Abstract
Background
Functional tricuspid regurgitation (FTR) is frequent in patients with mitral valve disease. Untreated tricuspid regurgitation (TR) may cause poor clinical outcomes. The surgical factors involved in annuloplasty for FTR remain controversial. Our objective was to compare effectiveness of different tricuspid annuloplasty (TVP), and reveal the risk factors of recurrence.
Methods
We analyzed the clinical details of 399 consecutive patients who underwent mitral surgery with concomitant TVP, from 2006 to 2011, in two Chinese single-centers. Three methods were used for TVP: De Vega surgery was completed in 242 patients; annuloplasty using a flexible band was completed in 98 patients; and surgery with a rigid ring was performed in 59 patients.
Results
The operative mortality rate was 2.3%. After surgery, the TR grade of all patients decreased significantly. At three years postoperatively, 13.7% of patients were diagnosed with recurrent FTR. At the three year time point, severe TR in the De Vega group was 18%, which was higher than those in the flexible (8.4%) and rigid planner ring groups (5.2%). During follow-up, the recurrent rates in the rigid group were significantly lower than in the flexible group. Multivariate analysis revealed that pre-operative atrial fibrillation, severe TR, large left atrial, ejection fraction (EF) < 40%, De Vega annuloplasty, and postoperative permanent pacemaker installation were independent risk factors for severe recurrent TR.
Conclusions
Rigid ring annuloplasty efficaciously improved post-operative tricuspid valve function in patients with FTR. Atrial fibrillation, a large left atrium, low EF and postoperative permanent pacemaker installation were independent risk factors for severe recurrent TR.
Keywords
Functional tricuspid regurgitation;Tricuspid annuloplasty;Follow-up
1. Background
Tricuspid regurgitation (TR) is always present in patients with mitral valve (MV) disease, and over one-third of the patients with mitral stenosis have at least moderate TR [1]. Patients with pre-operative severe TR have severe MV disease, higher pulmonary vascular resistance, and poorer outcomes. The functional label referring to TR, which is secondary to either left-sided heart disease (LHD) or pulmonary hypertension, could be possibly a misnomer [2]. If untreated at the time of MV surgery, TR may progress, negatively impacting functional class, and survival [3]. Patients with severe TR after MV surgery and who are undergoing isolated tricuspid valve (TV) surgery, usually have high operative mortality, and no significant improvement in functional capacity [4] ; [5]. On the contrary, many investigators have reported that even if there was an absence of any organic pathology, TR would not reliably resolve after the correction of the primary disorder [6]; [7] ; [8].
Due to the common belief that TR will resolve itself once the primary LHD has been treated, cardiac surgeons have placed more attention on intra-operative concomitant TR treatment. In the United States, the total number of TV procedures more than doubled over the last 10-year period [9]. Although corrective surgery of severe functional TR (FTR) showed trends toward improved survival, either significant residual or recurrent TR has been reported in 15% to 40% of patients after different TV surgery [10]; [11] ; [12]. Tricuspid valve replacement, which allows adequate surface area of co-aptation impossible, is associated with higher mortality [13] ; [14]. Several studies have shown better long-term freedom from recurrent TV regurgitation and repeat operation in those who underwent TV repair [15]; [16]; [17]; [18]; [19] ; [20]; however, data regarding the outcome of such an approach and the optimal surgical technique for TV repair is lacking [6] ; [21].
The present article is based on our experience over the past five years with Chinese patients in our institution to advance the understanding of the effectiveness, the durability of different TV annuloplasty methods, and the risk factors involved with the surgical outcome.
2. Methods
We retrospectively analyzed the clinical reports of 399 consecutive patients (188 males, 211 females; age 46.7 years; range: 33–75 years) who underwent mitral surgery with concomitant tricuspid annuloplasty, from January 2006 to June 2011, in two Chinese single-centers (Changzheng Hospital and Changhai Hospital). Our exclusion criteria included those with: an organic disease of the TV, either congenital or infective tricuspid diseases, and either single or traumatic TR. Our institutional ethics committee approved the present study and all patients were given a written informed consent.
Each of the patients underwent pre-operative transthoracic echocardiography (TTE) within one month prior to surgery. The severity of FTR was evaluated using an apical four-chamber view, and graded from 0 to 4 + (0: none, 1 +: mild, 2 +: moderate, 3 +: moderate-to-severe, 4 +: severe).
All patients also underwent mitral surgery with cardiopulmonary bypass (CPB) established between both the venae cavae and the ascending aorta. Mild systemic hypothermia was reported in all cases. The myocardial protection used was identical for all patients, and consisted of an anterograde cold blood crystalloid cardioplegia with topical ice slush. We first corrected the mitral valve disease, and then proceeded, after aorta de-clamping, to perform a TV annuloplasty under the beating heart. There were three procedures used to apply the tricuspid annuloplasty: (1) De Vega group (DV Group): 242 patients under either traditional or modified De Vega surgery; (2) flexible band group (Flexible Group): annuloplasty with either the Duran ring (Medtronic) or Cosgrove band (Cosgrove–Edwards annuloplasty system; Edwards Lifesciences) in 98 patients; (3) and the rigid planner ring group (Rigid Group): annuloplasty with MC3 ring (Edwards Lifesciences) in 59 patients. The type of tricuspid annuloplasty depended on a preference of the surgeon. The diameter of the annuloplasty was measured from the anteroseptal commissure to the antero-posterior commissure using a sterile supple ruler [22]. And the surgeon would choose the size of ring exactly according to the tricuspid diameter. We also performed a direct injection test, and utilized transesophageal echocardiography for intra-operative evaluation of TV function. Seven days after the valve repair, and at discharge, the temporal trend of the TR grades was assessed by TTE.
Individual patient contact after discharge was performed during either the outpatient process (69%), or with questionnaires (27%). Patients who did not respond were contacted by telephone (4%), and if no further information about the patients was available, we contacted their family physicians. The echocardiography data was evaluated based on TR grade and images were obtained at three months, six months, one year, and three years, postoperatively. The mean follow-up time was 3.3 years (range, 6 months–5.5 years). Seven patients were lost during follow-up. A total of 1467 TTE copies in 383 (96%) patients were analyzed during the follow-up.
2.1. Statistical analyses
Results are expressed as either a number (percentages), mean (standard deviation), or as a median (range) when the distribution of variables was not normal. Continuous variables were compared using either the Student t test or the Mann–Whitney test and categorical outcomes by either χ2 or Fishers exact test. Univariate and multivariate Cox proportional hazard models were used to examine the risk factors for recurrent or persistent significant TR. Variables with a p value < 0.20 in univariate analyses were used for the multivariate models. Multivariate analyses involved a backward elimination technique and only variables with a p value < 0.10 were included in the final model. All reported p values are two-sided and a p value < 0.05 was considered statistically significant. Analyses were performed using the SPSS version 13.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Baseline profiles
The etiology of the mitral lesion was rheumatic heart disease in 251 (62.9%), regressive in 108 (27.1%), and infective endocarditis in 40 patients (10.0%). When classified by using the pre-operative New York Heart Association (NYHA) functional class, 255 patients (64%) were in both classes III and IV. By TTE, a TR grade of 1 + or 2 + was in 119 patients (29.8%) and a grade of 3 + or 4 + was in 280 patients (71.2%); mean right ventricular systolic pressure (mRVSP) was 44.2 ± 16.1 mm Hg and mean pulmonary arterial pressure (mPAP) was 35.2 ± 12.1 mm Hg. There were no significant differences in the pre-operative clinical details of patients in the three groups, such as age, gender, NYHA class, TTE results, or the percent of TR 3 + or 4 + grades (Table 1).
| Variable | De Vega group (n = 242, %) | Flexible band group (n = 98, %) | Rigid ring group (n = 59, %) |
|---|---|---|---|
| Age | 46.2 ± 15.4 | 46.1 ± 14.7 | 47.7 ± 16.3 |
| Gender | |||
| Males | 114 (47.1) | 46 (46.9) | 28 (47.5) |
| Females | 128 (52.9) | 52 (53.1) | 31 (52.5) |
| NYHA⁎ | |||
| Class II | 90 (37.2) | 35 (35.7) | 20 (33.9) |
| Class III | 92 (38.0) | 38 (38.8) | 23 (40.0) |
| Class IV | 60 (24.8) | 25 (25.5) | 16 (27.1) |
| Atrial fibrillation | 109 (45.0) | 46 (46.9) | 29 (49.2) |
| Echocardiographic variables | |||
| Mitral stenosis | 39 (16.1) | 15 (15.3) | 10 (16.9) |
| Mitral incompetence | 88 (36.4) | 37 (37.8) | 23 (40.0) |
| Mitral mix lesions | 115 (47.5) | 46 (46.9) | 26 (44.1) |
| mPAP⁎ | 34.4 ± 12.3 | 35.7 ± 14.6 | 37.1 ± 16.1 |
| mRVSP⁎ | 43.9 ± 11.0 | 45.7 ± 12.9 | 45.1 ± 19.0 |
| LVEF⁎ | 55.1 ± 5.9 | 53.4 ± 4.1 | 56.2 ± 5.9 |
| TR⁎ grade | |||
| 1 + | 3 (1.2) | 3 (3.0) | 2 (3.4) |
| 2 + | 67 (27.7) | 27 (27.6) | 16 (27.1) |
| 3 + | 89 (36.8) | 35 (35.7) | 21 (35.6) |
| 4 + | 83 (34.3) | 33 (33.7) | 20 (33.9) |
The results of qualitative variables are expressed in absolute values (percentages) and the results of continuous variables are expressed as mean (SD).
⁎. NYHA = New York Heart Association; mPAP = mean pulmonary arterial pressure; mRVSP = mean right ventricular systolic pressure; LVEF = left ventricular ejection fraction; TR = tricuspid regurgitation.
3.2. Clinical outcomes
During surgery, there were no significant differences found within groups regarding CPB, aorta clamping time, and concomitant surgery. Due to the size of the annuloplasty ring, a 28# flexible band and MC3 ring were more commonly used. There was more 30# rings utilized in the Flexible Group, and a greater number of 26# bands in the Rigid Group.
The operative mortality was 2.3% (9/399). The main cause of death was multiple organ dysfunction syndrome in five patients. Two patients died of low output syndrome and the other two died of severe infection. The cause of death was attributed to pre-operative poor condition (Table 2).
| Variable | De Vega group (n = 242, %) | Flexible band group (n = 98, %) | Rigid ring group (n = 59, %) |
|---|---|---|---|
| Intraoperative outcome | |||
| Mitral surgery | |||
| Mitral replacement | 189 (78.1) | 72 (73.5) | 45 (76.3) |
| Mitral repair | 50 (20.1) | 25 (25.5) | 13 (22.0) |
| Size of TV⁎ prosthetic ring | |||
| 26 | / | 4 (4.1) | 6 (10.2) |
| 28 | / | 60 (61.2) | 39 (66.1) |
| 30 | / | 32 (32.3) | 14 (23.7) |
| 32 | / | 2 (2.0) | 0 (0.00) |
| Concomitant surgery | |||
| CABG⁎ | 3 (1.2) | 1 (1.0) | 1 (1.7) |
| CPB⁎ duration, minutes | 75.0 ± 10.0 | 80.2 ± 10.7 | 81.4 ± 9.1 |
| Aorta clamping time, minutes | 42.1 ± 9.3 | 40.9 ± 6.5 | 39.2 ± 7.3 |
| Mechanical ventilation time, hours | 9.1 ± 1.1 | 8.3 ± 1.6 | 8.6 ± 1.5 |
| ICU⁎ duration, days | 2.4 ± 0.2 | 2.5 ± 0.4 | 2.4 ± 0.3 |
| Hospital stay, days | 16.1 ± 1.1 | 15.5 ± 1.4 | 15.0 ± 1.1 |
| Postoperative outcome | |||
| Early death | 5 (2.1) | 2 (2.1) | 2 (3.4) |
| Low output syndrome | 2 | 0 | 0 |
| MODS⁎ | 2 | 1 | 2 |
| Serve infection | 1 | 1 | 0 |
| Installation of permanent pacemaker | 8 (3.3) | 3 (3.1) | 1 (1.7) |
| Reoperation due to TR⁎ in 5 years | 5 | 2 | 0 |
| Suture avulsion | 4 | 1 | / |
| Pacing wires | 1 | 1 | / |
The results of qualitative variables are expressed in absolute values (percentages) and the results of continuous variables are expressed as mean (SD).
⁎. TV = tricuspid valve; CABG = coronary artery bypass graft; CPB = cardiopulmonary bypass; ICU = intensive care unit; MODS = multiple organ dysfunction syndrome; TR = tricuspid regurgitation.
3.3. Efficiency of a different annuloplasty in treating FTR
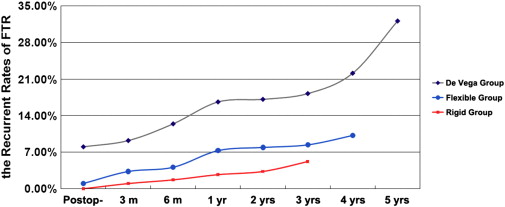
After surgery, the TR grade and mPAP of all patients significantly decreased (Table 3). According to the TTE results, at three and six months, and one and three years during the follow-up, 4.7% (18/383), 8.6% (33/382), 11.9% (45/378) and 13.7% (51/373) patients were found to have recurrent TR, respectively. During follow-up, the recurrent rate of the TR 3 + grade was always higher in the DV Group than the Flexible and Rigid Groups. At three years, there were 18.2% of patient graded as TR 3 + in the DV Group, which was much higher than those in the Flexible (8.4%, p < 0.05) or Rigid Groups (5.2%, p < 0.05). The recurrent rates were significantly lower in the Rigid Group than in the Flexible Group at six months, one year, and three years (Fig. 1).
| Variable | De Vega group (n = 242, %) | Flexible band group (n = 98, %) | Rigid ring group (n = 59, %) |
|---|---|---|---|
| TRa grade | |||
| Preop-a | 2.84 ± 1.1 | 2.70 ± 0.9 | 2.61 ± 0.7 |
| Postop-a | 0.71 ± 0.6b | 0.32 ± 0.1b ; c | 0.20 ± 0.1b ; c |
| mPAPa (mm Hg) | |||
| Preop- | 34.4 ± 10.3 | 35.7 ± 11.6 | 37.1 ± 12.1 |
| Postop- | 23.1 ± 9.1b | 25.2 ± 9.4b | 24.9 ± 10.6b |
| mRVSPa (mm Hg) | |||
| Preop- | 43.9 ± 11.0 | 45.7 ± 12.9 | 45.1 ± 19.0 |
| Postop- | 35.4 ± 13.8 | 36.3 ± 11.4 | 35.9 ± 12.7 |
a. TR = tricuspid regurgitation; Preop- = preoperative; Postop- = postoperative; mPAP = mean pulmonary arterial pressure; mRVSP = mean right ventricular systolic pressure.
b. Comparing with preoperative value: p < 0.01.
c. Comparing with De Vega Group: p < 0.05.
|
|
|
Fig. 1. The recurrent rates of tricuspid regurgitation after three different annuloplasty.
|
3.4. Risk factors for recurrence TR after tricuspid annuloplasty
Seven patients had a repeat operation to alleviate the recurrent TR. Four patients underwent TV replacement; in three patients the repair was successful. One patient died after the repeat operation.
Multivariate analysis revealed that atrial fibrillation, either a preoperative 3 + or 4 + grade of TR, LAD ≥ 60 mm, LVEF < 40%, De Vega annuloplasty, and a postoperative permanent pacemaker installation were independent risk factors for severe recurrent TR upon tricuspid repair (Table 4).
| Recurrent TR⁎ grade | p value | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| TR ≤ 2 + (n = 322) | TR ≥ 3 + (n = 51) | RR⁎ | 95% CI | p value | ||
| Age (year) | 45.4 ± 14.7 | 47.7 ± 16.4 | 0.68 | |||
| Female | 175 (54.3%) | 26 (50.9%) | 0.54 | |||
| Atrial fibrillation | 119 (27.0%) | 49 (96.1%) | 0.0001 | 9.4 | 2.3–65.0 | 0.001 |
| NYHA⁎ class IV | 74 (23.1%) | 15 (29.5%) | 0.43 | |||
| Rheumatic heart disease | 221 (68.6%) | 30 (58.8%) | 0.043 | |||
| Mitral stenosis | 49 (15.2%) | 11 (21.6%) | 0.31 | |||
| Mitral incompetence | 125 (38.8%) | 15 (29.4%) | 0.16 | |||
| Mitral mix lesions | 148 (54.0%) | 25 (49.0%) | 0.09 | |||
| LVEF⁎< 40% | 89 (27.7%) | 31 (60.1%) | 0.001 | 3.4 | 1.6–12.1 | 0.04 |
| Left atrial diameter ≥ 60 mm | 87 (27.0%) | 36 (70.6%) | 0.01 | 2.7 | 1.2–7.9 | 0.03 |
| mPAP⁎≥ 50 mm Hg | 94 (29.2%) | 18 (35.3%) | 0.05 | |||
| mRVSP⁎≥ 60 mm Hg | 97 (30.1%) | 18 (35.3%) | 0.06 | |||
| Preoperative TR grade ≥ 3 + | 207 (64.3%) | 47 (92.1%) | 0.001 | 3.6 | 1.7–8.7 | 0.002 |
| Tricuspid annulus diameter ≥ 45 mm | 140 (43.5%) | 28 (54.9%) | 0.04 | |||
| Mitral repair | 79 (24.5%) | 9 (17.6%) | 0.07 | |||
| De Vega annuloplasty | 202 (62.7%) | 40 (78.4%) | 0.001 | 7.2 | 2.7–15.4 | 0.002 |
| Tricuspid prosthetic ring size ≤ 28 mm | 102 (29.3%) | 7 (13.7%) | 0.32 | |||
| Installation of permanent pacemaker | 1 (0.3%) | 11 (21.5%) | 0.02 | 2.5 | 1.1–8.7 | 0.015 |
⁎. TR = tricuspid regurgitation; RR = risk ratio; NYHA = New York Heart Association; LVEF = left ventricular ejection fraction; mPAP = mean pulmonary arterial pressure; mRVSP = mean right ventricular systolic pressure.
4. Discussion
Our results showed that though rigid ring annuloplasty did not significantly decrease the incidence of adverse clinical outcomes, it was effective in improving the post-operative TV function in patients with FTR undergoing mitral surgery. Either the traditional or modified De Vega annuloplasty did not show a beneficial effect on long-term TV function. Furthermore, a preoperative 3 + or 4 + grade of TR, AF, LAD ≥ 60 mm, LVEF < 40%, De Vega annuloplasty, and postoperative permanent pacemaker installation were independent risk factors for severe recurrent TR upon tricuspid repair.
The most common etiologies of FTR are right ventricular (RV) dilation and dysfunction from LHD. As in cases of MV disease [23], mild FTR was reported in up to 74–86% of patients with left heart valve disease [12]. In our series, 62.9% patients had rheumatic MV pathology, which was similar to previous series [24]. MV disease leads to mitral stenosis, either regurgitation or mix lesion, causing an increase in left atrial pressure and, in some severe cases, secondary pulmonary hypertension. Increased afterload may lead to RV dysfunction and cardiomyopathy remodeling, resulting in either tricuspid annulus dilatation or tethering of the TV leaflets, leading to FTR [1]. By increasing left atrial size and pressure, MV disease might also cause AF, which gradually causes right atrial enlargement and leads to further tricuspid annular dilation. AF was recognized as an important risk factor for the development of TR in patients with MV disease as well as for either the occurrence or progression of TR after MV surgery [1]. Our results show that AF is a primary risk factor of recurrent moderate to severe TR. Furthermore, patients who had a concomitant successful Maze procedure during their MV surgery were reported to have significantly less TR at follow-up; however, patients with AF in our series did not routinely undergo Maze surgery.
FTR is primarily treated with a valve reconstruction that carries a much lower operative risk than valve replacement [15] ; [25]; however, there is still a heated debate regarding the superior method of repair (either suture based or a prosthetic ring annuloplasty). McCarthy [26] reported that both flexible bands and rigid planner rings had much less recurrent TR than the De Vega procedures. During our follow-up, it showed the similar results: at three years during follow-up, there were 18.2% of patient graded as TR 3 + in the DV Group, which was much higher than those in the Flexible (8.4%) or Rigid Groups (5.2%). Filsoufi [27] and De Bonis [8] found excellent reductions in TR severity early and mid-term after surgery with the MC3 ring. Ghoreishi [28] showed that the rigid three-dimensional ring could be the most reliable and durable treatment for FTR. This ring annuloplasty provides an early and sustained reduction of TR secondary to LVD, which has been reported by Navia [29]. In our series, the recurrent rates were significantly lower in the Rigid Group than in the Flexible Group postoperatively. However, though the MC3 ring was found to be superior to conventional techniques, about 14% of patients had TR graded greater than moderate one year after surgery: post-operative TR severity was associated with preoperative TR severity and extensive leaflet tethering [30]. And Pfannmuller [4] found that though both rigid and flexible systems could provide acceptable results, but that the use of a rigid ring may significantly increase the risk of early annular dehiscence. These results revealed that all of the annuloplasty methods did not permanently eliminate secondary TR. Thus, more studies are needed to explore the superior method of TV repair.
The incidence of a post-operative pacemaker implantation was reported from 3% to 6% after valve interventions [9]. A few studies have found that the need of pacemakers after a TV operation is higher than after other valve interventions [2]; [7] ; [31]; however, the need and clinical implications of pacemaker implantation after a TV operation are less documented. In our series, the rate of pacemaker implantation was 3% (much lower than other TV operation studies [31] ; [32]) and permanent pacemaker implantation was found to be an independent risk factor of recurrent TR during follow-up. One study showed that the survival of patients who needed a pacemaker after a TV operation was outstandingly higher than of those who did not [31]. More data about the general results and the need for a pacemaker after the TV operations are essential for further development of useful methods.
Our study is unique and summarizes the Chinese experience of treating FTR concomitant with mitral surgery over the past five years; however, we realize the potential limitations of our study. First, this is a retrospective cohort study containing different surgical techniques that were used in part because of the dissatisfaction with effectiveness, and surgeon preference. Secondly, the surgical preference may change in favor of rigid TV annuloplasty over the passage of time. This may cause some selection bias. Furthermore, a longer follow-up will be required to confirm our results.
5. Conclusions
Neither traditional nor modified De Vega annuloplasty did not show a beneficial effect on long-term TV function. Compared with a flexible system, MC3 rigid ring annuloplasty may provide better effectiveness in improving postoperative TV function in patients with FTR undergoing mitral surgery. Atrial fibrillation, a large left atrium, low LVEF and postoperative permanent pacemaker installation were independent risk factors for severe recurrent TR.
Conflict of interest
None.
Acknowledgments
None.
References
- [1] A. Shiran, A. Sagie; Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management; J Am Coll Cardiol, 53 (2009), pp. 401–408
- [2] M. Taramasso, H. Vanermen, F. Maisano, A. Guidotti, G. La Canna, O. Alfieri; The growing clinical importance of secondary tricuspid regurgitation; J Am Coll Cardiol, 59 (2012), pp. 703–710
- [3] M. Di Mauro, A. Bivona, A.L. Iaco, M. Contini, M. Gagliardi, E. Varone, et al.; Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation; Eur J Cardiothorac Surg, 35 (2009), pp. 635–639
- [4] B. Pfannmuller, M. Misfeld, M.A. Borger, C.D. Etz, A.K. Funkat, J. Garbade, et al.; Isolated reoperative minimally invasive tricuspid valve operations; Ann Thorac Surg, 94 (2012), pp. 2005–2010
- [5] J. Mascherbauer, G. Maurer; The forgotten valve: lessons to be learned in tricuspid regurgitation; Eur Heart J, 31 (2010), pp. 2841–2843
- [6] J.L. Navia, N.A. Brozzi, A.L. Klein, L.F. Ling, C. Kittayarak, E.R. Nowicki, et al.; Moderate tricuspid regurgitation with left-sided degenerative heart valve disease: to repair or not to repair?; Ann Thorac Surg, 93 (2012), pp. 59–67
- [7] D.J. LaPar, D.P. Mulloy, M.L. Stone, I.K. Crosby, C.L. Lau, I.L. Kron, et al.; Concomitant tricuspid valve operations affect outcomes after mitral operations: a multiinstitutional, statewide analysis; Ann Thorac Surg, 94 (2012), pp. 52–57
- [8] M. De Bonis, E. Lapenna, M. Taramasso, M. Manca, M.C. Calabrese, N. Buzzatti, et al.; Mid-term results of tricuspid annuloplasty with a three-dimensional remodelling ring; J Card Surg, 27 (2012), pp. 288–294
- [9] C.M. Vassileva, J. Shabosky, T. Boley, S. Markwell, S. Hazelrigg; Tricuspid valve surgery: the past 10 years from the nationwide inpatient sample (nis) database; J Thorac Cardiovasc Surg, 143 (2012), pp. 1043–1049
- [10] S. Fukuda, J.M. Song, A.M. Gillinov, P.M. McCarthy, M. Daimon, V. Kongsaerepong, et al.; Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty; Circulation, 111 (2005), pp. 975–979
- [11] A. Garatti, G. Nano, G. Bruschi, A. Canziani, T. Colombo, A. Frigiola, et al.; Twenty-five year outcomes of tricuspid valve replacement comparing mechanical and biologic prostheses; Ann Thorac Surg, 93 (2012), pp. 1146–1153
- [12] B. Mahesh, F. Wells, S. Nashef, S. Nair; Role of concomitant tricuspid surgery in moderate functional tricuspid regurgitation in patients undergoing left heart valve surgery; Eur J Cardiothorac Surg, 43 (2013), pp. 2–8
- [13] G. Vigano, A. Guidotti, M. Taramasso, A. Giacomini, O. Alfieri; Clinical mid-term results after tricuspid valve replacement; Interact Cardiovasc Thorac Surg, 10 (2010), pp. 709–713
- [14] F. Filsoufi, A.C. Anyanwu, S.P. Salzberg, T. Frankel, L.H. Cohn, D.H. Adams; Long-term outcomes of tricuspid valve replacement in the current era; Ann Thorac Surg, 80 (2005), pp. 845–850
- [15] T. Guenther, D. Mazzitelli, C. Noebauer, I. Hettich, P. Tassani-Prell, B. Voss, et al.; Tricuspid valve repair: is ring annuloplasty superior?; Eur J Cardiothorac Surg, 43 (2013), pp. 58–65
- [16] S. Ugaki, N.S. Khoo, D.B. Ross, I.M. Rebeyka, I. Adatia; Tricuspid valve repair improves early right ventricular and tricuspid valve remodeling in patients with hypoplastic left heart syndrome; J Thorac Cardiovasc Surg, 145 (2012), pp. 446–450
- [17] S.K. Ro, J.B. Kim, S.H. Jung, S.J. Choo, C.H. Chung, J.W. Lee; Mild-to-moderate functional tricuspid regurgitation in patients undergoing mitral valve surgery; J Thorac Cardiovasc Surg, 146 (2012), pp. 1092–1097
- [18] R.R. Desai, L.M. Vargas Abello, A.L. Klein, T.H. Marwick, R.A. Krasuski, Y. Ye, et al.; Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure; J Thorac Cardiovasc Surg, 146 (2013), pp. 1126–1132
- [19] S.W. Chen, F.C. Tsai, F.C. Tsai, Y.K. Chao, Y.K. Huang, J.J. Chu, et al.; Surgical risk and outcome of repair versus replacement for late tricuspid regurgitation in redo operation; Ann Thorac Surg, 93 (2012), pp. 770–775
- [20] R. Jeganathan, S. Armstrong, B. Al-Alao, T. David; The risk and outcomes of reoperative tricuspid valve surgery; Ann Thorac Surg, 95 (2013), pp. 119–124
- [21] G. Marquis-Gravel, D. Bouchard, L.P. Perrault, P. Page, H. Jeanmart, P. Demers, et al.; Retrospective cohort analysis of 926 tricuspid valve surgeries: clinical and hemodynamic outcomes with propensity score analysis; Am Heart J, 163 (2012), pp. 851–858
- [22] G.D. Dreyfus, P.J. Corbi, K.M. Chan, T. Bahrami; Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair?; Ann Thorac Surg, 79 (2005), pp. 127–132
- [23] N.S. Braunwald, J. Ross Jr., A.G. Morrow; Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement; Circulation, 35 (1967), pp. I63–I69
- [24] J.J. Kwak, Y.J. Kim, M.K. Kim, H.K. Kim, J.S. Park, K.H. Kim, et al.; Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations; Am Heart J, 155 (2008), pp. 732–737
- [25] G.H. Tang, T.E. David, S.K. Singh, M.D. Maganti, S. Armstrong, M.A. Borger; Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes; Circulation, 114 (2006), pp. I577–I581
- [26] P.M. McCarthy, S.K. Bhudia, J. Rajeswaran, K.J. Hoercher, B.W. Lytle, D.M. Cosgrove, et al.; Tricuspid valve repair: durability and risk factors for failure; J Thorac Cardiovasc Surg, 127 (2004), pp. 674–685
- [27] F. Filsoufi, S.P. Salzberg, M. Coutu, D.H. Adams; A three-dimensional ring annuloplasty for the treatment of tricuspid regurgitation; Ann Thorac Surg, 81 (2006), pp. 2273–2277
- [28] M. Ghoreishi, J.M. Brown, C.E. Stauffer, C.A. Young, M.J. Byron, B.P. Griffith, et al.; Undersized tricuspid annuloplasty rings optimally treat functional tricuspid regurgitation; Ann Thorac Surg, 92 (2011), pp. 89–95
- [29] J.L. Navia, E.R. Nowicki, E.H. Blackstone, N.A. Brozzi, D.E. Nento, F.A. Atik, et al.; Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure?; J Thorac Cardiovasc Surg, 139 (2010), pp. 1473–1482
- [30] S. Fukuda, A.M. Gillinov, P.M. McCarthy, Y. Matsumura, J.D. Thomas, T. Shiota; Echocardiographic follow-up of tricuspid annuloplasty with a new three-dimensional ring in patients with functional tricuspid regurgitation; J Am Soc Echocardiogr, 20 (2007), pp. 1236–1242
- [31] J.J. Jokinen, A.K. Turpeinen, O. Pitkanen, M.J. Hippelainen, J.E. Hartikainen; Pacemaker therapy after tricuspid valve operations: implications on mortality, morbidity, and quality of life; Ann Thorac Surg, 87 (2009), pp. 1806–1814
- [32] J.E. Molina, C.L. Roberts, D.G. Benditt; Long-term follow-up of permanent transvenous pacing systems preserved during tricuspid valve replacement; Ann Thorac Surg, 89 (2010), pp. 318–320
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?