Summary
Background/Objective
Standard laparoscopic adrenalectomy requires early control of the main adrenal vein; however, the small retroperitoneal working space is challenging for beginners to perform this maneuver. We report a technical modification of retroperitoneal laparoscopic adrenalectomy (RLA) for primary hyperaldosteronism (PHA) and the clinical outcomes.
Methods
A total of 38 RLAs were performed for the patients with PHA. The patients were placed in true lateral position with mild bending to expand the surgical field. Instead of attempting to control the main adrenal vein initially, we adopted a technical modification that manipulating and freeing the gland first before controlling the main adrenal vein.
Results
The RLAs were successfully performed in all but one case, which was converted to open surgery due to pancreatic injury. Mean operative time was 124 minutes and estimated blood loss was 74 ml. Mean maximal fluctuation of systolic blood pressure was 29 mmHg. For the right-side RLA, less operative time (113.5 vs. 137.9 minutes) and estimated blood loss (59.5 vs. 91.2 ml) were noted compared with the left-side procedure. Postoperative complications included cerebrovascular accident in one patient, one surgical site hematoma, and two patients had postoperative fever. Potassium level returned to normal in all patients and 70% of the patients reduced their antihypertensives.
Conclusion
Technical modification RLA for PHA without initial control of the main adrenal vein is a safe and feasible procedure. No vigorous blood pressure fluctuation was intraoperatively noted. No vascular injury occurred. Moreover, the right-side procedure became easier.
Keywords
laparoscopic adrenalectomy;outcome;primary hyperaldosteronism;retroperitoneum
1. Introduction
The first laparoscopic adrenalectomy (LA) was reported by Gagner et al 1 in 1992 for Cushing syndrome and pheochromocytoma. Since then, issues regarding the safety, feasibility, and efficacy of LA have been reported and confirmed in several series compared with open adrenalectomy.2 In addition, LA is more preferable than open adrenalectomy because of less pain, more rapid recovery, less morbidity, and superior cosmesis.3 With the world-wide acceptation and maturity of this technique, LA has been the standard care for benign adrenal tumors.
Recent studies in hypertensive populations have demonstrated a high prevalence of primary hyperaldosteronism (PHA) around 5%–15%.4 Once computed tomography shows an adrenal nodule simultaneously, the aldosteronoma should be considered the cause of PHA. This disease is surgically correctable and adrenalectomy is the treatment of choice. Accumulating data regarding LA for PHA shows comparable results with the open adrenalectomy on the postoperative improvement on the potassium level and hypertension control.5
Retroperitoneal laparoscopic adrenalectomy (RLA) is preferred by some urologists with the advantages of avoidance of bowel manipulation, direct access to the adrenal gland, and more rapid convalescence. However, a smaller working space, poor surgical landmarks, and a steeper learning curve are concerns with the procedure.6 Previously reported LAs have been performed with early ligation of the adrenal vein.7 The main reason for early vascular control in LA is to prevent the fluctuation of blood pressure; this is especially true in patients with pheochromocytoma. However, beginners may fail to approach the vessels initially due to the obscure anatomy. Herein, we report our experience of a technical modification on RLA and the clinical outcomes in patients with PHA.
2. Materials and methods
A total of 38 patients with PHA were recruited and underwent RLA at our institution. We retrospectively reviewed their medical records. Hypertension with or without limb weakness was found in the initial clinic visit for all patients. The diagnosis was confirmed by elevated serum aldosterone and decreased plasma renin activity. All patients had image studies such as computed tomography or magnetic resonance imaging performed to indicate the existence of an adrenal tumor.
2.1. Surgical procedures
Under satisfactory general anesthesia, the patients were put in a true lateral position with mild bending. An incision of 1.5 cm was made on the midaxillary line three fingers' breadth above the iliac crest. After opening the dorsal-lumbar fascia, finger dissection was employed to create the retroperitoneal space according to a previous report.8 A 0-degree telescope was introduced and the other two ports were set on the anterior and posterior axillary line at the level of 1 fingerbreadth cephalad to the first port. Using the telescope, blunt dissection proceeded between Gerota fascia and psoas muscle with care. Gerota fascia was then opened at the level around the midpole of the kidney. Different from the traditional LA, we did not intend to control the adrenal vessels at the beginning of the procedure since the pedicle is not easy to approach for the beginners. Dissection was performed along the psoas muscle to the level of diaphragm. Adrenal gland could be identified after the dissection proceeded medially. The upmost portion of the adrenal gland was dissected and the superior adrenal vessels were carefully controlled with Endoclip (Autosuture, USSC, Norwalk, CT, USA). The adrenal gland was dissected away from the peritoneum with care. A 30-degree telescope was then introduced and the dissection proceeded along the junction of kidney and adrenal gland. The adrenal gland was mobilized from the adjacent tissue except for the medial portion and could have been retracted for further dissection. For the right side (Fig. 1), the dissection was carried on carefully along the inferior vena cava. The right adrenal vein was identified and clipped with Endoclip. For the left side (Fig. 2), the dissection was undertaken along the medial side of the kidney to the left renal vein. The left adrenal vein was identified and clipped. The principle of dissection remained the same if minor anatomic variation was encountered. The specimen was put in a self-made bag (made by a surgical glove) and extracted. The bleeders were checked meticulously under the pneumo-retroperitoneum pressure of 5 mmHg.
|
|
|
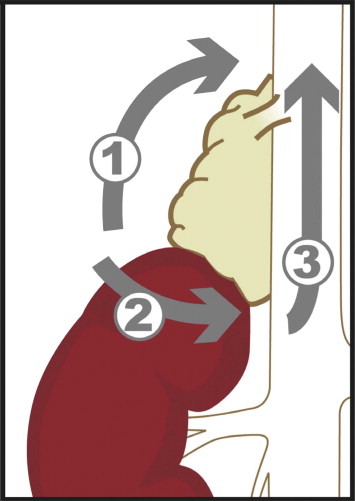
Figure 1. Sequence of dissection for right adrenalectomy. Initial dissection started at the posterior and posterolateral aspects of the adrenal gland (arrow 1). Dissection proceeded to the junction of the adrenal gland and kidney (arrow 2). Finally, the dissection was performed along the IVC and the main adrenal vein was clipped after the adrenal gland could be mobilized (arrow 3). |
|
|
|
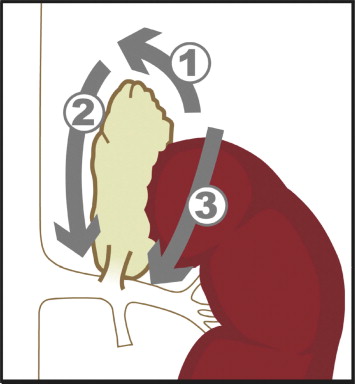
Figure 2. Sequence of dissection for left adrenalectomy. The dissection started at the posterolateral aspect of the adrenal gland (arrow 1). Then dissection was proceeded from the upmost portion of the gland along the aorta (arrow 2). Finally, the adrenal gland and kidney was separated (arrow 3). Clipping of the left adrenal vein was done after the whole gland was mobilized. |
During the operation, blood pressure was recorded from arterial line every 15 minutes and additional recording was performed when necessary. Blood pressures including pre- and postoperative systolic blood pressure (SBP) and diastolic blood pressure were collected. The maximal intraoperative fluctuation of blood pressure was defined as the difference between the highest SBP and lowest measurements during the operation. The upward and downward fluctuation of the SBP was also recorded compared with the baseline SBP taken at ward. The perioperative parameters of complications and functional outcomes were evaluated and analyzed. The pre and post-perative biochemical parameters, including potassium level, serum aldosterone, and plasma renin activity, were collected and compared. The perioperative parameters were collected and compared between left and right RLA. A two-sample t-test was used for the comparisons, and p < 0.05 was considered to be statistically significant.
3. Results
Of these 38 patients, 13 are men and 25 are women; their mean age was 50.6 ± 10.5 years old and their mean body mass index (BMI) was 25.0 ± 2.7. The duration of hypertension was 1.8 ± 1.5 yr and the average types of medication used for controlling hypertension were 2.0 ± 0.8 (Table 1). Perioperative parameters and pathologic data are shown in Table 2. The mean operative time was 124.4 ± 28.2 minutes and estimated blood loss (EBL) was 73.7 ± 38.1 ml. Mean maximal fluctuation of SBP was 22.9 ± 13.0 mmHg. Six out of the 38 patients were recorded to have upward fluctuation of SBP more than 20 mmHg but none had more than 50 mmHg. One intraoperative complication (pancreatic injury) was noted resulting in open conversion. Distal pancreectomy was performed after adrenalectomy in this patient. Four postoperative complications were identified [two postoperative fevers, one retroperitoneal hematoma, and one postoperative cerebrovascular accident (CVA)]. The postoperative CVA occurred in the same patient who experienced an intraoperative pancreatic injury. The fluctuation of the blood pressure was 22.5 mmHg during the operation and the highest measured blood pressure for this patient was 165/90 mmHg. The postoperative blood pressure control was not satisfactory and the CVA occurred on the third postoperative day. All complications were managed conservatively and no patient needed an additional surgical intervention.
| Demographics | |
|---|---|
| No. of patients (male:female) | 38 (13:25) |
| Laterality (right:left) | 21:17 |
| Age (yr) | 50.6 ± 10.5 |
| BMI | 25.0 ± 2.7 |
| Mean follow-up (mo) | 14.6 |
| Interval between operation and diagnosis of HTN (yr) | 1.8 ± 1.5 |
| No. of patients with hypokalemia (%) | 17/38 (44.7) |
| No. of patients with hypertension (%) | 38/38 (100) |
| Mean no. of anti-hypertensives used before surgery (yr) | 2.0 ± 0.8 |
BMI = body mass index; HTN = hypertension.
| Operative time (min) | 124.4 ± 28.2 |
| Estimated blood loss (ml) | 73.7 ± 38.1 |
| Maximal intraoperative systolic pressure (mmHg) | 144.4 ± 8.4 |
| Fluctuation of SBP during operation (mmHg) | 22.9 ± 13 |
| Tumor size (cm) | 2.3 ± 0.5 |
| Gland weight (g) | 7.5 ± 1.2 |
| Pathological diagnosis | Cortical adenoma (37) |
| Nodular hyperplasia (1) | |
| Time to oral intake (d) | 2.0 ± 1.3 |
| Hospital stay (d) | 4.3 ± 2.2 |
| Intraoperative complication (%) | 1 (2.6%) Pancreatic injury (1) |
| Open conversion (%) | 1 (2.6%) |
| Postoperative complications (%) | 4 (10.5%) |
| CVA (1) | |
| Surgical site hematoma (1) | |
| Postoperative fever (2) |
CVA = cerebrovascular accident; SBP = systolic blood pressure.
Comparing the pre- and postoperative biochemical parameters (Table 3), significant differences can be found on SBP, diastolic blood pressure, aldosterone, renin, potassium, and antihypertensives used. Preoperatively, 100% of the patients needed one or more antihypertensives to control the blood pressure. After the operation, 39.4% (15/38) of the patients did not need any medication for the hypertension, while 68.4% (26/38) of the patients reduced the need for antihypertensives. Postoperatively, the potassium level increased to 3.8 ± 0.3 meq/l.
| Preoperative | Postoperative | p value | |
|---|---|---|---|
| SBP (mmHg) | 160.3 ± 8.5 | 133.8 ± 9.3 | <0.001 |
| DBP (mmHg) | 86.2 ± 10.1 | 68.3 ± 7.9 | <0.001 |
| Aldosterone (ng/l) | 362.3 ± 65.5 | 109.1 ± 39.2 | <0.001 |
| Renin (ng/l) | 2.03 ± 1.4 | 2.68 ± 1.1 | 0.01 |
| Potassium (mmol/l) | 3.37 ± 0.4 | 3.8 ± 0.3 | <0.001 |
| Antihypertensive used (n) | 2.0 ± 0.8 | 0.9 ± 1.1 | <0.001 |
DBP = diastolic blood pressure; SBP = systolic blood pressure.
Histopathologic results showed 37 cortical adenomas and one adrenal hyperplasia. The mean tumor size was 2.3 ± 0.5 cm in diameter and the gland weight was 7.5 ± 1.2 g. Time to oral intake was 2.0 ± 1.3 days and hospital stay was 4.3 ± 2.2 days, exclusive of the patient with pancreatic injury.
When comparing the left LA and right LA in this series (Table 4), age, BMI, tumor size, and gland weight were comparable among the patients. Significant differences were shown on the operative time, EBL, and maximal SBP during the operation but not in the fluctuation of blood pressure during the operation. The right LA was noted to have a shorter operative time (113.5 vs. 137.9 minutes; p = 0.003), less EBL (59.5 vs. 91.2 ml; p = 0.004), and lower maximal SBP (141.4 vs. 148.2 mmHg; p = 0.005). Regarding the postoperative parameters, the time to oral intake and hospital stay were comparable and a higher complication rate was associated with the left LA.
| Left (n = 17) | Right (n = 21) | p value | |
|---|---|---|---|
| Age (yr) | 50.4 ± 10.6 | 50.9 ± 10.6 | 0.44 |
| Body mass index | 24.7 ± 2.9 | 25.3 ± 2.5 | 0.26 |
| Tumor size (cm) | 2.4 ± 0.6 | 2.3 ± 0.5 | 0.24 |
| Gland weight (g) | 7.1 ± 1.2 | 7.8 ± 1.1 | 0.05 |
| Operative time (min) | 137.9 ± 29.5 | 113.5 ± 22.2 | 0.003 |
| EBL (ml) | 91.2 ± 47.2 | 59.5 ± 20.1 | 0.004 |
| Maximal systolic blood pressure (mmHg) | 148.2 ± 7.7 | 141.4 ± 7.8 | 0.01 |
| Fluctuation (mmHg) | 23.8 ± 14.9 | 22.1 ± 11.7 | 0.35 |
| Hospital stay (d) | 4.6 ± 3.1 | 4.1 ± 1.0 | 0.25 |
| Time to oral intake (d) | 2.3 ± 1.7 | 1.8 ± 0.8 | 0.1 |
| Complications | |||
| Intraoperative | 1 (5.9%) | 0 (0%) | |
| Postoperative | 3 (17.6%) | 1 (4.8%) | |
EBL = estimated blood loss.
4. Discussion
Several approaches have been reported in LA, including transperitoneal, lateral retroperitoneal,7 posterior retroperitoneal,9 and transthoracic transdiaphragmatic.10 Transperitoneal approach is advantageous because of better visible, identifiable surgical landmarks and large working spaces with a better mobility of the trocars and instruments. However, the possibility of visceral organ injuries can be encountered. The RA is preferred mainly by urologists with merits of avoiding intraperitoneal organ injury and providing direct access to the adrenal gland. It is suitable for patients with previous abdominal surgery or who are morbidly obese.11 However, drawbacks were reported such as limited working space, poorly recognized landmarks, and a steep learning curve.7 Gill et al. 6 reported a prospective, randomized study that compared the transperitoneal and retroperitoneal approaches for LA and they concluded that LA can be performed safely and effectively by either approach. The choice mainly depends on the preference and experience of the surgeon.
Five series of LA in managing PHA in the English literature were reported on the perioperative and functional outcomes.12; 13; 14; 15 ; 16 The results are summarized in Table 5. Our results are comparable with other series in operative time, EBL, hospital stay, complication rate, and conversion rate. Regarding to the functional outcomes, almost all the patients are in eucalcemic status after operation and 39.4%–73.3% of patients are normotensive. Our series showed that the intraoperative maximal SBP fluctuations were acceptable while manipulating the adrenal gland before vessels control. The operation time, EBL, and postoperative parameters are comparable with the previous series. Persisted hypertension after operation was related to the age at the time of surgery and duration of hypertension.17 However, we could not show this tendency in our series. Tsujihata et al. 14 have reported the hormonal dynamics with a normalization of the hormonal level in 2 months postoperatively. Although we could not show the exact time to normalization, all our patients had a normal hormone level 3 months after operation.
| Author (no. of patients) | Approach | Mean follow-up (mo) | Mean operative time (min) | Mean blood loss (ml) | Time to oral intake (d) | Hospital stay (d) | Complication rate (%) | Conversion rate (%) |
|---|---|---|---|---|---|---|---|---|
| Meria et al. (212) | TLA (212) | 44.0 (6–84) | 102.0 (30–260) | 85.0 (10–1300) | 0.9 (0–4) | 3.6 (2–20) | 4.7 | 14.0 |
| Kalady et al. (27) | NA | 153.0 | 88.0 (20–400) | NA | 3.4 | 7.4 | 14.8 | |
| Tsujihata et al. (60) | TLA (31) | 68.8 (3–147) | 261.7 (95–835) | 204.2 (10–3740) | 1.5 (1–6) | 10.1 (5–20) | 9.4 | 1.7 |
| RLA (29) | ||||||||
| Goh et al. (47) | TLA (47) | 21 (1–60) | 127 (70–240) | NA | NA | 2.6 (1–5) | 2.1 | 0 |
| Present study (38) | RLA (38) | 14.6 (6–22) | 124.4 (90–215) | 73.7 (50–200) | 2 (1–8) | 4.3 (3–16) | 10.5 | 2.6 |
RLA = retroperitoneal laparoscopic adrenalectomy; TLA = transperitoneal laparoscopic adrenalectomy.
Traditional LA is performed with initial control of the feeding vessels for the adrenal gland as the open method.7 However, it is sometimes difficult to approach the vessels in the initial procedure, especially for beginners. The reason to control the feeding vessels initially is that it prevents the fluctuation of blood pressure, especially in patients with pheochromocytoma. No previous study has reported that manipulation of the adrenal gland prior to vessel control may cause fluctuation of blood pressure in patients with PHA. In a series of RLA for pheochromocytoma, they adopted the technique without initial control of the adrenal vein with hemodynamic observation.18 They reported 37.5% of upward fluctuation of blood pressure: more than 20 mmHg and 14.3% more than 50 mmHg. They concluded that the procedure was safe and the hemodynamic change is acceptable. The fluctuation of blood pressure in our series was not as vigorous as previously reported.
The most common complication reported in LA was vascular injury especially for the veins surrounding the adrenal gland including adrenal vein, renal vein, and vena cava.19 Vascular injuries usually occurred in an obscure anatomy during dissection. Right adrenal veins are predisposed to injury for the relative shortness and direct drainage into the IVC. Right LA is thought to be more difficult than the left-side procedure due to the above reasons. With the technical modification, no vascular injury was noted. The anatomy of the adrenal vessels can be clearly identified as the key factor. With retraction of the whole gland before controlling the vessels, we had a better view for dissection and a larger space for applying Endoclip. The operative time is shorter and no postoperative or intraoperative complications occurred during the right LA procedure. Even though the difficulty score is not assessed in this series, the above results suggest that the right LA with this method may be easier than the left-side procedure.
Pancreatic injury is not a common complication in laparoscopic surgery. Vakarakis et al. 20 reported a series of pancreatic injuries during laparoscopic surgery. All of the complication happened in left-sided laparoscopic procedures and one-half of the procedures were adrenalectomies. They concluded that the complication could be managed conservatively with adequate drainage. However, the prolonged hospital course can be expected. As for our patient, the complication was recognized intraoperatively and open conversion with distal pancreectomy was preformed. Unfortunately, a cerebrovascular accident then developed. The patient discharged from the hospital without severe sequelae 40 days after the operation.
The limitation of this study is its relatively small number of cases. More cases should be collected to verify the ultimate feasibility of this technical modification. This method is somewhat against the surgical principle for adrenalectomy and may be not suitable for patients with pheochromocytoma since catecholamines can be released during manipulation of the tumor. However, Zhang et al. 18 report a series of pheochromocytoma underwent laparoscopic adrenalectomy with the similar procedure. They did not found prominent fluctuation of the blood pressure. In our series, the fluctuation of blood pressure is smaller than the previous series and we believe this modification is safe and feasible.
5. Conclusion
RLA using the technical modification without initial control of the adrenal vein is a safe and effective procedure for the management of PHA with an adrenal tumor. Potassium levels returned to normal in all patients, and approximately 70% of patients reduced their antihypertensives. Moreover, no complication of vascular injury can be found, and the right LA procedure appears to become easier with time.
References
- 1 M. Gagner, A. Lacroix, E. Bolte; Laparoscopic adrenalectomy in Cushings syndrome and pheochromocytoma; N Engl J Med, 327 (1992), p. 1033
- 2 G. Guazzoni, F. Montorsi, A. Bocciardi, et al.; Transperitoneal laparoscopic versus open adrenalectomy for benign hyperfunctioning adrenal tumors: a comparative study; J Urol, 153 (1995), pp. 1597–1600
- 3 I.S. Gill; The case for laparoscopic adrenalectomy; J Urol, 166 (2001), pp. 429–436
- 4 L. Mosso, C. Carvajal, A. Gonzalez, et al.; Primary aldosteronism and hypertensive disease; Hypertension, 42 (2003), pp. 161–165
- 5 L.M. Brunt, J.F. Moley, G.M. Doherty, T.C. Lairmore, M.K. DeBenedetti, M.A. Quasebarth; Outcomes analysis in patients undergoing laparoscopic adrenalectomy for hormonally active adrenal tumors; Surgery, 130 (2001), pp. 629–634
- 6 M. Rubinstein, I.S. Gill, M. Aron, et al.; Prospective, randomized comparison of transperitoneal versus retroperitoneal laparoscopic adrenalectomy; J Urol, 174 (2005), pp. 442–445
- 7 G.T. Sung, T.H. Hsu, I.S. Gill; Retroperitoneoscopic adrenalectomy: lateral approach; J Endourol, 15 (2001), pp. 505–511
- 8 A.W. Chiu, Y.L. Huang, S.K. Huan, S.H. Huang, W.L. Lin; Comparison study on two different accessing methods for retroperitoneoscopic adrenalectomy; Urology, 60 (2002), pp. 988–992
- 9 S. Baba, A. Miyajima, A. Uchida, H. Asanuma, A. Miyakawa, M. Murai; A posterior lumbar approach for retroperitoneoscopic adrenalectomy: assessment of surgical efficacy; Urology, 50 (1997), pp. 19–24
- 10 I.S. Gill, A.M. Meraney, J.C. Thomas, G.T. Sung, A.C. Novick, I. Lieberman; Thoracoscopic transdiaphragmatic adrenalectomy: the initial experience; J Urol, 165 (6 Pt 1) (2001), pp. 1875–1881
- 11 R. Viterbo, R.E. Greenberg, T. Al-Saleem, R.G. Uzzo; Prior abdominal surgery and radiation do not complicate the retroperitoneoscopic approach to the kidney or adrenal gland; J Urol, 174 (2005), pp. 446–450
- 12 P. Meria, B.F. Kempf, J.F. Hermieu, P.F. Plouin, J.M. Duclos; Laparoscopic management of primary hyperaldosteronism: clinical experience with 212 cases; J Urol, 169 (2003), pp. 32–35
- 13 M.F. Kalady, R. McKinlay, J.A. Olson Jr., et al.; Laparoscopic adrenalectomy for pheochromocytoma. A comparison to aldosteronoma and incidentaloma; Surg Endosc, 18 (2004), pp. 621–625
- 14 M. Tsujihata, N. Nonomura, A. Tsujimura, K. Nishimura, K. Yoshimura, A. Okuyama; Laparoscopic adrenalectomy for primary hyperaldosteronism: clinical experience with 60 cases; J Endourol, 20 (2006), pp. 262–265
- 15 B.K. Goh, Y.H. Tan, S.K. Yip, P.H. Eng, C.W. Cheng; Outcome of patients undergoing laparoscopic adrenalectomy for primary hyperaldosteronism; JSLS, 8 (2004), pp. 320–325
- 16 A.W. Kim, R.M. Quiros, J.B. Maxhimer, A.R. El-Ganzouri, R.A. Prinz; Outcome of laparoscopic adrenalectomy for pheochromocytomas vs aldosteronomas; Arch Surg, 139 (2004), pp. 526–531
- 17 A. Meyer, G. Brabant, M. Behrend; Long-term follow-up after adrenalectomy for primary aldosteronism; World J Surg, 29 (2005), pp. 155–159
- 18 X. Zhang, B. Lang, J.Z. Ouyang, et al.; Retroperitoneoscopic adrenalectomy without previous control of adrenal vein is feasible and safe for pheochromocytoma; Urology, 69 (2007), pp. 849–853
- 19 L. Salomon, M. Soulie, P. Mouly, et al.; Experience with retroperitoneal laparoscopic adrenalectomy in 115 procedures; J Urol, 166 (2001), pp. 38–41
- 20 I.M. Varkarakis, M.E. Allaf, S.B. Bhayani, et al.; Pancreatic injuries during laparoscopic urologic surgery; Urology, 64 (2004), pp. 1089–1093
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?