal of Ethnopharmacology, 2014. 153(1): p. 133-141.
238. 任德权, 临床实用中成药. 2002: 人民卫生出版社.
239. Liu, M.J., et al., Effects of zuogui pill (see text) on Wnt singal transduction in rats with glucocorticoid-induced osteoporosis. J Tradit Chin Med, 2011. 31(2): p. 98-102.
240. Xiong, X.J., et al., Shenqi pill, a traditional Chinese herbal formula, for the treatment of hypertension: A systematic review. Complementary Therapies in Medicine, 2015. 23(3): p. 484-493.
241. 唐容川, 血证论. 1977: 上海人民出版社.
242. Belefant-Miller, H. and S.C. Grace, Variations in Bran Carotenoid Levels within and between Rice Subgroups. Plant Foods for Human Nutrition, 2010. 65(4): p. 358-363.
243. Paine, J.A., et al., Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nature Biotechnology, 2005. 23(4): p. 482-487.
244. 丁光迪, 诸病源候论校注. 1992: 人民卫生出版社.
245. Yildiz, B., et al., Increased serum levels of insulin-like growth factor (IGF)-1 and IGF-binding protein-3 in Henoch-Schonlein purpura. Tohoku Journal of Experimental Medicine, 2008. 214(4): p. 333-340.
246. Tojo, A., et al., Spironolactone with ACE inhibitor is effective in gross hematuria caused by nephroptosis. International Journal of Urology, 2006. 13(7): p. 990-992.
247. Morrone, L.F., et al., Interference of angiotensin-converting enzyme inhibitors on erythropoiesis in kidney transplant recipients: role of growth factors and cytokines. Transplantation, 1997. 64(6): p. 913-8.
248. 李东垣, 脾胃论. 2007: 中国中医药出版社.
249. Gower, W.R., et al., Gastric atrial natriuretic peptide regulates endocrine secretion in antrum and fundus of human and rat stomach. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2003. 284(4): p. G638-G645.
250. Inui, A., et al., Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. Faseb Journal, 2004. 18(3): p. 439-456.
251. 李时珍, 本草纲目. 2002: 中国经济出版社.
252. Chung, Y.C., Y.C. Hou, and A.C.H. Pan, Endoglin (CD105) expression in the development of haemorrhoids. European Journal of Clinical Investigation, 2004. 34(2): p. 107-112.
253. Lee, W.T., et al., Increased prevalence of constipation in pre-school children is attributable to under-consumption of plant foods: A community-based study. J Paediatr Child Health, 2008. 44(4): p. 170-5.
254. Ghambarali, Z., et al., Ethanolic extract of Ficus carica leave Suppresses Angiogenesis by Regulating VEGF-A and Integrin beta3 mRNA Expression in Human umbilical vein endothelial cells. Indian J Physiol Pharmacol, 2014. 58(4): p. 407-15.
255. USDA, N.N.D. Statistics Report 09089, Figs, raw. Available from: https://ndb.nal.usda.gov/ndb/foods/show/2201?format=Stats&reportfmt=pdf...
256. Andersson, K.E. and K. Persson, Nitric-Oxide Synthase and Nitric Oxide-Mediated Effects in Lower Urinary-Tract Smooth Muscles. World Journal of Urology, 1994. 12(5): p. 274-280.
257. Balat, A., et al., Nitric oxide synthase gene polymorphisms in children with primary nocturnal enuresis: A preliminary study. Renal Failure, 2007. 29(1): p. 79-83.
258. Sudo, H., et al., Nicorandil, a Potassium Channel Opener and Nitric Oxide Donor, Improves the Frequent Urination without Changing the Blood Pressure in Rats with Partial Bladder Outlet Obstruction. Biological & Pharmaceutical Bulletin, 2008. 31(11): p. 2079-2082.
259. 孙世发, 中医肝胆病良方. 2005: 金盾出版社.
260. Geetha, A., Evidence for oxidative stress in the gall bladder mucosa of gall stone patients. J Biochem Mol Biol Biophys, 2002. 6(6): p. 427-32.
261. Pomelov, V.S., et al., [Glutathione levels and the activity of the enzymes of glutathione metabolism in erythrocytes of patients with acute cholecystitis]. Sov Med, 1991(8): p. 27-30.
262. 何任,谭日强等, 高血压证治. 中医杂志, 1986. 2: p. 11-14.
263. Vaziri, N.D., Mechanism of erythropoietin-induced hypertension. American Journal of Kidney Diseases, 1999. 33(5): p. 821-828.
264. Morishita, R., et al., Hepatocyte Growth Factor (HGF) as a potential index of severity of hypertension. Hypertension Research-Clinical and Experimental, 1999. 22(3): p. 161-167.
265. Conn, J.W., D.R. Rovner, and E.L. Cohen, Suppression of Plasma Renin Activity in Primary Aldosteronism - Distinguishing Primary from Secondary Aldosteronism in Hypertensive Disease. Jama-Journal of the American Medical Association, 1964. 190(3): p. 213-&.
266. Nozik-Grayck, E., et al., Selective depletion of vascular EC-SOD augments chronic hypoxic pulmonary hypertension. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2014. 307(11): p. L868-L876.
267. Tyther, R., et al., Protein carbonylation in kidney medulla of the spontaneously hypertensive rat. Proteomics Clinical Applications, 2009. 3(3): p. 338-346.
268. Cheng, P.W., et al., Wnt Signaling Regulates Blood Pressure by Downregulating a GSK-3 beta-Mediated Pathway to Enhance Insulin Signaling in the Central Nervous System. Diabetes, 2015. 64(10): p. 3413-3424.
Document information
Published on 01/01/2017
Licence: CC BY-NC-SA license