Summary
Splenic vein occlusion caused by abdominal lymphadenopathy is rare. We herein present the case of a 80-year-old man with refractory isolated gastric variceal bleeding in the absence of pancreatic or liver disease. Left-sided portal hypertension was confirmed by angiography, and para-aortic lymphadenopathy compressing the splenic vein was identified by serial abdominal computed tomography. Endoscopic sclerosing therapy failed to treat the recurring gastric variceal hemorrhage. Therefore, splenectomy was suggested and the patient was successfully treated. The patient had been variceal bleeding free for 12 months since the surgery. In patients with isolated gastric varices but without advanced liver disease, a variety of diagnostic techniques should be attempted to elucidate the nature of portal hypertension, and left-sided portal hypertension should be suspected. For those cases in which endoscopic treatment failed to treat refractory gastric variceal bleeding, splenectomy can be an effective option.
Keywords
Gastric varices ; Splenectomy ; Splenic vein ; Lymph node
Introduction
Gastric variceal bleeding is a serious complication of portal hypertension. Gastric varices (GVs) are found in about 20% patients with portal hypertension [1] . GV can occur in patients with intrahepatic portal hypertension caused by liver cirrhosis. It can also develop in patients with extrahepatic portal hypertension (e.g., left-sided portal hypertension).
Left-sided portal hypertension is associated with the formation of isolated GVs (IGVs) in the gastric fundus without esophageal varices. It can be caused by thrombosis, obstruction, or stenosis of the splenic vein. The most common cause of splenic vein obliteration is pancreatic pathology including acute or chronic pancreatitis and pancreatic neoplasm [2] .
Abdominal lymphadenopathy is a rare cause of splenic vein occlusion (SVO). We herein report a case of recurrent IGV bleeding related to SVO caused by para-aortic lymphadenopathy, possibly related to tuberculosis, compressing the splenic vein. The patient was successfully treated by splenectomy. The literature was reviewed for this uncommon cause and presentation of GV bleeding.
Case Report
A 80-year-old man, who worked as a coal miner, was diagnosed with pulmonary tuberculosis about 20 years ago. He had received a complete course of tuberculosis treatment with isoniazid, ethambutol, rifampin, and pyrazinamide for 6 months. He did not have any history of liver or pancreatic disease. He experienced hematemesis, the first episode of gastrointestinal hemorrhage, 7 years prior to the most recent hospitalization. Esophagogastroduodenoscopy (EGD) revealed engorged GVs in the gastric cardia and fundus without esophageal varices. The GVs were obliterated by injection of a mixture of N -butyl-2-cyanoacrylate and Lipiodol (ethiodized oil). Despite a smooth recovery, he had been hospitalized a dozen times since the first presentation. However, the intervals between each hospitalization progressively shortened. Rebleeding of GV was confirmed by EGD and endoscopic obliterative therapy was attempted each time. Blood tests of liver biochemistries and serum levels of amylase and lipase had been within normal levels during these hospitalizations. There was no coagulopathy and the serological markers of hepatitis B or C virus were negative. Active pulmonary tuberculosis was excluded, as both sputum cultures and smears for tuberculosis were negative.
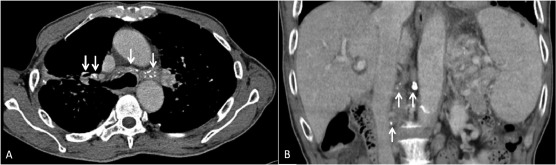
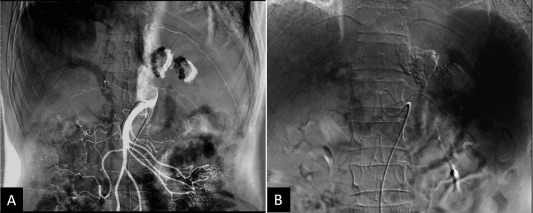
An abdominal ultrasound evaluation revealed portal hypertension by engorged portal vein and collateral vessels from the splenic hilum to the stomach without liver cirrhosis or ascites. A multiphase contrast-enhanced computed tomography (CT) scan revealed splenomegaly with prominent GVs, perigastric varices, and a small caliber of the splenic vein. CT revealed many enlarged lymph nodes with calcifications in the celiac axis and para-aortic regions (Figure 1 B). The splenic vein was markedly narrowed and a lymph node was found compressing the splenic vein (Figure 2 ). The angiography showed patent portal veins and superior mesenteric artery (Figure 3 ). A superselective splenic portogram showed complete obliteration of the splenic vein with multiple tortuous dilated collateral circulations from the splenic hilum and the gastric cardiac portion to the gastric antrum, with the terminal flow connecting to the main portal vein (Figure 3 B). Based on these findings, left-sided portal hypertension was suggested.
|
|
|
Figure 1. Computed tomography (CT) scan images. (A) Chest CT scan showed partially calcified mediastinal and bilateral hilar lymph nodes (arrows). (B) Coronal reconstruction of CT revealed enlarged calcified lymph nodes in the celiac axis and para-aortic region (arrows). |
|
|
|
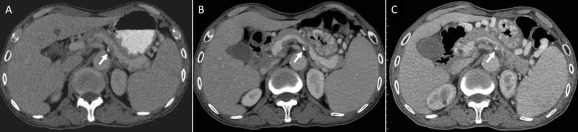
Figure 2. Serial contrast-enhanced abdominal computed tomography scans of the patient acquired in the past (A) 7 years, (B) 5 years, and (C) 1 year showed the progression of splenic vein occlusion (arrow) related to calcified para-aortic lymph node. |
|
|
|
Figure 3. Selective abdominal angiography for characterization of portal hypertension. (A) Selective superior mesenteric artery arteriography showed patency of the portal veins and superior mesenteric artery. (B) Superselective splenic portogram showed tortuous collateral vessels connecting from the splenic hilum and the gastric cardiac portion to the main portal vein. |
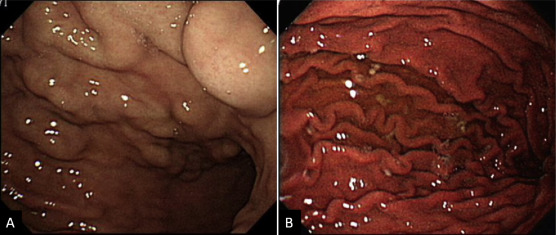
Splenectomy was performed. Engorged veins were found at the perisplenic area and these were devascularized. Pathologically, the spleen was enlarged with thickening of the splenic capsule and congestion in the red pulps and prominence of the sinusoidal endothelial cells. Congestion of the perisplenic vessels and aggregation of hemosiderin-laden macrophages were noted. The splenic vessels from the hilum were congested without thrombus. The pathology confirmed congestive splenomegaly. The GVs had regressed in the follow-up EGDs (Figure 4 ). The patient had been variceal bleeding free for 12 months since splenectomy.
|
|
|
Figure 4. Esophagogastroduodenoscopy before and after splenectomy. (A) Prominent gastric varices extending from the gastric cardia to the fundus were found prior to the surgery. (B) Endoscopic evaluation 8 months after splenectomy showed regression of gastric varices. |
Discussion
GVs can be classified into gastroesophageal varices (GOVs) and IGVs according to the classification proposed by Sarin and Kumar [3] . IGV is a rare condition associated with either generalized or left-sided portal hypertension. Generalized portal hypertension can be caused by obstruction to the portal vein due to external compression, thrombosis, or tumor invasion, or by increased vascular resistance in the liver caused by cirrhosis or noncirrhotic portal fibrosis. In generalized portal hypertension, increased pressure in the portal and splenic veins is transmitted through the short gastric vein and the gastroepiploic veins into the fundal venous plexus to form fundal GVs; GOVs are produced if the pressure is transmitted through the coronary vein. The extrahepatic portal vein is patent and the portal pressure is usually normal in left-sided portal hypertension due to SVO. The increased splenic venous pressure leads to reversal of blood flow through the short gastric vein, and the flow can enter the portal venous system via the coronary vein without resulting in esophageal varices [4] . It is important to identify the cause of IGVs as those related to general portal hypertension need more extensive management and cannot be effectively treated with splenectomy alone. IGV can be further subclassified as Type 1 IGV (IGV1), which is located in the gastric fundus, and Type 2 IGV (IGV2), which is located in the gastric antrum, corpus, or pylorus. IGV1 and IGV2 account for 8% and 2% of GVs, respectively [1] . Our patient is classified as a case of IGV1. Such subclassification is important in patients with IGV because the risk of bleeding is much greater in IGV1 (78%) than in that with IGV2 (10%) [1] ; [3] .
Left-sided portal hypertension should be suspected if IGV presented with splenomegaly but without evidence of liver diseases. The pancreatic diseases are the most common causes. Koklu et al [5] found that 39% of the cases were caused by pancreatitis and 22% by pancreatic tumors. Our patient, however, did not have any evidence of liver or pancreatic disease. The diagnosis of left-sided portal hypertension can be made by clinical, biochemical, and diagnostic imaging. CT scan images of our patient showed multiple calcified lymph nodes at the mediastinum, bilateral hilum, and para-aortic region. One of the calcified lymphadenopathies was located just anterior to the celiac trunk and compressed the adjacent splenic vein. Serial CTs showed the progression of splenic vein stenosis (Figure 2 ). The association between abdominal lymphadenopathy and isolated SVO is very rare. Only few cases have been reported with etiologies of splenic [6] or pancreatic lymphoma [7] , and tuberculous lymphadenopathy [8] . Calcified lymph nodes are associated with a variety of clinical settings including treated lymphoma, tuberculosis, histoplasmosis, silicosis, sarcoidosis, and metastatic carcinoma. Our patient neither had a history nor clinical or radiographic findings of lymphoma, sarcoidosis, histoplasmosis, or malignancy. Eggshell calcification of lymph nodes is common in patients with silicosis and coal worker’s pneumoconiosis. Our patient may have had occupational exposure to inhaled crystalline silica as a coal miner. Inhaled silica particles may be immobilized in the lymph node and produce eggshell calcification. However, the calcified lymph nodes are frequently located in the hilar and mediastinal regions, and there are only few reports of extrathoracic involvement. In the presented case, there is no typical shell-like calcification nor complete ring-like shadow in the lymph nodes. The findings did not match the criteria of eggshell calcification proposed by Jacobson et al [9] . Tuberculosis might have a lymphohematogenous spread from a pulmonary origin, involving the peripancreatic and mesenteric lymph node groups, and although rare, can cause obstruction of the adjacent vascular structures. Caseation or necrosis of granulation tissue may initially show central low attenuation areas and peripheral rim enhancement of lymph nodes in a CT scan in tuberculous lymphadenitis. Based on the clinical history and image findings, our patient was suspected to have inactive lymphadenopathy related to tuberculosis compressing the splenic vein and causing SVO, left-sided portal hypertension, and IGV1. Following treatment for tuberculosis, the lymphadenopathy may disappear or regress with fibrotic tissue and calcifications [10] . For active tuberculous lymphadenitis, tuberculosis treatment may cause regression of lymphadenopathy and potential alleviation of related obstruction, but the benefits of treatment might not be significant in the case of an inactive disease. Our patient was more likely to have had inactive tuberculosis because the features of the pulmonary and extrapulmonary manifestations had been stable without signs of progression.
Surgical splenectomy with or without devascularization is the treatment of choice in patients with left-side portal hypertension-related refractory IGV bleeding, which persist despite repeated endoscopic treatments. Splenectomy can correct potentially life-threatening bleeding and reduce future bleeding. Splenic artery embolization is another treatment option for patients who are not good candidates for surgical treatments. Splenic artery embolization will decrease the splenic venous outflow and decrease gastric venous pressures. However, splenic artery embolization may have serious complications, including partial infarction of the stomach and pancreas, splenic abscess, septicemia, portal vein thrombosis, liver failure, and death, especially for those with complete splenic artery embolization. Balloon-occluded retrograde transvenous obliteration (BRTO), introduced by Kanagawa et al [11] , is another option for endovascular management of GV bleeding. It involves occlusion of draining vessel of the portosystemic shunt, using an occlusion balloon followed injection of sclerosant. Understanding the characteristics of draining veins is important to achieve a successful outcome with BRTO. Our patient had Saad–Caldwell Type 2 GVs, which correlated with endoscopic Sarin classification (IGV1) and the Fukuda–Hirota Type 1. The dominant portal venous feeders were the short gastric and posterior gastric veins coming off the splenic vein near the splenic hilum. BRTO is indicated in the presence of a large gastrorenal shunt (Saad–Caldwell Type 2b). However, our patient had no accessible gastrorenal shunt (Saad–Caldwell Type 2a), and the majority of the splenic outflow was through the small collaterals. In this situation, BRTO is limited, and partial splenic embolization or splenectomy is preferred [12] . Although our patient had higher risks for surgery due to poor pulmonary reserve, he was successfully treated with splenectomy.
The limitation of this case is that endoscopic ultrasound (EUS) could not be performed to evaluate the structures of pancreas, portal venous system, and patency of the splenic vein. EUS is more sensitive to ultrasound or CT in diagnosing small pancreatic and vascular lesions. EUS is also sensitive to determining GV or paragastric collaterals veins, and differentiating between engorged gastric fold and varices. EUS can contribute to early diagnosis of IGV and provide extensive information for complete devascularization of extragastric collaterals [10] . Another limitation is that pathological studies for abdominal lymphadenopathy are limited by extensive and complex collateral vessels within the abdomen, which made dissection of the para-aortic lymph nodes difficult and risky.
In summary, we reported a case of recurrent bleeding of IGV caused by SVO associated with abdominal lymphadenopathy, which is extremely rare. The lymphadenopathy might be related to tuberculosis or silicosis. Left-sided portal hypertension should be considered in patients with IGV without advanced liver disease. Splenectomy is the preferred treatment for patients with recurrent bleeding who failed endoscopic treatment.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] S.K. Sarin, D. Lahoti, S.P. Saxena, N.S. Murthy, U.K. Makwana; Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients; Hepatology, 16 (1992), pp. 1343–1349
- [2] S. Koklu, S. Coban, O. Yuksel, M. Arhan; Left-sided portal hypertension; Dig Dis Sci, 52 (2007), pp. 1141–1149
- [3] S.K. Sarin, A. Kumar; Gastric varices: profile, classification, and management; Am J Gastroenterol, 84 (1989), pp. 1244–1249
- [4] S. Nagral, S. Shah, M. Gandhi, S.K. Mathur; Bleeding isolated gastric varices: a retrospective analysis; Indian J Gastroenterol, 18 (1999), pp. 69–72
- [5] S. Koklu, O. Yuksel, M. Arhan, S. Coban, O. Basar, O.F. Yolcu, et al.; Report of 24 left-sided portal hypertension cases: a single-center prospective cohort study; Dig Dis Sci, 50 (2005), pp. 976–982
- [6] B.C. Chen, H.H. Wang, Y.C. Lin, Y.L. Shih, W.K. Chang, T.Y. Hsieh; Isolated gastric variceal bleeding caused by splenic lymphoma-associated splenic vein occlusion; World J Gastroenterol, 19 (2013), pp. 6939–6942
- [7] L. Vida Perez, A. Gonzalez Galilea, E. Fraga Rivas; Bleeding from gastric varices as the initial manifestation of primary pancreatic lymphoma; Gastroenterol Hepatol, 33 (2010), pp. 165–170 [Article in Spanish.]
- [8] H. Takeuchi, M. Suzuki, M. Unno, T. Kakita, S. Matsuno, H. Nakura; Splenic vein occlusion secondary to tuberculous lymphadenitis at the splenic hilum: report of a case; Surg Today, 30 (2000), pp. 383–385
- [9] G. Jacobson, B. Felson, E.P. Pendergrass, R.H. Flinn, W.S. Lainhart; Eggshell calcifications in coal and metal miners; Semin Roentgenol, 2 (1967), pp. 276–282
- [10] W.K. Moon, J.G. Im, K.M. Yeon, M.C. Han; Mediastinal tuberculous lymphadenitis: CT findings of active and inactive disease; AJR Am J Roentgenol, 170 (1998), pp. 715–718
- [11] H. Kanagawa, S. Mima, H. Kouyama, K. Gotoh, T. Uchida, K. Okuda; Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration; J Gastroenterol Hepatol, 11 (1996), pp. 51–58
- [12] W.E. Saad; Endovascular management of gastric varices; Clin Liver Dis, 18 (2014), pp. 829–851
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?