Summary
Background
Pyogenic granuloma (PG) is a polypoid form of capillary hemangioma. This study aimed to analyze the clinical and endoscopic features of gastric PG.
Methods
We retrospectively reviewed nine patients with gastric PGs who were evaluated by esophagogastroduodenoscopy and diagnosed by pathological study at the Chang Gung Medical Center (Taoyuan, Taiwan) between 2000 and 2009. Demographic data, clinical presentations, endoscopic findings, treatment, and outcome were collected and analyzed.
Results
The median age of the study patients was 62 years (range, 40–73 years) with a female preponderance. The most common symptom at presentation was overt gastrointestinal bleeding, followed by anemia and epigastralgia. Two patients were asymptomatic at diagnosis. The most common underlying diseases were liver cirrhosis [5 (56%) patients] and hypertension [5 (56%) patients]. Five (56%) cases of gastric PGs originated at the site of prior ulcer lesions. Most gastric PGs were solitary [7 (78%) patients] and located in the antrum [8 (89%) patients]. The gastric PGs typically appeared morphologically as smooth protruding hyperemic lesions with adherent white or yellow deposits. One patient received an endoscopic mucosal resection with complete excision of the lesion. Another patient received surgical intervention. Four gastric PG lesions were stationary or regressed with conservative management.
Conclusion
Overt gastrointestinal bleeding was the most common clinical presentation in patients with gastric PG. Gastric ulcers were the most common precursors of PG with the antrum being the most frequent site involved. Gastric PGs were characteristically protruding hyperemic lesions with adherent exudates. Conservative treatment may be considered for asymptomatic PG patients with major comorbidities. Endoscopic resection may be offered to patients with symptoms.
Keywords
Endoscopy ; Pyogenic granuloma ; Stomach
Introduction
Pyogenic granuloma (PG) is an inflammatory vascular lesion that is generally a red polypoid mass of apparent granulation tissue that bleeds easily [1] . The name PG is misleading because it is not infectious, it does not form pus, and it is not granulomatous. It is actually a polypoid form of capillary hemangioma [2] , which explains its tendency to bleed. The most commonly affected sites are the extremities and the oral cavity. Pyogenic granuloma is extremely rare in the alimentary tract, except in the oral cavity [3] . To date, only five case reports of gastric PG have been published [3] ; [4] ; [5] ; [6] ; [7] . The aim of the study is to evaluate the clinical presentations, endoscopic findings, treatment, and outcomes of gastric PG in our case series.
Methods
A retrospective chart review study was conducted that targeted patients diagnosed with gastric PG at the Chang Gung Medical Center (Taoyuan, Taiwan). We identified nine patients who were diagnosed as having gastric PG between January 2000 and September 2009.
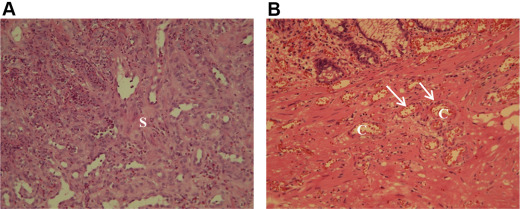
Pyogenic granuloma was diagnosed based on the histological identification of a lobular arrangement of multiple capillaries lined by endothelial cells, and edematous stroma with inflammatory cell infiltration and fibroblast proliferation in routine hematoxylin and eosin (HE)-stained sections of formalin-fixed, paraffin-embedded materials (Fig. 1 ) [4] ; [7] ; [8] ; [9] . The specimens in all patients were acquired by biopsy forceps from areas of polypoid lesions or ulcers.
|
|
|
Figure 1. (A) Edematous collagenous stroma (s ) with mixed inflammatory cell infiltration and fibroblast proliferation form the granulation tissue. The infiltrate is typically predominantly neutrophilic near the ulcerated surface of the lesion and chronic inflammatory mononucleated cells are scattered in the deeper zones. (B) The lobular arrangement of capillaries (c ), which are lined by a single layer of flattened or round endothelial cells (arrows) (hematoxylin and eosin stain; magnification, medium power). |
The clinical parameters were collected from the medical records and included age, sex, initial presentation, underlying disease, medications, prior lesions at the same site, Helicobacter pylori (HP) infection, treatment, and outcome. The endoscopic characteristics of gastric PG were collected such as the indications for endoscopy, and the location, size, number and gross appearance of lesions. Six of the nine patients underwent follow-up esophagogastroduodenoscopy (EGD). Data on the follow-up duration, the findings, and changes in the lesion size were recorded.
Results
The median age of the study patients was 62 years (range, 40–73 years). Six patients were women and three patients were men. The initial presentations included gastrointestinal (GI) bleeding in four patients; anemia in two patients; and epigastralgia in one patients. Two patients had no symptoms at diagnosis. Among the nine patients, five (55.56%) patients had liver cirrhosis, which included two patients with hepatitis B virus infection, two patients with hepatitis C virus infection, and one patient with primary biliary cirrhosis. Five (55.56%) patients had hypertension, three (33.33%) patients had diabetes mellitus, and two (22.22%) patients had hepatocellular carcinoma. With regard to the medication history, seven (77.78%) patients had used proton pump inhibitors (PPIs) prior to the diagnosis of PG and four (44.44%) patients had used nonsteroidal anti-inflammatory drugs (NSAIDs). Prior lesions at the PG site were gastric ulcer (GU) in five (55.56%) patients and subepithelial lesion in two patients. Endoscopic ultrasonography was performed on one subepithelial lesion, which presented as a well-defined inhomogeneous hypoechoic tumor arising from the second layer of the mucosa. Endoscopic ultrasonography was not performed on one lesion because of the patients unstable clinical condition. Two patients had not undergone EGD prior to the diagnosis of gastric PG. The median duration between the GU and the diagnosis of PG was 3 months. At the time of the diagnosis of PG, five patients had an HP infection, which was confirmed by pathological examination (Table 1 ; Table 2 ).
| Case | Age, sex | Initial presentation | Underlying disease | Medication history | Prior lesion (time prior to PG Dx) | HP infection | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 y F | GI bleeding | HTN Hypothyroidism | PPI NSAID | GU (3 mo) | P | Gastrectomy | Fatality |
| 2 | 62 y F | GI bleeding | Liver cirrhosis, HCV End-stage renal disease Coronary artery disease | PPI | SEL (40 mo) | N | Lost to f/u | — |
| 3 | 55 y M | GI bleeding | Liver cirrhosis, alcohol + HBV HTN DM HCC | PPI | GU (6 mo) | P | EGD f/u (30 mo) | Stationary |
| 4 | 59 y F | Asymptomatic | Liver cirrhosis, PBC DM | PPI | GU (5 mo) | P | EGD f/u (4 mo) | Regressive |
| 5 | 69 y F | GI bleeding | Liver cirrhosis, HCV HCC | PPI | GU (2 mo) | P | Lost f/u | — |
| 6 | 68 y M | Asymptomatic | Liver cirrhosis, HBV HTN HCC | PPI NSAID | GU (2.5 mo) | P | EGD f/u (10 mo) | Regressive |
| 7 | 73 y F | Epigastralgia | HTN Rectal cancer status post operation | PPI NSAID | SEL (1 mo) | N | EGD f/u (4 mo) | Regressive |
| 8 | 62 y F | Anemia | HTN Autoimmune thyroid disease | NSAID | Unknown | N | Endoscopic resection | Cure |
| 9 | 69 y M | Anemia | DM Large B cell lymphoma (bone marrow) | — | Unknown | N | Lost to f/u | — |
DM = diabetes mellitus; Dx = diagnosis; EGD = esophagogastroduodenoscopy; F = female; f/u = follow-up; GI = gastrointestinal; GU = gastric ulcer; HBV = hepatitis B virus; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; HP = Helicobacter pylori ; HTN = hypertension; M = male; mo = month; N = negative; NSAID = nonsteroidal anti-inflammatory drug; P = positive; PBC = primary biliary cirrhosis; PG = pyogenic granuloma; PPI = proton pump inhibitor; SEL = subepithelial lesion; y = years.
| Age (y) | 40–73 (median, 62) |
| Sex (male/female) | 3/6 |
| Initial presentation | |
| Gastrointestinal bleeding | 4 (44.44) |
| Epigastralgia | 3 (33.33) |
| Anemia | 2 (22.22) |
| No symptoms | 2 (22.22) |
| Underlying disease | |
| Liver cirrhosis | 5 (55.56) |
| Etiology of liver cirrhosis (HBV/HCV/PBC) | 2/2/1 |
| Hypertension | 5 (55.56) |
| Diabetes mellitus | 3 (33.33) |
| Hepatocellular carcinoma | 2 (22.22) |
| End-stage renal disease | 1 (11.11) |
| Hypothyroidism | 1 (11.11) |
| Coronary artery disease | 1 (11.11) |
| Autoimmune thyroid disease | 1 (11.11) |
| Bone marrow large B cell lymphoma | 1 (11.11) |
| Rectal cancer status post operation | 1 (11.11) |
| Medication history | |
| Proton pump inhibitor | 7 (77.78) |
| Nonsteroidal anti-inflammatory drug | 4 (44.44) |
| Prior lesion on the same site | |
| Gastric ulcer | 5(55.56) |
| Median time to the diagnosis of PG | 3 mo (2–6 mo) |
| Subepithelial lesion | 2 (22.22) |
| Helicobacter pylori infection at time of the diagnosis | 5 (55.56) |
With the exception of age and sex, the data are presented as n (%).
HBV = hepatitis B virus; HCV = hepatitis C virus; PBC = primary biliary cirrhosis.
The indications for endoscopy were bleeding survey in four patients, follow up for GU in two patients, anemia in two patients, and abdominal pain in one patient. The PGs were located in the antral area of the stomach in eight patients and in the body of the stomach in one patient. The median size of lesions was 2.5 cm (range, 0.9–3 cm). They were solitary lesions in seven patients, two lesions in one patient, and multiple (i.e., >3) lesions in one patient. All lesions were of the protruding type with pedunculated lesions in two patients and semipedunculated lesions or sessile lesions in seven patients. The lesions appeared hyperemic in six patients and pink in three patients. The surface of the lesions was smooth in five patients, erosive in two patients, and ulcerated in two patients. Seven patients had adherent white or yellow deposits on the lesions (Table 3 ).
| Case | Indication | Site | Size (cm) | No. of lesions | Gross appearance | Follow-up duration | Finding | Change in size (cm) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology | Color | Surface | Adherent white or yellow deposits | ||||||||
| 1 | Bleeding survey | Antrum | 3 | Single | Semipedunculated Lobulated | Pink | Smooth | P | 2 mo | Stationary | 3 → 3 |
| 2 | Bleeding survey | Antrum | 3 | Single | Semipedunculated Lobulated | Hyperemic | Ulcerated | P | Lost f/u | ||
| 3 | Bleeding survey | Antrum | 2.5 | Single | Semipedunculated Polypoid | Pink | Smooth | N | 26 mo | Stationary | 2.5 → 2.5 |
| 4 | GU f/u | Antrum | 0.9 and 3 | Two | Sessile Polypoid | Hyperemic | Erosive | P | 5 mo. | Regressive | 0.9 → 0.3 3 → 0.5 |
| 5 | Bleeding survey | Antrum | 2.5 | Single | Sessile Lobulated | Hyperemic | Smooth | P | Lost to f/u | ||
| 6 | GU f/u | Antrum | 2 | Single | Sessile Lobulated | Hyperemic | Erosive | P | 10 mo | Regressive | 2 → 0.3 |
| 7 | Abdominal pain survey | Antrum | 2.5 | Single | Sessile Polypoid | Pink | Ulcerated | N | 4 mo | Regressive | 2.5 → 1 |
| 8 | Anemia survey | Body | 1–3 | Multiple | Pedunculated Polypoid | Hyperemic | Smooth | P | 4 mo | Cure | 1–3 → 0 |
| 9 | Anemia survey | Antrum | 2.5 | Single | Pedunculated Polypoid | Hyperemic | Ulcerated | P | Lost to f/u | ||
f/u = follow-up; GU = gastric ulcer; N = negative; P = positive.
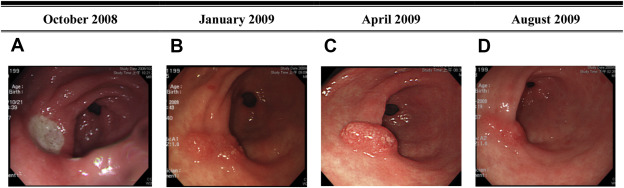
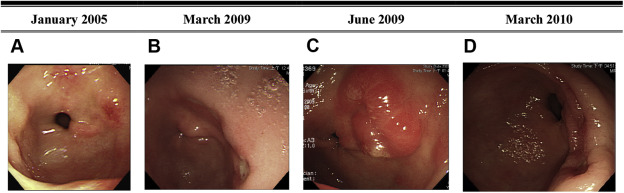
Six patients had follow-up EGD after diagnosis. The median follow-up duration was 4 months (range, 2–26 months). One patient who had underlying hypertension and hypothyroidism underwent gastrectomy because of intractable epigastric pain and GI bleeding. However, she eventually died because of septic shock after surgery. One patient received endoscopic resection of the lesion without recurrence. The other four patients took a histamine 2 receptor antagonist or proton pump inhibitor for >3 months after the index endoscopy. One lesion was stationary in size and three lesions regressed (Figure 2 ; Figure 3 ).
|
|
|
Figure 2. Case 4. A 59-year-old woman had GI bleeding. The EGD showed gastric ulcers in October 2008. At a regular follow-up EGD, PGs were diagnosed by pathological study in April 2009. Sequential endoscopic findings between October 2008 and August 2009 are shown. (A) One 2-cm active ulcer is present on the anterior wall of the antrum in October 2008. (B) Three months later, the ulcer has healed and a mild mucosal elevation with an irregularly polypoid surface is at the same site. (C) Five months later, the PG is at the same site of the prior ulcer. (D) The PG subsequently regressed 5 months later. EGD = esophagogastroduodenoscopy; GI = gastrointestinal; PG = pyogenic granuloma. |
|
|
|
Figure 3. Case 6. A 68-year-old man underwent regular follow-up EGDs for GU since January 2005. In June 2009, he was diagnosed by pathology as having PG. Sequential endoscopic findings between January 2005 and March 2010 are shown. (A) In January 2005, one tiny erosion was present on the posterior wall of the antrum. (B) In March 2009, two 1-cm active ulcers were present in the posterior wall of the antrum. (C) Three months later, one 2-cm hyperemic sessile lesion appeared at the same site of the prior ulcer. Endoscopic biopsy proved it was PG. (D) Nine months later, the PG had finally regressed. EGD = esophagogastroduodenoscopy; GU = gastric ulcer; PG = pyogenic granuloma. |
Discussion
In 1897, the cutaneous form of PG (i.e., “human botryomycosis”) was initially described by two French surgeons, [30] . However, in 1904, Hartzell coined the term “pyogenic granuloma” [3] . In 1967, Payson et al. [31] and associates published the first well-documented case report of the GI tract PG [4] . To our knowledge, approximately 65 cases of PG in the alimentary tract (other than in the oral mucosa) have been published in the literature. The reports have described 19 cases of PG in the esophagus [10] ; [11] ; [12] ; [13] ; [14] ; five cases in the stomach; 20 cases in the small intestine [10] ; [11] ; [14] ; [15] ; [16] ; [17] ; [18] ; 19 cases in the colon [10] ; [19] ; [20] ; [21] ; [22] ; [23] ; [24] ; [25] ; one case in an unidentified area of the alimentary tract [26] ; and one case in the common bile duct [1] . The patients' ages ranged from 1.5 years to 82 years (mean age, 49.97 years) and 56.36% of the patients were male. In our case series and in five other previously published reports on gastric PG (Table 4 ), the lesions occurred in middle-aged people and more frequently in female patients than in male patients with a female:male ratio of approximately 8:6.
| Year | Author | Age, sex | Clinical presentation | Underlying disease | Treatment | Site | Size (cm) | No. of lesions | Gross appearance | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology | Color | Surface | Adherent white or yellow deposits | |||||||||
| 1996 | Kogawa et al [6] | 50 y M | RUQ pain | Unknown | Endoscopic resection | Unknown | 1.8 | Solitary | NA | NA | NA | NA |
| 2005 | Kusakabe et al [3] | 82 y M | Melena | Nil | Embolotherapy + endoscopic resection | Fundus | 3 | Solitary | Semipedunculated | NA | Irregular | NA |
| 2007 | Antonio Quiros et al [4] | 67 y F | Anemia | Unknown | Endoscopic resection | Antrum | 1 | Solitary | Sessile Polypoid | Hyperemic | Ulcerated | P |
| 2009 | Malhotra et al [5] | 40 y F | Anemia | Nil | Endoscopic resection | Antrum & body | NA | Multiple | Sessile Polypoid | Hyperemic | Smooth | N |
| 2012 | Erarslan et al [7] | 64 y M | Hematemesis and melena | Unknown | Endoscopic resection | Cardia | 0.8 | Solitary | Pedunculated | Hyperemic | Irregular | N |
F = female; M = male; N = negative; NA = not available; No. = number; P = positive; RUQ = right upper quadrant; y = years old.
The clinical presentation of the GI tract PG depends on its site [4] . Gastrointestinal bleeding is the most common presenting symptom (in 34.55% of cases) [3] ; [11] ; [18] ; [19] ; [21] ; [23] ; [24] ; [26] . Overt GI bleeding was the most common clinical presentation in our series, and gastric PG should be considered a source of hemorrhage in patients presenting with overt GI bleeding or iron deficiency anemia.
Liver cirrhosis was one of the most common underlying diseases in the present study. To date, no analysis of the underlying disease in patients with gastric PG has been reported. Only two patients with PG in the small bowel—both of whom had liver cirrhosis—were mentioned previously, but the association or possible mechanisms that increase PG in cirrhotic patients remained unclear [18] . Two hypotheses have been suggested, based on the previous study: portal hypertension and hormone imbalance. Portal hypertension may form venous stasis with retrograde dilatation of the venous and capillary blood vessels and cause growth of preexistent vascular lesions (i.e., produce a hemangioma) in the GI tract [27] ; [28] . Hormone imbalance may also be suspected as a cause of PG, as in the case of spider angiomas. Increased plasma levels of vascular endothelial growth factor and basic fibroblast growth factor are the most significant predictors of the presence of spider angiomas in cirrhotic patients [28] .
In 55.56% of the patients in our case series, GU was previously identified at the same site of gastric PG prior to the index endoscopic examination. Previous studies suggest that GI tract PGs may emerge at sites of previous inflammation of ulceration [4] . Our findings support the hypothesis that PG may be reactive and induced by trauma with a subsequent overgrowth of granulation tissue.
Gastric PGs are mostly semipedunculated or sessile; less frequently they are pedunculated. This finding is slightly different from lesions at other sites of the GI tract where lesions are typically pedunculated or semipedunculated and, less frequently, sessile [4] . Adherent white or yellow deposits on the surface of the lesion is a characteristic feature of PG. Serban and Florescu [29] analyzed cases of PG in the colons of children. They believe that the endoscopic detection of an adherent white or yellow deposit on the mucosa or on polypoid lesions is suggestive of PG, and the endoscopic sensitivity and specificity are both 100% [29] .
Complete excision is reportedly the optimal management for GI tract PG [4] ; [29] . Endoscopic resection is the most favorable treatment for GI tract PG and has been reported in 63.33% of patients [3] ; [4] ; [5] ; [6] ; [7] ; [10] ; [12] ; [13] ; [14] ; [15] ; [16] ; [17] ; [18] ; [19] ; [21] ; [23] ; [24] . Only one patient developed perforation at the site of polypectomy and then underwent surgical repair; however, he recovered and was discharged 5 days later [23] . The second choice for the treatment of GI tract PG was surgical resection. Furthermore, successful combination therapy with embolization of the nutritional artery for the lesion and endoscopic resection has been reported for a large gastric PG [3] . In our series, four gastric PG lesions were stationary or regressed with conservative management. Therefore, conservative treatment may be an option in asymptomatic PG patients with major comorbidities.
In conclusion, overt GI bleeding was the most common clinical presentation, and liver cirrhosis was a common underlying disease in patients with gastric PG in our series. Gastric ulcer was the most common precursor of PG with the antrum being the most frequent site involved. Gastric PGs were characteristically protruding hyperemic lesions with adherent exudates. Endoscopic resection may be offered to patients with symptoms. Conservative treatment may be considered for asymptomatic patients with PG with major comorbidities.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] D.C. Moffatt, P. Warwryko, H. Singh; Pyogenic granuloma: an unusual cause of massive gastrointestinal bleeding from the small bowel; Can J Gastroenterol, 23 (2009), pp. 261–264
- [2] H. Jafarzadeh, M. Sanatkhani, N. Mohtasham; Oral pyogenic granuloma: a review; J Oral Sci, 48 (2006), pp. 167–175
- [3] A. Kusakabe, H. Kato, K. Hayashi, T. Igami, H. Hasegawa, T. Tsuzuki, et al.; Pyogenic granuloma of the stomach successfully treated by endoscopic resection after transarterial embolization of the feeding artery; J Gastroenterol, 40 (2005), pp. 530–535
- [4] J. Antonio Quiros, J. Van Dam, T. Longacre, S. Banerjee; Gastric pyogenic granuloma; Gastroenterol Hepatol (NY), 3 (2007), pp. 850–854
- [5] A. Malhotra, S. Jaganmohan, L.D. Scott; Clinical challenges and images in GI diagnosis: gastric pyogenic granuloma; Gastroenterology, 136 (2009), p. 1168 1463
- [6] T. Kogawa, H. Kogane, H. Kayama; A case of pyogenic granuloma in the stomach; Prog Dig Endosc, 49 (1996), pp. 112–114 [in Japanese]
- [7] E. Erarslan, F. Ekiz, H. Unverdi, B. Yılmaz, İ. Yüksel, Ş. Çoban, et al.; Unusual cause of acute gastrointestinal bleeding: gastric pyogenic granuloma; Dig Endosc, 24 (2012), p. 122
- [8] M. Carmen Gonzalez-Vela, J. Fernando Val-Bernal, M. Francisca Garijo, C. García-Suárez; Pyogenic granuloma of the sigmoid colon; Ann Diagn Pathol, 9 (2005), pp. 106–109
- [9] S. van Eeden, G.J. Offerhaus, F.H. Morsink, B.P. van Rees, O.R. Busch, C.J. van Noesel; Pyogenic granuloma: an unrecognized cause of gastrointestinal bleeding; Virchows Arch, 444 (2004), pp. 590–593
- [10] S.Y. Park, C.H. Park, W.S. Lee, H.S. Kim, S.K. Choi, J.S. Rew; Pyogenic granuloma of the duodenum treated successfully by endoscopic mucosal resection; Gut Liver, 3 (2009), pp. 48–51
- [11] C. Rolanda, R. Goncalves, G. Macedo; A metamorphic lesion; Gastroenterology, 137 (2009), p. 41 395
- [12] D. Ludlow, J. Thibodeaux, C.A. Nathan; Pyogenic granuloma of the esophagus: a cause of dysphagia; Otolaryngol Head Neck Surg, 143 (2010), p. 171
- [13] R. Drut; Pyogenic granuloma of the esophagus; Patología Revista Latinoamericana, 49 (2011), pp. S11–S13
- [14] H.G. Seoung, G.H. Kim, G.A. Song, J.H. Kim, M.Y. Oh, J.C. Choi, et al.; Esophageal pyogenic granuloma: endosonographic findings and endoscopic treatments; Clin Endosc, 46 (2013), pp. 81–84
- [15] R. Drut; Multiple pyogenic granuloma of the duodenum. A case report in a child; Rev Esp Patol, 42 (2009), pp. 243–245
- [16] R. Kuga, C.K. Furuya Jr., S.N. Fylyk, P. Sakai; Solitary pyogenic granuloma of the small bowel as the cause of obscure gastrointestinal bleeding; Endoscopy, 41 (Suppl. 2) (2009), pp. E76–E77
- [17] H. Nagoya, S. Tanaka, A. Tatsuguchi, K. Mitsui, A. Ehara, T. Kobayashi, et al.; Rare cause of obscure gastrointestinal bleeding due to pyogenic granuloma in the ileum detected by capsule endoscopy and treated with double balloon endoscopy; Dig Endosc, 22 (2010), pp. 71–73
- [18] J.W. Chou, S.F. Chen, C.Y. Yii, Y.S. Shih, K.S. Cheng, H.W. Chang; Jejunal pyogenic granuloma diagnosed and treated with spiral enteroscopy; Endoscopy, 44 (Suppl. 2) (2012) UCTN: E2–3
- [19] P. Mandhan; Sigmoidoscopy in children with chronic lower gastrointestinal bleeding; J Paediatr Child Health, 40 (2004), pp. 365–368
- [20] T. Morita, S. Tamura, K. Okawauchi, Y. Yokoyama, T. Tadokoro, Y. Higashidani, et al.; A case of pyogenic granuloma in the sigmoid colon treated with argon plasma coagulation; Dig Endosc, 17 (2005), pp. 253–256
- [21] S.S. Blanchard, G. Chelimsky, S.J. Czinn, R. Redline, J. Splawski; Pyogenic granuloma of the colon in children; J Pediatr Gastroenterol Nutr, 43 (2006), pp. 119–121
- [22] V. Giaccaglia, A. Stefanuto, C. Cavallotti, A. Quintiliani, F. Stipa; Transanal excision of rectal pyogenic granuloma: case report and literature review; Surg Laparosc Endosc Percutan Tech, 21 (2011), pp. e91–e92
- [23] G. Veres, P. Lukovich, H. Gyorrffy; Pyogenic granuloma; J Pediatr Gastroenterol Nutr, 52 (2011), p. 1
- [24] S.L.1 Castle, B.J. Naik-Mathuria, A.L. Kawaguchi, D.B. Shaul; Management of rectal pyogenic granuloma with transanal mucosal sleeve resection; J Pediatr Surg, 47 (2012), pp. 1754–1756
- [25] E. Altamirano, R. Drut; Pyogenic granuloma of the transverse colon. Report of a pediatric case; Patología Revista Latinoamericana, 46 (2008), pp. 263–265
- [26] K.J. Lin, T.C. Yen, K.Y. Tzen; Multiple pyogenic granuloma demonstrated by SPECT using 99Tcm-labelled red blood cells; Br J Radiol, 72 (1999), pp. 397–399
- [27] J. Santos, J. Ruiz-Tovar, A. López, A. Arroyo, R. Calpena; Simultaneous massive low gastrointestinal bleeding and hemoperitoneum caused by a capillary hemangioma in ileocecal valve; Int J Colorectal Dis, 26 (2011), pp. 1363–1364
- [28] C.P. Li, F.Y. Lee, S.J. Hwang, R.H. Lu, W.P. Lee, Y. Chao, et al.; Spider angiomas in patients with liver cirrhosis: role of vascular endothelial growth factor and basic fibroblast growth factor; World J Gastroenterol, 9 (2003), pp. 2832–2835
- [29] D.E. Serban, P. Florescu; Colonic pyogenic granuloma in children: a rare or rarely recognized entity; Am J Gastroenterol, 98 (2003), pp. 2106–2107
- [30] A. Poncet, L. Dor; Botryomycose humaine; Rec Chir., 18 (1897), pp. 996–1003
- [31] B.A. Payson, C.M. Karpas, P. Exelby; Intussusception due to pyogenic granuloma of ileum; N Y State J Med., 67 (1967), pp. 2135–2138
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?