Summary
Gallbladder perforation is a rare complication of cholecystitis. Similarly, septic thrombophlebitis of the portal vein, also called pylephlebitis, is another rare complication of intra-abdominal infections including cholecystitis. Both gallbladder perforation and pylephlebitis are associated with significantly higher morbidity and mortality. Herein, we report a patient with an atypical presentation of gallbladder perforation and liver abscess formation. A 68-year-old man suffered from malaise, poor appetite, and body weight loss for 1 month. Liver mass lesion and portal vein thrombosis were detected by ultrasound at a local clinic. He was referred to our institution under the tentative diagnosis of hepatocellular carcinoma. He underwent abdominal ultrasound and computed tomography examinations at our hospital. Cholecystitis with gallbladder perforation was highly suspected. Broad-spectrum antibiotics were administered immediately. Percutaneous transhepatic gallbladder drainage was performed in this case, and pigtail drainage for liver abscess was done later. The patients condition—cholecystitis, liver abscess, and pylephlebitis (followed by ultrasound)—improved after treatment. Furthermore, the patient recovered his appetite and his body weight increased.
Keywords
Gallbladder perforation ; Liver abscess ; Septic thrombophlebitis
Introduction
In 1934, Niemeier [1] classified gallbladder perforations (GBPs) into three types: Type I, acute perforation into the free peritoneal cavity; Type II, subacute perforation with abscess formation; and Type III, chronic perforation with cholecystoenteric fistula formation. In order to avoid heterogeneity with regard to data and to facilitate future research, Date et al [2] suggested that reporting types of GBP should be detailed according to the original Niemeier classification in future studies [2] . Thus, our case should be classified as type II, arising after intrahepatic GBP.
Patients with type II perforations are more complicated to manage, because of the atypical presentation of acute cholecystitis and a clinically insidious onset. Initially, a liver mass with portal vein thrombosis in the background of liver cirrhosis was detected by ultrasound in our case. The hepatocellular carcinoma was impressed according to the clinical presentation and imaging findings. The treatment between the infection and malignancy was different. An accurate and early diagnosis of GBP with liver abscess formation is important so as to start the treatment as soon as possible and thereby improve the prognosis.
Case report
A 68-year-old man had been previously healthy without any history of significant systemic diseases and regular medications. Three episodes of fever (up to 38.2°C) without chills developed for 1 day followed by malaise and poor appetite, with nausea. He did not consult a doctor until his weight dropped from 75 kg to 64.3 kg within 1 month. An evaluation of the abdominal ultrasound at another hospital revealed that he had liver cirrhosis with splenomegaly, a space-occupying lesion of about 70 mm × 60 mm over the left lobe of the liver, and portal vein thrombosis. He was transferred to our hospital for treatment of a suspected hepatocellular carcinoma. The initial clinical findings at our hospital were as follows: pulse rate, 116 beats/minute; systolic blood pressure, 135 mmHg; diastolic blood pressure, 82 mmHg; temperature, 37.1°C. An abdominal examination revealed mild distention without abdominal tenderness. The laboratory findings were as follows: white blood cell count, 16,400/mm3 ; differential count, band 1% and neutrophil 91%; C-reactive protein, 6.68 mg/dL; platelet count, 215,000/cumm; prothrombin time, 14.1 seconds (control value 8.0–12.0 seconds); activated partial thromboplastin time, 28.8 seconds (control value 23.9–35.5 seconds); protein S, 67.0% (normal value >60%); protein C, 62.8% (normal value >70%). The liver profile was as follows: alanine aminotransferase, 55 IU/L; aspartate aminotransferase, 83 IU/L; alkaline phosphatase, 356 IU/L; gamma glutamyl transpeptidase, 213 IU/L; total bilirubin, 1.43 mg/dL; albumin, 2.2 mg/dL. He denied smoking and alcoholism. Hepatitis B surface antigen and antihepatitis C virus antibody were nonreactive, whereas the anti-HBc antibody was reactive. His Child–Pugh score was 7 (Class B).
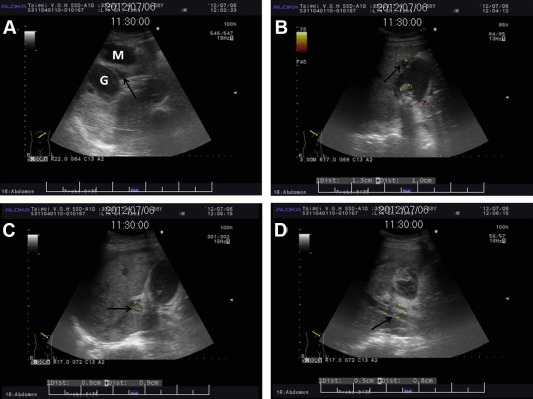
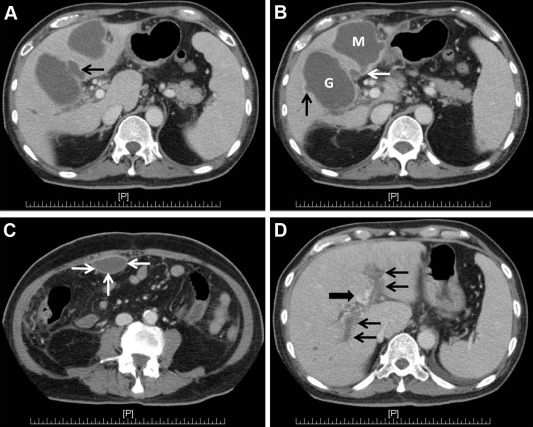
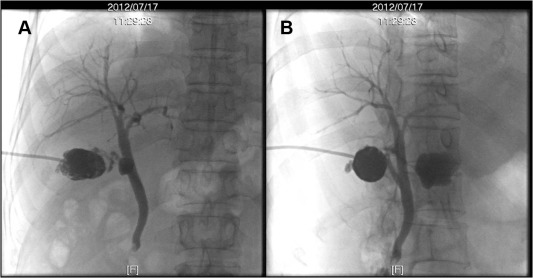
The ultrasound (Fig. 1 ) showed multiple gallstones with heterogeneous contents and an irregular hypoechoic lesion near the gallbladder. Liver cirrhosis with mild ascites and splenomegaly was also noted. An esophagogastroduodenoscopy showed evidence of esophageal and gastric varices. A computed tomography (CT) scan (Fig. 2 ) revealed gallstones and marked gallbladder distention with wall thickening and a 68-mm hypodense lesion at S4 of the liver abutting the distended gallbladder with postcontrast rim enhancement. The findings were suggestive of suppurative cholecystitis with intrahepatic perforation and liver abscess formation. An empiric antibiotic was administered, cefuroxime 1500 mg Q8H (intravenously). The patient underwent ultrasound-guided percutaneous transhepatic gallbladder drainage but refused pigtail drainage for liver abscess simultaneously. Turbid milky pus (Fig. 3 ) was drained, and the culture showed growth of Escherichia coli , Proteus vulgaris , and Pseudomonas aeruginosa . Antibiotic treatment was shifted to ceftazidime (2 g Q8H, intravenously) according to the sensitivity test. The blood culture showed a negative result. The patients appetite and malaise improved after treatment. To confirm the communication between the gallbladder and liver abscess, a contrast medium was infused via the gallbladder drainage catheter; results showed the flow of the contrast medium from the gallbladder into the abscess ( Fig. 4 ). The ultrasound, which was repeated after ceftazidime treatment for 5 days, revealed an improvement of the portal vein thrombosis but poor regression of the liver abscess (32 mm × 44 mm in size). Ultrasound-guided pigtail drainage for liver abscess was then performed with the patients consent. The culture of the liver abscess grew out P. aeruginosa only. The gallbladder drainage catheter and the abscess pigtail catheter were maintained for 20 days and 3 days, respectively. They were removed after regression of abscess and scarce pus drainage. Ceftazidime was administered for 2 weeks during the admission followed by oral Ciprofloxacin (500 mg, BID) for 1 week after discharge. The ultrasound follow-up at the outpatient department revealed the disappearance of liver abscess and contracted gallbladder with gallstones. Cholecystectomy was recommended to the patient, who expressed his hesitation to undergo this procedure.
|
|
|
Figure 1. Abdominal ultrasound. (A) One irregular hypoechoic mass lesion (M) 5.5 cm × 6.6 cm in size near the gallbladder (G) and pericholecystic hypoechoic fluid (arrow). (B) Multiple gallstones with acoustic shadow and a small bulging lesion with gallbladder wall defect (arrow). (C, D) Heterogenic contents within the gallbladder and portal vein thrombus (arrow). |
|
|
|
Figure 2. Contrast-enhanced CT scan. (A, B) Marked gallbladder distention (G), 55 mm in maximal diameter, with wall thickening and an evident 68-mm abscess (M) at S4 of the liver abutting the distended gallbladder; two gallbladder wall defects (black arrow), compatible with intrahepatic gallbladder perforation. A suspected communication tract (white arrow) is found between the gallbladder and abscess. (C) Another abscess formation at the anterior peritoneum (white arrows), 45 mm × 22 mm in size. (D) Total thrombosis of bilateral portal veins (black arrows) and cavernous transformation (thick black arrow). |
|
|
|
Figure 3. Turbid milky pus with tiny gallstones is drained from the gallbladder. |
|
|
|
Figure 4. Contrast medium is infused via the gallbladder drainage catheter and shows flow of contrast medium from the gallbladder into the abscess. This confirms the communication between the gallbladder and liver abscess. |
Discussion
The most common symptom of GBP is right upper quadrant pain combined with nausea and vomiting. Diagnosis is often delayed or made during an operation because of the difficulty in differentiating between the symptoms of this condition and those of cholecystitis without perforation based on symptoms alone. Delayed diagnosis is the major cause of high morbidity and mortality of GBP [3] ; [4] ; [5] .
When evaluating gallbladder disorders, ultrasound is helpful as a first-line imaging tool and is widely used in clinics. An ultrasound can easily detect gallbladder wall thickening, gallstones, as well as intraperitoneal and pericholecystic-free fluid, and may also be used to detect common bile duct stones, intraperitoneal gallstones, and perforation sites [6] . The gallbladder wall defect can be detected by ultrasound in up to 70% of cases based on one study [7] . Contrast-enhanced CT scanning is also an important imaging tool especially if one wishes to obtain more information with regard to an equivocal result of ultrasound and also in regards to the occurrence of complications in cholecystitis. CT scan has a higher accuracy rate than ultrasound [8] ; [9] . The rate of gallbladder wall perforations identified using CT scans is 81.3–88.2% [8] ; [10] . Male sex, older age, cholelithiasis, fever (>38°C), marked leukocytosis greater than 18,000/cumm, and greater cardiovascular comorbidity are the risk factors for GBP in cholecystitis. By contrast, immunocompromised status, corticosteroid therapy, and diabetes mellitus are still controversial risk factors [3] ; [4] ; [11] ; [12] . The reported incidence of GBP in cholecystitis is very low and varies considerably (0.8–10.6% among several case series [3] ; [4] ; [6] ; [11] ). The incidence shows a reduction over time because of an increase in the number of cholecystectomies owing to symptomatic cholelithiasis as compared with the past. The average patient age is around 60–70 years with a wide range in age distribution [3] ; [4] ; [6] ; [11] . Among the incidences of the three types, types I and II have roughly similar incidence rates and are higher than type III in recent studies [2] ; [6] ; [11] ; [12] . With the assistance of imaging tools, an earlier diagnosis can be made, and with improved anesthesiology, surgical techniques, and intensive care conditions, these factors can lead to decreased mortality of GBP in cholecystitis over time [2] ; [12] . The overall median mortality rate in a systemic review was 10.8%, and male sex was weakly related to mortality [2] . Moreover, mortality is not significantly different with age, cholelithiasis, and perforation type [2] ; [12] .
Septic thrombophlebitis of the portal vein, also called pylephlebitis, is a complication with high mortality of intra-abdominal infectious or inflammatory process. It is found most commonly in diverticulitis and has also been reported in appendicitis, pancreatitis, cholecystitis, cholangitis, as well as autoimmune diseases such as inflammatory bowel disease and Behcets disease [13] ; [14] . Diagnosis of pylephlebitis always depends on contrast-enhanced CT because of varying clinical presentations [13] ; [15] . Blood culture is 85% positive, and the most common isolated organism is Bacteroides fragilis[14] . The treatment for patients with type I perforations and acute presentation of illness is urgent surgery directly with open or laparoscopic cholecystectomy. Late operative intervention is associated with increased morbidity, mortality, number of intensive care unit admissions, and long postoperative hospital stays [12] . Recently, percutaneous drainage has been reported as an alternative therapy for GBP. In type II GBP with abscess formation, percutaneous drainage of both gallbladder and abscess can be the appropriate treatment strategy or used as a bridge to surgery. This procedure is reasonable, especially for those in poor physical condition and owing to the high mortality involved with anesthesia and emergency surgery [16] . In type III perforations, patients with gastrointestinal tract obstruction should undergo enterotomy and cholecystectomy to remove the gallstones. Noteworthy, in the presence of pylephlebitis, laparoscopic surgery may aggravate the portal vein thrombosis because of capnoperitoneum-induced decreased visceral venous return and mesenteric venous constriction [17] .
In the presented case, our patient presented with an insidious onset of cholecystitis followed by an obscure course of chronic inflammation and infection that led to pyogenesis of the gallbladder, intrahepatic perforation of the gallbladder wall, liver abscess formation with communication to gallbladder, and septic thrombophlebitis of the portal vein. His predominant symptoms were malaise, anorexia, nausea. and body weight loss instead of the symptoms and signs related to cholecystitis such as fever, abdominal pain, and jaundice. These symptoms, coupled with the findings of the initial ultrasound, are typical presentations in patients with hepatocellular carcinoma. After the CT scan and additional laboratory data, the infectious process was favored and the diagnosis was established by pus drainage.
In conclusion, the early and rapid diagnosis of GBP and septic thrombophlebitis of the portal vein is important in decreasing morbidity and mortality. Broad-spectrum antibiotics need to be prescribed immediately. However, early surgical intervention to treat GBP is preferred with lower morbidity and mortality. Percutaneous drainage with elective cholecystectomy is another treatment modality that is nearly as safe as immediate surgery especially for critical patients.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] O.W. Niemeier; Acute free perforation of the gall-bladder; Ann Surg, 99 (1934), pp. 922–924
- [2] R.S. Date, S.G. Thrumurthy, S. Whiteside, M.A. Umer, K.G. Pursnani, J.B. Ward, et al.; Gallbladder perforation: case series and systematic review; Int J Surg, 10 (2) (2012), pp. 63–68 http://dx.doi.org/:10.1016/j.ijsu.2011.12.004
- [3] J.A. MacDonald; Perforation of the gallbladder associated with acute cholecystitis: 8-year review of 20 cases; Ann Surg, 164 (1966), pp. 849–852
- [4] N.F. Williams, T.K. Scobie; Perforation of the gallbladder: analysis of 19 cases; Can Med Assoc J, 115 (1976), pp. 1223–1225
- [5] M. Aljiffry, M. Walsh, K. Peltekian, M. Molinari; Type II gall bladder perforation with abdominal wall abscess in a cirrhotic patient: case report and review of the literature; J Surg Educ, 65 (5) (2008), pp. 367–371 http://dx.doi.org/:10.1016/j.jsurg.2008.07.004
- [6] H. Derici, C. Kara, A.D. Bozdag, O. Nazli, T. Tansug, E. Akca; Diagnosis and treatment of gallbladder perforation; World J Gastroenterol, 12 (48) (2006), pp. 7832–7836
- [7] B.P. Sood, N. Kalra, S. Gupta, R. Sidhu, M. Gulati, N. Khandelwal, et al.; Role of sonography in the diagnosis of gallbladder perforation; J Clin Ultrasound, 30 (5) (2002), pp. 270–274 http://dx.doi.org/:10.1002/jcu.10071
- [8] M.J. Tsai, J.D. Chen, C.M. Tiu, Y.H. Chou, S.C. Hu, C.Y. Chang; Can acute cholecystitis with gallbladder perforation be detected preoperatively by computed tomography in ED? Correlation with clinical data and computed tomography features; Am J Emerg Med, 27 (5) (2009), pp. 574–581 http://dx.doi.org/:10.1016/j.ajem.2008.04.024
- [9] R. Singal, A. Mittal, S. Gupta, B. Singh, P. Jain; Management of gall bladder perforation evaluation on ultrasonography: report of six rare cases with review of literature; J Med Life, 4 (4) (2011), pp. 364–371
- [10] B.S. Morris, P.R. Balpande, A.C. Morani, R.K. Chaudhary, M. Maheshwari, A.A. Raut; The CT appearances of gallbladder perforation; Br J Radiol, 80 (959) (2007), pp. 898–901 http://dx.doi.org/:10.1259/bjr/28510614
- [11] D. Stefanidis, K.R. Sirinek, J. Bingener; Gallbladder perforation: risk factors and outcome; J Surg Res, 131 (2006), pp. 204–208
- [12] H. Derici, E. Kamer, C. Kara, H.R. Unalp, T. Tansug, A.D. Bozdag, et al.; Gallbladder perforation: clinical presentation, predisposing factors, and surgical outcomes of 46 patients; Turk J Gastroenterol, 22 (5) (2011), pp. 505–512
- [13] J.T. Ames, M.P. Federle; Septic thrombophlebitis of the portal venous system: clinical and imaging findings in thirty-three patients; Digest Dis Sci, 56 (2011), pp. 2179–2184
- [14] J.A. Chirinos, J. Garcia, M.L. Alcaide, G. Toledo, G.J. Baracco, D.M. Lichtstein; Septic thrombophlebitis: diagnosis and management; Am J Cardiovasc Drugs, 6 (1) (2006), pp. 9–14
- [15] S.H. Choi, J.M. Lee, K.H. Lee, S.H. Kim, Y.J. Kim, S.K. An, et al.; Relationship between various patterns of transient increased hepatic attenuation on CT and portal vein thrombosis related to acute cholecystitis; AJR Am J Roentgenol, 183 (2) (2004), pp. 437–442
- [16] K. Kochar, K. Vallance, G. Mathew, V. Jadhav; Intrahepatic perforation of the gall bladder presenting as liver abscess: case report, review of literature and Niemeiers classification; Eur J Gastroenterol Hepatol, 20 (3) (2008), pp. 240–244 http://dx.doi.org/:10.1097/MEG.0b013e3282eeb520
- [17] S.C. Hsu, C.H. Hsieh, S.C. Wu, H.C. Huang, H.C. Lo, C.C. Yeh; Successful staged treatment for acute cholecystitis complicated by portal vein thrombosis; Am Surg, 78 (1) (2012), pp. E19–E21
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?