Summary
Background/aims
Transcatheter arterial chemoembolization (TACE) is a main therapy for patients with intermediate-stage hepatocellular carcinoma (HCC). The purpose of our study was to determine the risk factors for 1-year mortality in patients treated solely with TACE.
Methods
A total of 123 patients with intermediate-stage HCC treated solely with TACE were recruited from Ren-ai Branch, Taipei City hospital during the period from January 1998 to June 2013. Baseline characteristics and factors associated with 1-year mortality were analyzed.
Results
There were 94 men (76.4%) and 29 women (23.6%) among 123 newly diagnosed intermediate-stage HCC patients treated solely with TACE. The mean age was 63 ± 11 years (range, 31–92 years). The 1–5-year overall cumulative survival rates were 65.9%, 46%, 33.2%, 22%, and 18.4% [median: 23 months, 95% confidence interval (CI): 16.4–29.6 months], respectively. Of these, 42 (34.1%) and 81 (65.9%) patients had survival time shorter (Group 1) and longer (Group 2) than 1 year, respectively. There were no significant differences in sex, age, hepatitis B virus/hepatitis C virus positive rate and tumor number between Group 1 and Group 2 patients. Compared to Group 2, Group 1 patients had a significantly larger mean maximum tumor size (6.8 ± 3.2 cm vs. 5.3 ± 3.1 cm, p = 0.024), lower serum albumin level (3.4 ± 0.45 g/dL vs. 3.6 ± 0.46 g/dL, p = 0.011), higher serum bilirubin level (1.52 ± 1.07 mg/dL vs. 1.07 ± 0.59 mg/dL, p = 0.023), higher ratio of serum alpha-fetoprotein (AFP) > 400 ng/mL (52.4% vs. 24.7%, p = 0.003), and higher ratio of Child-Turcotte-Pugh (CTP) class B cirrhosis (26.2% vs. 6.2%, p = 0.003). Multivariate analysis revealed that AFP level > 400 ng/mL [hazard ratio (HR): 2.663, 95% CI: 1.143–6.205, p = 0.023], CTP class B cirrhosis (HR: 4.69, 95% CI: 1.399–15.715, p = 0.012) and tumor size (HR: 1.153 for each 1 cm increase, 95% CI: 1.015–1.310, p = 0.029) were independently associated with 1-year mortality.
Conclusion
One-year mortality in patients with intermediate-stage HCC treated solely with TACE is not uncommon. High serum AFP level (> 400 ng/mL), CTP class B cirrhosis, and tumor size are independent risk factors for 1-year mortality in those patients.
Keywords
Alpha-fetoprotein ; Cirrhosis ; Intermediate-stage hepatocellular carcinoma ; Transcatheter arterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related deaths in the world [1] . Although periodic screening in a definable high risk population would benefit in detecting HCC in an early curable stage and yield a long-term survival [2] , the worldwide 5-year survival rate of HCC only slightly increased from 5% to 15% over the past 2 decades [3] . The major causes of unsatisfactory prognosis of HCC include that curative treatment is possible in only a small percentage of patients [4] and the heterogeneous nature of HCC affects treatment outcome. Cumulative evidence has suggested that the stage of HCC and efficacy of optimal treatment may be used to predict the survival rate at the time of HCC diagnosis [5] . Currently, the American Association for the Study of Liver Diseases (AASLD) guidelines, a revised version of the Barcelona Clinic Liver Cancer (BCLC) staging system by AASLD, are most widely used in HCC management [5] ; [6] . According to the guidelines, transcatheter arterial chemoembolization (TACE) has been recognized as an effective option for those with intermediate-stage HCC (BCLC stage B) [5] ; [6] ; [7] . TACE has been considered a palliative treatment for unresectable HCC. However, cumulative meta-analysis of all published randomized, controlled trials indicates that patient survival is significantly improved after TACE [8] . A nationwide, multicenter study from four medical centers in Taiwan reported that the 1-, 3-, and 5-year survival rates for unresectable HCC patients who underwent TACE alone were significantly higher than for those who underwent supportive treatment (60.2%, 39.3%, and 11.5% vs. 37.3%, 17.6%, and 2%, respectively) [9] . Nonetheless, the improvement in survival in treated patients is not satisfactory, and ranges from 20% to 60% at 2 years [10] . It is therefore important to clarify the risk factors that affect short-term mortality in HCC patients treated with TACE. The purpose of our study was to determine the risk factors for 1-year mortality in intermediate-stage HCC patients treated solely with TACE.
Materials and methods
Patients
A total of 123 newly diagnosed intermediate-stage HCC patients treated solely with TACE were retrospectively enrolled from Ren-ai Branch, Taipei City Hospital, Taiwan between January 1998 and June 2013. The severity of cirrhosis was assessed by the Child-Turcotte-Pugh (CTP) score [11] . The diagnosis of HCC was histologically confirmed in 74 (60.2%) patients. The remaining 49 (39.8%) patients were confirmed by typical imaging studies, such as enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Typical HCC exhibited arterial enhancement and portal venous washout on multiphasic CT or MRI [5] ; [6] . According to the BCLC staging system, intermediate-stage HCC was defined as CTP class A and B cirrhotic patients with large or multifocal HCC who do not have cancer related symptoms and do not have macrovascular invasion or extrahepatic spread [7] . This study protocol was approved by the Institutional Review Board of Taipei City Hospital.
Biochemical and serological testing
The biochemical tests were measured by using routine automated methods. The Hepatitis B surface antigen (HBsAg) and anti-hepatitis C virus (HCV) were assayed using commercial kits (General Biological HBsAg radioimmunoassay, General Biological Cooperation, Hsinchu, Taiwan. HCV enzyme immunoassay (EIA) II; Abbot Laboratories, North Chicago, IL, USA).
Procedure of TACE
TACE was performed in a dedicated angiography suite DFP8000D (Toshiba Medical Systems, Otawara, Japan) with 4Fr J-Curve catheters (Terumo Medical Corporation, Tokyo, Japan) inserted via right external femoral artery puncture, guided by a matching mandrel (Terumo Medical Corporation). The J-Curve catheter tip was placed in the celiac axis orifice and one set of digital subtraction angiography was performed with a bolus of 21 mL of nonionic iodinated contrast medium Iopamiro 370 (Bracco Imaging, Milan, Italy) injected by ProVis power injector (Medrad Inc., Warrendale, PA, USA) at 3mL/second. For patients with S5 or S6 tumors, which might draw their tumor vessels from superior mesenteric branches, digitally-subtracted superior mesenteric angiography was also performed using exactly the same parameters.
If the tumor stains and the supplying vessels were not well demonstrated by the above angiograms, further superselective angiograms were performed, including angiograms of common hepatic artery, proper hepatic artery, right or left hepatic arteries, and sometimes even smaller segmental branches. Only after identifying expected tumor stains and vessels was embolization performed from an appropriate site, which was usually a proper hepatic artery for multiple bilateral tumors, and right or left hepatic arteries for unilateral tumors. Occasionally embolization was performed more superselectively to avoid concomitant embolization of the right gastric or cystic arteries, as well as to minimize collateral damage in a patient with poor liver reserve. Superselection was usually performed with additional coaxial microcatheters and guide wires, usually ranging from 2.5Fr to 3Fr in the outer diameter and with malleable mandrel tips. The embolization agents were ethiodized oil Lipiodol (Guerbet, Villepinte, France) and absorbable gelatin sponge Surgifoam (Ethicon, Somerville, NJ, USA), and the chemotherapy agents were mitomycin and doxorubicin. The dosage of Lipiodol and gelatin sponge was based on sufficient slowing of arterial flow as demonstrated by fluoroscopy, typical dose range 3–15 mL of Lipiodol. Exact dose calculation of gelatin sponge was not possible as it is usually shredded from a larger piece. The dosages of mitomycin and doxorubicin were determined by patient weight; the typical dose of mitomycin was 4–8 mg and for doxorubicin was 20–30 mg. The presence of viable tumor was confirmed by multiphasic CT or MRI, 1–2 months after TACE. TACE was repeated with an interval of 3 months when viable tumor was found.
Statistical analysis
Data were analyzed by Chi-square test, Fishers exact test, Student t test, and logistic regression where appropriate. Overall survival was measured from the day of HCC diagnosis and patient survival was confirmed through June 2013. Cumulative survival was calculated according to the Kaplan-Meier method. The association of potential risk factors with 1-year mortality was determined by univariate and multivariate logistic regression. All tests were two-tailed and p < 0.05 was considered statistically significant.
Results
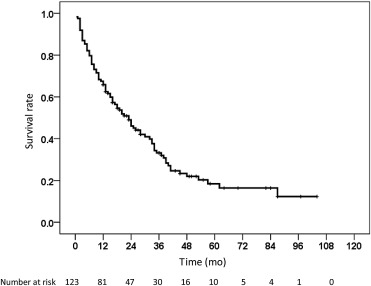
Baseline clinical characteristics of the 123 intermediate-stage HCC patients treated solely with TACE are shown in Table 1 . There were 94 men (76.4%) and 29 women (23.6%). The mean age was 63 ± 11 years (range: 31–92 years). Among them, 20 patients (16.3%) had solitary lesions, 27 patients (21.9%) had two tumors, and 76 patients (61.8%) had more than three tumors. The mean of maximum tumor size was 5.8 ± 3.2 cm (range: 1–16 cm). The dominant etiologies were hepatitis B virus (HBV) infection (63/123, 51.2%) and HCV infection (39/123, 37.1%). Regarding the liver function reserve, 107 patients (87%) were classified as CTP class A cirrhosis and 16 patients (13%) had CTP class B cirrhosis. Eighty-one and 42 patients (65.9% and 34.1%) had serum alpha-fetoprotein (AFP) levels ≤ 400 ng/mL and > 400 ng/mL, respectively. The mean follow-up duration was 22 (range: 1–104) months. The 1–5-year overall cumulative survival rates were 65.9%, 46%, 33.2%, 22%, and 18.4% [median: 23 months, 95% confidence interval (CI): 16.4–29.6 months], respectively (Fig. 1 ). The baseline characteristics of the patients stratified by 1-year survival are shown in Table 1 . There were 42 (34.1%) and 81 (65.9%) patients with survival time < 1 year (Group 1) and > 1 year (Group 2), respectively. There were no significant differences in terms of sex, age, prevalence of HBV/HCV infection, prevalence of diabetes mellitus, and tumor number between Group 1 and Group 2 patients. Compared to Group 2 patients, Group 1 patients had a significantly larger mean of maximum tumor size (6.8 ± 3.2 cm vs. 5.3 ± 3.1 cm, p = 0.024), lower serum albumin level (3.4 ± 0.45 g/dL vs. 3.6 ± 0.46 g/dL, p = 0.011), higher serum total bilirubin level (1.52 ± 1.07 mg/dL vs. 1.07 ± 0.59 mg/dL, p = 0.023), higher ratio of AFP level > 400 ng/mL (52.4% vs. 24.7%, p = 0.003), and higher ratio of CTP class B cirrhosis (26.2% vs. 6.2%, p = 0.003). The causes of death of Group 1 patients included uncontrolled HCC (25 patients), hepatic failure (15 patients), and esophageal variceal bleeding (2 patients).
| Characteristics | Group 1a (n = 42) | Group 2b (n = 81) | p |
|---|---|---|---|
| Sex | |||
| Male | 29 (69) | 65 (80.2) | 0.184 |
| Female | 13 (31) | 16 (19.8) | |
| Mean age (y) | 61.6 ± 12.1 | 64 ± 10.9 | 0.272 |
| ≤ 60 | 18 (42.9) | 30 (37) | 0.563 |
| > 60 | 24 (57.1) | 51 (63) | |
| Etiology | |||
| HBV infection | 26 (61.9) | 37 (45.7) | 0.302c |
| HCV infection | 12 (28.6) | 27 (33.3) | |
| HBV/HCV coinfection | 0 | 5 (6.2) | |
| Missing data | 4 (9.5) | 12 (14.8) | |
| Child-Turcotte-Pugh class | |||
| A | 31 (73.8) | 76 (93.8) | 0.003 |
| B | 11 (26.2) | 5 (6.2) | |
| Laboratory data | |||
| Albumin (g/dL) | 3.4 ± 0.45 | 3.6 ± 0.46 | 0.011 |
| Total bilirubin (mg/dL) | 1.52 ± 1.07 | 1.07 ± 0.59 | 0.023 |
| ALT (U/L) | 94 ± 136 | 77 ± 58 | 0.312 |
| PT (s) | 12.9 ± 1.5 | 12.4 ± 1.8 | 0.213 |
| AFP (ng/mL) | |||
| ≤ 400 | 20 (47.6) | 61 (75.3) | 0.003 |
| > 400 | 22 (52.4) | 20 (24.7) | |
| Maximum tumor size in cm (range) | 6.8 ± 3.2 (2–15) | 5.3 ± 3.1 (1.0–16.0) | 0.024 |
| Number of tumors | |||
| 1 | 11 (26.2) | 9 (11.1) | 0.104 |
| 2–3 | 15 (35.7) | 35 (43.2) | |
| > 3 | 16 (38.1) | 37 (45.7) | |
| DM | |||
| Yes | 12 (28.6) | 27 (33.3) | 0.718 |
| No | 30 (71.4) | 54 (66.7) | |
Data are presented as n (%) or mean ± SD, unless otherwise indicated.
AFP = alpha-fetoprotein; ALT = alanine aminotransferase; DM = diabetes mellitus; HBV = hepatitis B virus; HCV = hepatitis C virus; PT = prothrombin time.
a. Group 1: patients with 1-year mortality.
b. Group 2: patients with survival time longer than 1 year.
c. HBV infection vs. HCV infection.
|
|
|
Figure 1. The cumulative survival curve of 123 intermediate-stage hepatocellular carcinoma patients treated solely with transcatheter arterial chemoembolization. |
Results of logistic regression for risk factors against 1-year mortality are shown in Table 2 . AFP level > 400 ng/mL [hazard ratio (HR):3.355, 95% CI:1.525–7.381, p = 0.003], CTP class B cirrhosis (HR:5.394, 95% CI:1.731–16.808, p = 0.004), and tumor size (HR:1.145 for each 1 cm increase, 95% CI:1.015–1.291, p = 0.028) were significantly associated with 1-year mortality in univariate logistic regression analysis. However, only AFP level > 400 ng/mL (HR: 2.663, 95% CI:1.143–6.205, p = 0.023), CTP class B cirrhosis (HR: 4.69, 95% CI:1.399–15.715, p = 0.012), and tumor size (HR: 1.153 for each 1 cm increase, 95% CI:1.015–1.310, p = 0.029) were independently associated with 1-year mortality in the subsequent multivariate logistic regression analysis.
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Sex | 0.168 | |||
| Male | 1 | |||
| Female | 1.821 (0.776–4.272) | |||
| Age | 0.531 | |||
| ≤ 60 y | 1 | |||
| > 60 y | 0.784 (0.367–1.676) | |||
| AFP | 0.003 | 0.023 | ||
| ≤ 400 | 1 | 1 | ||
| > 400 | 3.355 (1.525–7.381) | 2.663 (1.143–6.205) | ||
| Etiology | 0.288 | |||
| B | 1 | |||
| C | 0.632 (0.272–1.476) | |||
| Child-Turcotte-Pugh | 0.004 | 0.012 | ||
| A | 1 | 1 | ||
| B | 5.394 (1.731–16.808) | 4.69 (1.399–15.715) | ||
| Tumor size | 0.028 | 0.029 | ||
| Increase 1 cm | 1.145 (1.015–1.291) | 1.153 (1.015–1.310) | ||
AFP = alpha-fetoprotein; CI = confidence interval.
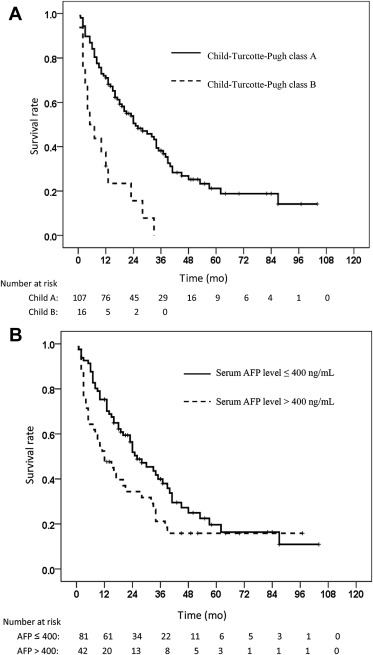
A comparison of the long-term survival of patients stratified by CTP classification and serum AFP levels is shown in Fig. 2 . Patients with CTP class A cirrhosis had significantly higher 1-, 2-, and 3-year cumulative survival rates compared with those who had CTP class B cirrhosis (71%, 50.0%, 38.2% vs. 31.2%, 15.6%, 0%, respectively, p < 0.001). Similarly, patients with serum AFP levels ≤ 400 ng/mL had significantly higher 1-, 2-, and 3-year cumulative survival rates than those with serum AFP levels > 400 ng/mL (75.3%, 51.9%, 39.9% vs. 47.6%, 34.4%, 21.2%, respectively, p = 0.039).
|
|
|
Figure 2. Comparison of cumulative long-term survival curves of intermediate-stage hepatocellular carcinoma patients treated solely with transcatheter arterial chemoembolization according to (A) Child-Turcotte-Pugh cirrhosis and (B) serum alpha-fetoprotein (AFP) levels. |
Discussion
The major reasons for poor prognosis of HCC include the heterogeneous nature of HCC and the difficulty in making the most appropriate treatment planning decision for an individual patient. Therefore, staging-related treatment planning is important for predicting prognosis and guiding the therapeutic approach. In addition to BCLC [12] , current HCC staging systems include the Okuda system [13] , the Cancer of the Liver Italian Program (CLIP) score [14] , the Japan Integrated Scoring system [15] , and the tumor-node-metastasis (TNM) staging system [16] . Among these, the BCLC staging system and recommended treatment strategy (AASLD guideline), which is linked to the underlying liver function and the extension of the tumor, is the most widely used in the management of HCC [5] ; [6] . According to AASLD guidelines, patients with intermediate-stage HCC are asymptomatic with multinodular tumors, but without vascular invasion or extrahepatic metastasis, and are eligible for TACE.
TACE is the most widely used palliative treatment for patients with unresectable HCC [17] . A previous meta-analysis, including seven randomized trials and 545 patients, revealed that TACE improved the 2-year survival of unresectable HCC patients compared with supportive treatment. However, the 1-year survival varies from 24% to 82% [18] . Therefore, the selection of ideal candidates for TACE should improve the long-term survival effect of the treatment. In our retrospective study, records of 123 intermediate-stage HCC patients treated solely with TACE were analyzed and assessed with survival as a primary endpoint. Our study showed that the 1-year mortality still remains at a disappointing 34.1%. Importantly, CTP class B cirrhosis was associated with 1-year mortality. Further long-term survival analysis revealed that patients with CTP class A cirrhosis had a significantly higher cumulative survival rate than those with CTP class B cirrhosis. CTP classification was already a known independent prognostic factor for HCC [19] ; [20] ; [21] . In addition, CTP classification has been incorporated to current prognostic staging systems (CLIP and BCLC staging systems) [12] ; [14] . According to the BCLC staging system, intermediate-stage HCC includes both CTP class A and B patients; the discriminating survival of patients in intermediate-stage HCC is expected. Our results indicated that individual HCC patients in a single BCLC stage have inconsistent responses to specific therapy.
The prognostic role of baseline AFP values for HCC patients has been confirmed in multiple studies and incorporated in the CLIP system [14] ; [22] . Furthermore, serum AFP level is associated with poor tumor biological behavior of HCC, such as tumor multiplicity, low grade differentiation and carcinoma cell embolus, as well as moderate/severe cirrhosis [23] . Recent studies of patients with BCLC stage A and B HCC revealed that serum AFP level was an independent predictor of worse survival [24] ; [25] . Consistent with previous studies, we found that the serum AFP level was significantly associated with 1-year mortality of intermediate-stage HCC patients treated solely with TACE. Taken together, incorporation of AFP level to current HCC staging systems in evaluating prognosis should be considered in clinical practice.
The treatment effects of TACE in unresectable HCC include local tumor control and prolonged survival [26] . However, TACE only allows complete local tumor control of 25–35% [27] . Furthermore, TACE may induce hepatic function deterioration especially in cirrhotic patients and lead to an increased incidence of liver failure [28] ; [29] . For this reason, uncontrolled HCC and hepatic failure were possibly the causes of death in two-thirds of our patients.
There are several limitations of this study. First, as a retrospective study design, data collection depends on the availability and accuracy of the medical record. Thus, the existence of patient selection bias should be considered. In addition, the frequency of TACE administration for individual patients varies in our study, which was influenced by tumor progression and the severity of cirrhosis during the follow-up period. Therefore, large cohort studies are needed to further evaluate the optimum TACE treatment strategy.
In conclusion, the treatment strategies of HCC are well developed during the past 2 decades. Based on the BCLC staging system, TACE is the recommended treatment for patients with intermediate-stage HCC. Unfortunately, the long-term effects of TACE still remain unsatisfactory. The 1-year mortality in patients with intermediate-stage HCC treated solely with TACE is common. High serum AFP level (> 400 ng/mL) and CTP class B cirrhosis are independent risk factors for 1-year mortality in those patients. Careful patient selection is the key point to improve the early and long-term results of TACE in patients with intermediate-stage HCC.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
The study was supported by grants from Taipei City Hospital (96001-62-009 ).
References
- [1] J.H. Kao, D.S. Chen; Changing disease burden of hepatocellular carcinoma in the Far East and Southeast Asia; Liver Int, 25 (2005), pp. 696–703
- [2] B.H. Zhang, B.H. Yang, Z.Y. Tang; Randomized controlled trial of screening for hepatocellular carcinoma; J Cancer Res Clin Oncol, 130 (2004), pp. 417–421
- [3] American Cancer Society; Cancer facts & figures 2012; American Cancer Society, Atlanta (2012)
- [4] F. Farinati, A. Sergio, A. Baldan, A. Giacomin, M.A. Di Nolfo, P. Del Poggio, et al.; Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study; BMC Cancer, 9 (2009), pp. 33–45
- [5] J. Bruix, M. Sherman; Management of hepatocellular carcinoma; Hepatology, 42 (2005), pp. 1208–1236
- [6] J. Bruix, M. Sherman; Management of hepatocellular carcinoma: an update; Hepatology, 53 (2011), pp. 1020–1022
- [7] A. Forner, M.E. Reig, C.R. de Lope, J. Bruix; Current strategy for staging and treatment: the BCLC update and future prospects; Semin Liver Dis, 30 (2010), pp. 61–74
- [8] J.M. Llovet, J. Bruix; Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival; Hepatology, 37 (2003), pp. 429–442
- [9] Y.H. Huang, C.H. Chen, T.T. Chang, S.C. Chen, J.H. Chiang, H.S. Lee, et al.; The role of transcatheter arterial embolization for patients with unresectable hepatocellular carcinoma: a nationwide, multicentre study evaluated by cancer stage; Aliment Pharmacol Ther, 21 (2005), pp. 687–694
- [10] R. Lencioni, X.P. Chen, L. Dagher, A.P. Venook; Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved?; Oncologist, 15 (Suppl. 4) (2010), pp. 42–52
- [11] R.N.H. Pugh, I.M. Muray-Lyon, J.L. Dawson, M.C. Pietroni, R. Williams; Transection of the esophagus in the bleeding esophageal varices; Br J Surg, 60 (1973), pp. 648–652
- [12] J.M. Llovet, C. Brù, J. Bruix; Prognosis of hepatocellular carcinoma: the BCLC-staging classification; Semin Liver Dis, 19 (1999), pp. 329–338
- [13] K. Okuda, T. Ohtsuki, H. Obata, M. Tomimatsu, N. Okazaki, H. Hasegawa, et al.; Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients; Cancer, 56 (1985), pp. 918–928
- [14] The Cancer of Liver Italian Program (CLIP) Investigators; A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients; Hepatology, 28 (1998), pp. 751–755
- [15] M. Kudo, H. Chung, Y. Osaki; Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score); J Gastroenterol, 38 (2003), pp. 207–215
- [16] F.L. Greene, D.L. Page, I.D. Fleming (Eds.), et al. , AJCC cancer staging manual (6th ed), Springer, New York (2002)
- [17] P.M. Lopez, A. Villanueva, J.M. Llovet; Systematic review: evidence-based management of hepatocellular carcinoma-an updated analysis of randomized controlled trials; Aliment Pharmacol Ther, 23 (2006), pp. 1535–1547
- [18] J.M. Llovet, J. Bruix, Barcelona Clinic Liver Cancer Group; Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival; Hepatology, 37 (2003), pp. 429–442
- [19] Y. Yamashita, M. Takahashi, Y. Koga, R. Saito, S. Nanakawa, Y. Hatanaka, et al.; Prognostic factor in the treatment of hepatocellular carcinoma with transcatheter arterial embolization and arterial infusion; Cancer, 67 (1991), pp. 385–391
- [20] L. Mondazzi, R. Bottelli, G. Brambilla, A. Rampoldi, I. Rezakovic, C. Zavaglia, et al.; Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors; Hepatology, 19 (1994), pp. 1115–1123
- [21] R. Lencioni, C. Bartolozzi, D. Caramella, A. Paolicchi, M. Carrai, G. Maltinti, et al.; Treatment of small hepatocellular carcinoma with percutaneous ethanol injection; Cancer, 76 (1995), pp. 1737–1746
- [22] S. Chevret, J.C. Trinchet, D. Mathieu, A.A. Rached, M. Beaugrand, C. Chastang; A new prognostic classification for predicting survival in patients with hepatocellular carcinoma: Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire; J Hepatol, 31 (1999), pp. 133–141
- [23] J. Zhou, T. Yan, X. Bi, H. Zhao, Z. Huang, Y. Zhang, et al.; Evaluation of seven different staging systems for alpha-fetoprotein expression in hepatocellular carcinoma after hepatectomy; Tumour Biol, 34 (2013), pp. 1061–1070
- [24] R. Santambrogio, E. Opocher, M. Costa, M. Barabino, M. Zuin, E. Bertolini, et al.; Hepatic resection for “BCLC stage A” hepatocellular carcinoma. The prognostic role of alpha-fetoprotein; Ann Surg Oncol, 19 (2012), pp. 426–434
- [25] J.H. Zhong, B.D. Xiang, W.F. Gong, Y. Ke, Q.G. Mo, L. Ma, et al.; Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization; PLoS One, 8 (2013), p. e68193
- [26] T.J. Vogl, N.N. Naguib, N.E. Nour-Eldin, P. Rao, A.H. Emami, S. Zangos, et al.; Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications; Eur J Radiol, 72 (2009), pp. 505–516
- [27] M. Biolato, G. Marrone, S. Racco, C. Di Stasi, L. Miele, G. Gasbarrini, et al.; Transarterial chemoembolization (TACE) for unresectable HCC: a new life begins?; Eur Rev Med Pharmacol Sci, 14 (2010), pp. 356–362
- [28] Y.S. Huang, J.H. Chiang, J.C. Wu, F.Y. Chang, S.D. Lee; Risk of hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma: predictive value of the monoethylglycinexylidide test; Am J Gastroenterol, 97 (2002), pp. 1223–1227
- [29] Y.W. Min, J. Kim, S. Kim, Y.K. Sung, J.H. Lee, G.Y. Gwak, et al.; Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma; Liver Int, 33 (2013), pp. 197–202
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?