Summary
Objective
To investigate the clinical application value of intraoperative magnetic resonance imaging (iMRI) in large invasive pituitary adenoma surgery.
Methods
A total of 30 patients with large pituitary adenoma underwent microscopic tumor resection under the assistance of an iMRI system; 26 cases received surgery through the nasal–transsphenoidal approach, and the remaining four cases received surgery through the pterion approach. iMRI was performed one or two times depending on the need of the surgeon. If a residual tumor was found, further resection was conducted under iMRI guidance.
Results
iMRI revealed residual tumors in 12 cases, among which nine cases received further resection. Of these nine cases, iMRI rescanning confirmed complete resection in six cases, and subtotal resection in the remaining three. Overall, 24 cases of tumor were totally resected, and six cases were subtotally resected. The total resection rate of tumors increased from 60% to 80%.
Conclusion
iMRI can effectively determine the resection extent of pituitary adenomas. In addition, it provides an objective basis for real-time judgment of surgical outcome, subsequently improving surgical accuracy and safety, and increasing the total tumor resection rate.
Keywords
intraoperative magnetic resonance imaging;invasion;microsurgery;pituitary adenoma
1. Introduction
The nasal-transsphenoidal approach is the most commonly used approach in microsurgery for treatment of pituitary adenoma. However, the operative field of this approach is small, which makes it impossible to completely discover the condition of important nerves, cavernous sinus, and the carotid artery invaded by the tumor, as well as identify the relationship of the tumor with the hypothalamus. Therefore, it is difficult to deal with a tumor which has invaded the aforementioned structures. Because of the lack of objective test indicators, clinical judgments mainly rely on the subjective experience and anatomical knowledge of the surgeon. This increases the chances of a residual tumor, and at times may even damage the important neurovascular tissues.
In the past decade, intraoperative imaging has become one of the favored techniques in neurosurgery. Intraoperative magnetic resonance imaging (iMRI) is the most advanced means of intraoperative imaging. As a good adjunct to neurosurgery, iMRI can provide a more objective assessment for controlling the intraoperative quality,1; 2; 3; 4; 5 ; 6 and effectively determining the resection level of a tumor and the relationship of the lesion with surrounding functional tissues.7 ; 8 Several reports have pointed out that iMRI identifies residual tumors in approximately 30% of the cases in which the surgeons believed that the tumors were totally resected.5 ; 8 iMRI is helpful in observing and discovering the residual tumor invading the parasellar structures and cavernous sinus.9; 10; 11 ; 12 Application of iMRI to the treatment of pituitary adenoma for achieving maximal tumor removal and minimal damage to surrounding normal functional tissues is seldom reported. In this study, 30 patients with large invasive pituitary adenoma received surgery under the assistance of iMRI. We aimed to investigate the clinical application value of iMRI in large invasive pituitary adenoma surgery.
2. Patients and methods
2.1. Patients
A total of 30 patients (13 men and 17 women) were enrolled in this study. The patients were aged 21–65 years (mean age, 36 years). Of these 30 patients, pituitary adenoma was found for the first time in 26 patients, whereas the remaining four patients suffered from postoperative recurrence or residual pituitary adenoma. There were 22 cases of headache, 26 cases of visual disturbance, four cases of galactorrhea, seven cases of menstrual irregularity or menopause, eight cases of acromegaly, and nine cases of declining sexual function. This study was conducted with approval from the Ethics Committee of Jinling Hospital, School of Medicine, Nanjing University (Nanjing, China). Written informed consent was obtained from all patients or their families.
2.2. Imaging, endocrinological, and pathological examinations
Head computed tomography (CT), MRI, and endocrinological, routine pathological, and immunohistochemical examinations were performed on all patients. The maximum tumor diameter was 18–65 mm (≤30 mm in 10 cases; 31–50 mm in 17 cases; and ≥50 mm in three cases). Based on preoperative tumor morphology identified on magnetic resonance images, the tumors were classified according to the Knosp–Steiner carotid arterial line classification: consequently, 16 cases were diagnosed as invasive pituitary adenoma, and the remaining 14 cases as noninvasive pituitary adenoma. According to clinical manifestations and endocrinological and pathological examination results, nine, four, eight, and nine cases were diagnosed as nonfunctioning pituitary adenoma, prolactin-secreting pituitary adenoma, growth hormone-secreting pituitary adenoma, and gonadal hormone-secreting adenoma, respectively.
2.3. Surgery and iMRI examination
All 30 patients received general anesthesia prior to microscopic tumor resection under the assistance of an iMRI system; 26 cases received surgery through the nasal–transsphenoidal approach, and the remaining four cases received surgery through the pterion approach. For the iMRI examination, the patients lay in a suitable position, and were fixed with a titanium head frame. The incision was made based on tumor size and invasive-growth direction identified on preoperative MRI. The surgical procedure was basically the same as that of conventional microsurgery.
The iMRI system used a 1.5-T superconducting magnet (MAGNETOM Espree; Siemens Healthcare, PA, USA), with dual-chamber design, namely, an operating room and a diagnostic room, which were separated by a metal screen door. If no iMRI was performed, the MRI magnet was in the diagnostic room. When iMRI was needed, the screen door was opened, and the patient was brought into the diagnostic room and placed on a long bed that slides into the MRI chamber. After completion of iMRI, the patient was shifted back to the operating room. Based on the needs of the surgeon, the iMRI was performed one or two times to understand the extent of tumor resection required. If the iMRI revealed a residual tumor, further resection was carried out under iMRI guidance. The scanning was repeated until complete tumor resection was confirmed by iMRI.
3. Results
3.1. Extent of tumor resection
The extent of tumor resection needed was determined by iMRI examination. Residual tumor was identified in 12 cases, among which nine cases required further resection. We did not perform further resection in the remaining three cases because the tumor had extensively invaded the cavernous sinus, sphenoid sinus, and clivus, with hard or tough texture, and the extent of resection had reached the preoperative expectation. Among the nine cases that required further resection, iMRI rescanning confirmed complete resection in six cases, and subtotal resection in the remaining three (the tumor invaded the cavernous sinus and surrounded the carotid artery, and the resection range had achieved the expectation). Overall, 24 cases achieved complete tumor resection, and six cases achieved subtotal resection. The complete tumor resection rate increased from 60% to 80% (Figure 1 ; Figure 2).
|
|
|
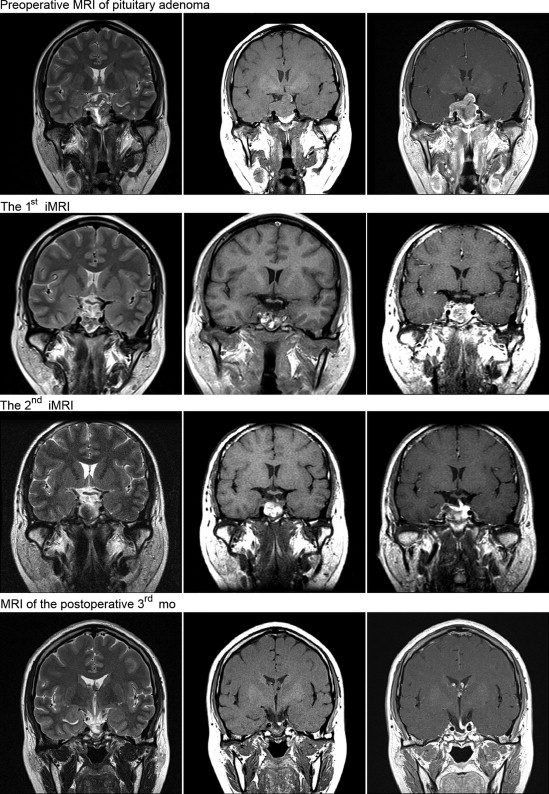
Figure 1. Resection of a large invasive pituitary adenoma under the assistance of intraoperative magnetic resonance imaging (iMRI) using the nasal-transsphenoidal approach. |
|
|
|
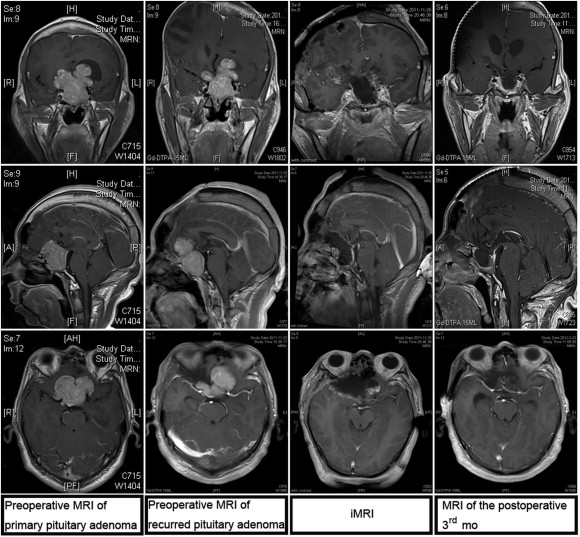
Figure 2. Resection of a recurred large invasive pituitary adenoma under the assistance of intraoperative magnetic resonance imaging (iMRI) using the pterion approach. |
3.2. Pituitary hormone change
The pituitary hormone level of all patients was rechecked 7 days later. In eight cases with growth hormone-secreting pituitary adenoma, the growth hormone level was reduced to normal in six cases, and in the remaining two cases, it had decreased by >50%. In four cases with prolactin-secreting pituitary adenoma, the prolactin level was reduced to normal in two cases, with a >50% decrease. There was a <50% decrease in the other two cases.
3.3. Postoperative complications
After the surgery, diabetes insipidus occurred in three patients, which disappeared after 1–2 weeks of pituitary hormone therapy. Cerebrospinal fluid rhinorrhea occurred in four cases, which was cured by continuous lumbar drainage of cerebrospinal fluid for 1 week. Suprasellar cistern subarachnoid hemorrhage was detected by iMRI in one case. However, the postoperative head CT demonstrated that the suprasellar cistern subarachnoid hemorrhage did not increase, and there was no decreased visual acuity. After continuous lumbar drainage of cerebrospinal fluid for 1 week, the headache symptom was mitigated, and the patient was discharged with good recovery. The iMRI was performed one or two times, which extended the total time of surgery to approximately 1 hour, but there was no iMRI-associated secondary infection or adverse event. The mean duration of hospitalization was 12 days.
3.4. Follow up
Postoperative follow up was performed on 30 patients by outpatient review or telephone interview. The follow-up time was 3–24 months. In 22 cases of preoperative headache, the symptom disappeared in 13 patients, and was significantly mitigated in the other nine cases. The visual disturbance was ameliorated in 22 cases, and acromegaly was ameliorated in six cases. Galactorrhea stopped in two cases, and there was recovery of the menstrual cycle. Patients with a residual tumor or pituitary hormone abnormalities were treated by radiation or medical therapy, and had satisfactory results.
4. Discussion
4.1. Necessity of iMRI in pituitary adenoma surgery
In treatment of pituitary adenoma, the nasal–transsphenoidal approach has been widely used, because it can shorten the operation path, reduce the surgical trauma, and avoid the exposure and stretch of brain tissues. However, this approach has a small operative field and there is disturbance due to the “blind field”. Thus, the surgeons cannot completely remove the tumors under direct vision. Therefore, the outcome of resection is often subjective, which increases the chances of residual tumor. The pterional approach is the most widely used procedure in transcranial pituitary adenoma surgery. This approach is applicable to the suprasellar-extended tumor, dumbbell-shaped huge tumor, and invasive tumor extending to the parasellar and postsellar regions and anterior skull base. In craniotomy, the region among the ipsilateral optic nerve, optic tract bottom, and interior of the clinoid process of the ipsilateral internal carotid artery is the “blind field.” The tumors in the ipsilateral optic nerve bottom and lateral walls of the cavernous sinus are often invasive tumors, and thus the explosion of tumor and resection of these tumors is difficult. In addition, the tumors in the sellar region and those invading into the sphenoid sinus are also easily missed due to visual limitations. Therefore, the surgical process and outcome mainly depends on surgeons' subjective experience, and there is a lack of objective detection indicators and basis.
The aforementioned problems can best be solved by making use of real-time intraoperative imaging technology. In the mid-1990s, with the development of hardware and software of MRI technology, Black et al1 pioneered the introduction of MRI technology in neurosurgery. In 1995, the Department of Neurosurgery of Erlangen and Heidelberg University, in cooperation with Siemens Company, developed the first iMRI system.13 The combination of iMRI technology and neurosurgery not only possesses the three-dimensional positioning function, but also has the real-time navigation function, which effectively assists the surgeon to identify and resect the diseased tissue accurately, while avoiding damage to the major blood vessels, nerves, or functional areas of the brain. This technology has enabled real-time judgment and objective evaluation of the results of surgery. Therefore, iMRI can greatly improve the accuracy, safety, and objectivity of neurosurgery, and has important clinical significance for improving the quality of surgery.
In general, according to magnetic-field strength, the iMRI system is divided into low-field strength (<0.5 T) and high-field strength (≥1.5 T). The high-field-magnet iMRI system can be further divided into the fixed magnet type and the movable magnet type, based on whether the magnet can be moved or not.14 ; 15 The magnet of the former is fixed, and the patient is moved into the magnet through a movable bed; by contrast, in the latter case, the patient is fixed, and the magnet is shifted to the operating room for scanning. Our hospital introduced the German Siemens fixed magnet-rotating bed high-field (1.5 T) iMRI system.
4.2. Role of iMRI in pituitary adenoma surgery
Wirtz et al16 reported that iMRI scanning revealed residual tumors in 62.1% of high-grade glioma patients and in 41.4% of low-grade glioma patients. Nimsky et al5 also reported that among 48 patients who received complete pituitary tumor resection, iMRI discovered 19 cases (39.6%) of residual tumors that needed further resection. With the assistance of iMRI, the total resection rate of tumors increased from 56.2% to 87.5%. In our study, with the assistance of iMRI, the complete tumor resection rate increased from 60% to 80%, suggesting that iMRI could significantly increase the total resection rate of pituitary adenoma.
Large invasive pituitary adenoma is bulky and often develops toward the lateral, posterior, superior, or anterior part of the sella. It involves a variety of neurovascular structures, which increases the difficulty of surgery and postoperative complications. Although the transsphenoidal and pterional approaches are very mature, it is still difficult to completely resect large invasive pituitary adenomas due to the visual limitations. iMRI can provide three-dimensional stereoscopic images, which allow the surgeon to observe the relationship and distance between a pituitary tumor and important structures.17; 18 ; 19 Therefore, the surgeon can resect the tumor as much as possible while ensuring the safety of surgery, thereby avoiding damage to the important blood vessels and nerves around the sella turcica.
iMRI offers real-time surgical evaluation, which enables surgeons to identify acute surgical complications and reduce postoperative complications. In this study, iMRI identified a case of suprasellar cistern subarachnoid hemorrhage when operating using the transsphenoidal approach. The postoperative head CT demonstrated no increase of subarachnoid hemorrhage in this patient. The patient had postoperative headache without vision loss. After continuous drainage of blood cerebrospinal fluid by lumbar puncture for 1 week, the symptoms were mitigated, and the patient had good recovery.
Whether the iMRI system extends the operation time or not is a controversial issue. The movement of patients and their preparation in the operating room before and after scanning takes some time. It needs approximately 20 minutes to complete intraoperative routine scanning with the 1.5-T magnet, and the movement of the patient and preparation requires 5 minutes. Thus, one intraoperative scan can be completed within 25 minutes, which is an acceptable duration. The applications of iMRI and functional neural navigation enable the surgeon to better distinguish between the tumor border and important brain functional structures, and thus resect the lesions much more quickly, which saves on operation time. In addition, iMRI can actually reduce the total medical costs by reducing the risk of complications, secondary surgery, and average hospitalization duration (although its actual operation time is longer than the traditional surgery).20 In this study, iMRI scanning was performed one or two times, which extended the total surgery time to approximately 1 hour; however, none of the patients had iMRI-associated secondary infection and adverse events caused by secondary surgery and magnetic fields. The average length of hospital stay and total medical costs also did not increase.
4.3. Prospect of iMRI
With the continuous development of iMRI technology, it is possible that iMRI could be combined with many other technologies such as laser, endoscopy, focused ultrasound, freezing, radiofrequency ablation, and intraoperative brain functional evaluation. Endoscopic operating direction and depth can be freely controlled using a multiangle lens, which can make up for the restrictions of single tubular vision in surgical microscopes and benefit the tumor resection.21 In this study, iMRI revealed residual tumors in three cases of invasive pituitary adenoma. After further resection of the tumor using neuroendoscopic procedures, iMRI rescanning showed complete tumor resection. The results suggest that iMRI could be combined with endoscopic techniques to improve the resection extent of pituitary adenoma.
At present, iMRI is a new technology with continuous development. With increasingly growing interest in its application and a higher rate of acceptance by neurosurgeons, the integration of iMRI with traditional neurosurgery will offer a wide range of application. iMRI technology provides the neurosurgeon with real-time guidance and real-time objective evaluation of surgical outcomes, thereby enhancing the accuracy and safety of neurosurgery, and thus increasing the degree of tumor resection. However, iMRI has some limitations such as high cost, loud noise, radiofrequency pulse-energy accumulation in body, and increased metal artifacts. These issues need to be addressed in further development and application.
Acknowledgments
This work was supported by the Grant from Jinling Hospital of Nanjing, School of Medicine, Nanjing University (No. 2010M023) and the Major Innovative Project of Medical Science and Technology of Nanjing Military Command (No.14ZX16).
References
- 1 P.M. Black, T. Moriarty, E. Alexander 3rd, et al.; Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications; Neurosurgery, 41 (1997), pp. 831–842
- 2 W.A. Hall, K. Kowalik, H. Liu, C.L. Truwit, J. Kucharezyk; Costs and benefits of intraoperative MR-guided brain tumor resection; Acta Neurochir Suppl, 85 (2003), pp. 137–142
- 3 W.A. Hall, H. Liu, A.J. Martin, C.H. Pozza, R.E. Maxwell, C.L. Truwit; Safety, efficacy, and functionality of high-field strength interventional magnetic resonance imaging for neurosurgery; Neurosurgery, 46 (2000), pp. 632–641
- 4 C. Nimsky, O. Ganslandt, R. Fahlbusch; Comparing 0.2 tesla with 1.5 tesla intraoperative magnetic resonance imaging analysis of setup, workflow, and efficiency; Acad Radiol, 12 (2005), pp. 1065–1079
- 5 C. Nimsky, O. Ganslandt, B. Von Keller, J. Romstöck, R. Fahlbusch; Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients; Radiology, 233 (2004), pp. 67–78
- 6 G.R. Sutherland, T. Kaibara, D. Louw, D.I. Hoult, B. Tomanek, J. Saunders; A mobile high-field magnetic resonance system for neurosurgery; J Neurosurg, 91 (1999), pp. 804–813
- 7 R. Fahlbusch, O. Ganslandt, C. Nimsky; Intraoperative imaging with open magnetic resonance imaging and neuronavigation; Childs Nerv Syst, 16 (2000), pp. 829–831
- 8 R.B. Schwartz, L. Hsu, T.Z. Wong, et al.; Intraoperative MR imaging guidance for intracranial neurosurgery: experience with the first 200 cases; Radiology, 211 (1999), pp. 477–488
- 9 R. Gerlach, R. du Mesnil de Rochemont, T. Gasser, et al.; Feasibility of polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases; Neurosurgery, 63 (2008), pp. 272–285
- 10 J. Jones, J. Ruge; Intraoperative magnetic resonance imaging in pituitary macroadenoma surgery: an assessment of visual outcome; Neurosurg Focus, 23 (2007), p. E12
- 11 M. Buchfelder, S.M. Schlaffer; Intraoperative magnetic resonance imaging during surgery for pituitary adenomas: pros and cons; Endocrine, 42 (2012), pp. 483–495
- 12 D. Bellut, M. Hlavica, C. Muroi, C.M. Woernle, C. Schmid, R.L. Bernays; Impact of intraoperative MRI-guided transsphenoidal surgery on endocrine function and hormone substitution therapy in patients with pituitary adenoma; Swiss Med Wkly, 23 (2012), p. w13699
- 13 V.M. Tronnier, C.R. Wirtz, M. Knauth, et al.; Intraoperative diagnostic and interventional magnetic resonance imaging in neurosurgery; Neurosurgery, 40 (1997), pp. 891–900
- 14 F.A. Jolesz; Future perspectives for intraoperative MRI; Neurosurg Clin N Am, 16 (2005), pp. 201–213
- 15 C. Nimsky, O. Ganslandt, R. Fahlbusch; 1.5T: intraoperative imaging beyond standard anatomic imaging; Neurosurg Clin N Am, 16 (2005), pp. 185–200
- 16 C.R. Wirtz, M. Knauth, A. Staubert, et al.; Clinical evaluation and follow-up results for intraoperative magnetic resonance imaging in neurosurgery; Neurosurgery, 46 (2000), pp. 1112–1120
- 17 A. Nabavi, P.M. Black, D.T. Gering, et al.; Serial intraoperative magnetic resonance imaging of brain shift; Neurosurgery, 48 (2001), pp. 787–797
- 18 P. Hastreiter, C. Rezk-Salama, G. Soza, et al.; Strategies for brain shift evaluation; Med Image Anal, 8 (2004), pp. 447–464
- 19 C. Nimsky, O. Ganslandt, P. Hastreiter, R. Fahlbusch; Intraoperative compensation for brain shift; Surg Neurol, 56 (2001), pp. 357–364
- 20 F.A. Jolesz, I.-F. Talos, G.R. Sutherland; The vision of intraoperative magnetic resonance imaging; Tech Neurosurg, 7 (2002), pp. 344–351
- 21 C.P. Hofstetter, R.H. Mannaa, L. Mubita, et al.; Endoscopic endonasal transsphenoidal surgery for growth hormone-secreting pituitary adenomas; Neurosurg Focus, 29 (2010), p. E6
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?