Summary
Background
Malignant gastric outlet obstruction causes significant malnutrition and morbidity. The implantation of a metallic stent is an alternative palliative treatment to allow the intake of food in these patients.
Patients and Methods
Thirty-eight consecutive patients with malignant gastric outlet obstruction who had received an uncovered metallic stent placement in our department from April 2010 to April 2012 were enrolled for analysis. The mean follow-up time was 6.3 months. Food intake, measured by the Gastric Outlet Obstruction Scoring System, complications, duration of stent patency, and survival were evaluated.
Results
The technical and clinical success rates of the procedure were 100% and 94.7%, respectively. The Gastric Outlet Obstruction Scoring System scores were significantly improved at 1 day, 7 days, and 30 days after the implantation compared with those prior to the procedure (p < 0.001). Aspiration pneumonia developed in two patients (5.2%) after the procedure. One of these patients developed respiratory failure and died 3 days later. Stent dysfunction developed in 11 of 38 patients (28.9%) during the follow-up period; one patient (2.6%) experienced migration of the stent 38 days later due to resolution of the stricture; 10 patients (26.3%) had stent restenosis. The median time of stent patency was 120 days. The presence of peritoneal carcinomatosis when the procedure was carried out was a significantly poor predictive factor of stent patency [hazard ratio (HR) 7.9, p = 0.039]. The median survival of the patients was 156 days. Poor performance status ≥3; HR 2.647, p = 0.012) and nongastric cancer origin (HR 3.466, p = 0.008) were associated with a significantly short survival time.
Conclusion
Metallic stent placement is an effective and relatively safe treatment for patients with malignant gastric outlet obstruction.
Keywords
Malignant gastric outlet obstruction ; Metallic stent ; Outcome ; Stent dysfunction
Introduction
Malignant gastric outlet obstruction (MGOO), a late complication of advanced carcinoma of the stomach, duodenum, periampulla, or pancreas, causes significant malnutrition and morbidity [1] ; [2] ; [3] . Persistent severe nausea and vomiting, poor oral intake, and weight loss develop in these patients [2] . These symptoms lead to dehydration, malnutrition, cachexia, and a poor quality of life [4] . In addition, palliative chemotherapy and radiotherapy cannot be administered because of the patients poor clinical condition. These patients usually die in a matter of weeks [1] .
Self-expandable metallic stents have been suggested as an alternative palliative treatment for MGOO in recent years [5] , especially in patients with an inoperable disease [6] . This endoscopic treatment has the advantages of low morbidity and mortality, early relief of symptoms, and no need for general anesthesia [5] . In Taipei Veterans General Hospital, Taipei, Taiwan, metallic stent placement started to be performed in patients with inoperable MGOOs in April, 2010. The aim of this study was to evaluate the clinical efficacy of stent placement in these patients.
Methods
Patients
We retrospectively reviewed 38 consecutive patients with inoperable malignant carcinoma causing MGOO who received metallic stent placement at Taipei Veterans General Hospital between April 2010 and April 2012. MGOO was confirmed by endoscopy or radiological studies. All patients had obstructive symptoms such as nausea, vomiting, and poor oral intake. This study was approved by the institutional review board of Taipei Veterans General Hospital.
Stent placement procedure
Prior to the procedure, all patients received nothing by mouth, with nasogastric tube drainage for at least 24 hours to relieve symptoms of obstruction and decrease the risk of aspiration.
All stents were deployed using a standard gastroscope (JF-240/260, Olympus, Shinjuku-ku, Tokyo, Japan) under fluoroscopic guidance without sedation. A guide wire (0.89 mm × 460 cm; Hydra Jagwire, Boston Scientific, Natick, MA, USA) was introduced through the working channel of the endoscope to more than 20 cm beyond the site of obstruction. Water-soluble radiographic contrast was injected to determine the length and location of the stricture. The stent was chosen by the length of about an additional 1–3 cm on either side of the stricture. The uncovered stent (WallFlex Duodenal Stent; Boston Scientific Corporation) was deployed under fluoroscopic guidance. Radiological follow-up was performed to evaluate the expansion and location of the metallic stent 1 day later.
Evaluation of the Gastric Outlet Obstruction Scoring System
The primary outcome of the study was the improvement of food intake. It was measured by the Gastric Outlet Obstruction Scoring System (GOOSS) score with 0 = no oral intake, 1 = liquid diet, 2 = soft diet, and 3 = regular diet [2] . Based on these data, clinical success was defined as a relief of obstructive symptoms and improvement of oral intake. Other outcomes, including technical success, complications, stent dysfunction, and survival time, were also evaluated.
Technical success of stent placement was defined as adequate deployment and positioning of the stent. Complications were both minor and major. Minor complications were defined as those that were not life-threatening and which did not require further aggressive treatment, such as abdominal pain, nausea, and vomiting. Major complications were defined as life-threatening or severe complications, such as aspiration pneumonia, bleeding, perforation, stent migration, and sepsis.
Data collection
Data were obtained from patients' notes, radiology reports, procedure notes, and telephone interviews. The collected data included patient demographics, procedural characteristics, complications, GOOSS score, duration of stent patency, re-interventions, and survival time.
Statistical analysis
Continuous variables are presented as mean ± standard deviation. The pre- and post-stent placement GOOSS scores were analyzed using the Wilcoxon signed rank test. Univariate analyses of survival time and the patency of the stent were performed by Kaplan–Meier analysis. Only variables with p < 0.05 in univariate analysis were selected for multivariate analysis, which was performed by Cox regression models. Patients were recorded on the last date of follow up, date of death, or development of in-stent restenosis. The time to restenosis of stents and the survival curves after stent placement in different groups were analyzed by the log-rank test. Statistical significance was considered for p < 0.05. All statistical analyses were performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Table 1 summarizes the characteristics of the enrolled patients. Of the 38 patients, 23 were men and 15 were women, with a mean age of 70.2 years. Most patients (76.3%) had a primary malignancy of gastric adenocarcinoma. The sites of obstruction were the antrum in 11 patients, the pylorus in 29 patients, the duodenum in seven patients, and the Billroth gastroduodenal anastomosis of palliative subtotal gastrectomy in one patient. Among the seven patients with the obstruction site at the duodenum, two had an obstruction in the second portion of duodenum due to pancreatic cancer, complicated with concomitant biliary obstruction that was treated by percutaneous transhepatic cholangial drainage the next day. Two patients received radiotherapy and 18 patients received chemotherapy prior to the procedure. After stent placement, 16 patients received further chemotherapy and two patients received further radiotherapy.
| Sex | |
| Male | 23 (60.5) |
| Female | 15 (39.5) |
| Age (y) | 70.2 ± 16.8a |
| BMI (kg/m2 ) | 21.2 ± 3.9 |
| Performance status | |
| 0 | 8 (21.1) |
| 1 | 8 (21.1) |
| 2 | 7 (18.4) |
| 3 | 11 (28.9) |
| 4 | 4 (10.5) |
| Primary malignancy | |
| Gastric cancer | 29 (76.3) |
| Pancreatic cancer | 4 (10.5) |
| Cholangiocarcinoma | 5 (13.2) |
| Stage | |
| IIIA | 1 (2.6) |
| IIIB | 1 (2.6) |
| IV | 36 (94.8) |
| Site of obstruction | |
| Antrum | 11 (28.9) |
| Pylorus | 19 (50.0) |
| Duodenum | 7 (18.4) |
| Anastomosis | 1 (2.6) |
| Prior radiation or chemotherapy | 20 (52.6) |
Data are expressed as n (%) or mean ± SD.
BMI = body mass index.
a. Range, 31–100 years.
Success of technique
The placement of the duodenal stents was uneventful in all patients. The mean ± standard deviation procedure time was 27.2 ± 8.8 minutes and the median procedure time was 25 minutes (range, 11–56 minutes).
Clinical success
Prior to stent placement, 26 patients were unable to tolerate intake by mouth and 11 patients tolerated only liquids. Thirty-seven patients started oral intake within 1 day after stent placement. One patient delayed oral intake until the 2nd day after the procedure due to abdominal pain. Another patient had nausea and vomiting after the procedure and an abdominal plain film radiograph showed a distended bowel loop. Abdominal computed tomography was performed and showed the ileus with a transitional zone around a splenic flexure without evidence of pneumoperitoneum. After conservative treatment, his condition improved and he started oral intake on the 3rd day after stent placement.
Overall, 36/38 patients (94.73%) had an improvement in their levels of dietary intake. Two patients (5.27%) had no improvement and none had a worse dietary status. The pre-procedure GOOSS scores were 0.34 ± 0.53. The GOOSS scores were significantly increased the next day (1.37 ± 0.59) and achieved the maximum level on Day 7 (2.24 ± 0.76), which was maintained on Day 30 (2.24 ± 0.86) when compared with the basal scores (p < 0.001). Table 2 gives the detailed GOOSS score distribution. However, the body mass index of the patients had still decreased at the 30-day follow ups (initial 21.14 ± 3.93 vs. 30-day 20.38 ± 3.86, p = 0.001) and the plasma albumin levels remained unchanged (initial 3.01 ± 0.46 vs. 30-day 2.94 ± 0.52, p = 0.209).
| GOOSS score | Pre-procedure | Postoperative | ||
|---|---|---|---|---|
| Day 1* | Day 7* | Day 30* | ||
| 0 | 26 | 0 | 0 | 1 |
| 1 | 11 | 26 | 7 | 5 |
| 2 | 1 | 10 | 14 | 13 |
| 3 | 0 | 2 | 16 | 15 |
Data are expressed as n .
- p < 0.001 vs. pre-procedure group.
Complications and re-interventions
Table 3 gives the procedure-related complications. Two patients (5.2%) had aspiration pneumonia. One of these two patients recovered after treatment with antibiotics and was discharged 7 days later; the other developed respiratory failure and died 3 days later because the patient had requested no resuscitation. One patient (2.63%) had sepsis and improved after empirical treatment with antibiotics. One patient (2.63%) receiving chemotherapy had late migration of their stent 38 days after the procedure. Endoscopy showed that the stent was lodged at the third portion of the duodenum, with an improvement of the previous obstruction. The stent was smoothly removed via over-tube and the patient had no obstructive symptoms by the end of the follow-up period. Thirteen patients (34.21%) experienced minor complications including abdominal pain (5 patients), nausea (3 patients), and vomiting (5 patients); these symptoms resolved within 1 week of conservative treatment. No patient was observed to have secondary biliary obstruction.
| Major complications | n (%) |
|---|---|
| Aspiration pneumonia | 2 (5.3) |
| Migration of stent | 1 (2.6) |
| Sepsis | 1 (2.6) |
| Minor complications | |
| Abdominal pain | 5 (13.2) |
| Nausea | 3 (7.9) |
| Vomiting | 5 (13.2) |
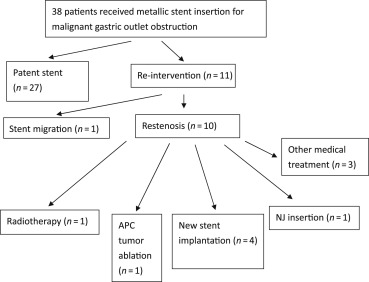
A total of 10 patients (26.31%) had recurrent obstruction due to tumor ingrowth after stent placement (Fig. 1 ), but only seven patients received further intervention. The remaining three patients were treated with hospice care because they were in the terminal stages of cancer. Four patients received a new stent placement, one patient received argon plasma coagulation ablation, one patient underwent radiotherapy, and one patient had a naso-jejunal (NJ) tube inserted. No further recurrent obstruction or endoscopic intervention was noted in these patients until death or the end of follow-up.
|
|
|
Figure 1. Outcomes of patients with malignant gastric outlet obstruction treated with metallic stent placement (n = 38). APC = argon plasma coagulation; NJ = nasojejunal. |
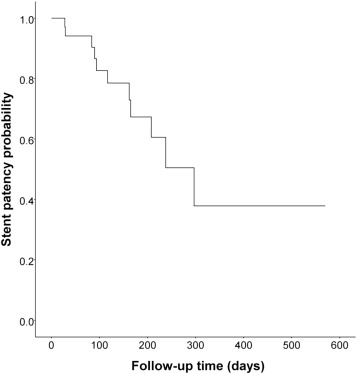
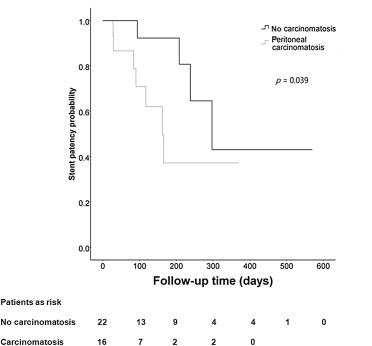
The median duration for the patency of the stents was 120 days (range, 3–570 days). Fig. 2 shows the time to restenosis after stent placement. On univariate analyses, peritoneal carcinomatosis was the only significantly predictive factor of a shorter patency time (Table 4 ). The patency times of patients with or without peritoneal carcinomatosis were 123 ± 93.24 days and 160.45 ± 142.02 days, respectively (Fig. 3 ).
|
|
|
Figure 2. Time to restenosis after stent placement. Incidence of recurrent obstructions recorded for patients still alive after treatment. |
| Variable | Univariate analysis | ||
|---|---|---|---|
| n | Restenosis, n (%) | p | |
| Age (y) | 0.436 | ||
| <70 | 18 | 5 (27.8) | |
| ≥70 | 20 | 6 (30) | |
| Sex | 0.139 | ||
| Male | 23 | 6 (26.1) | |
| Female | 15 | 5 (33.3) | |
| Tumor origin | 0.220 | ||
| Gastric cancer | 29 | 11(37.9) | |
| Nongastric cancer | 9 | 0 (0) | |
| Location of obstruction | 0.462 | ||
| Antrum | 11 | 3 (27.3) | |
| Pylorus | 19 | 8 (42.1) | |
| Duodenum | 7 | 0 (0) | |
| Length of stenosis (cm) | 0.748 | ||
| <5 | 10 | 2 (20) | |
| ≥5 | 12 | 2 (16.7) | |
| Length of stent (cm) | 0.147 | ||
| <9 | 6 | 3 (50) | |
| ≥9 | 32 | 8 (25) | |
| Previous RT | 0.445 | ||
| Yes | 2 | 0 (0) | |
| No | 36 | 11 (30.6) | |
| Post-procedure RT | 0.619 | ||
| Yes | 1 | 0 (0) | |
| No | 37 | 11 (29.7) | |
| Previous CT | 0.902 | ||
| Yes | 23 | 6 (26.1) | |
| No | 15 | 5 (33.3) | |
| Post-procedure CT | 0.924 | ||
| Yes | 14 | 5 (35.7) | |
| No | 24 | 6 (25) | |
| Peritoneal carcinomatosis | 0.039 | ||
| Yes | 16 | 7 (43.8) | |
| No | 22 | 4 (18.2) | |
CT = chemotherapy; RT = radiotherapy.
|
|
|
Figure 3. Time to restenosis of stents in patients with or without peritoneal carcinomatosis. |
Survival
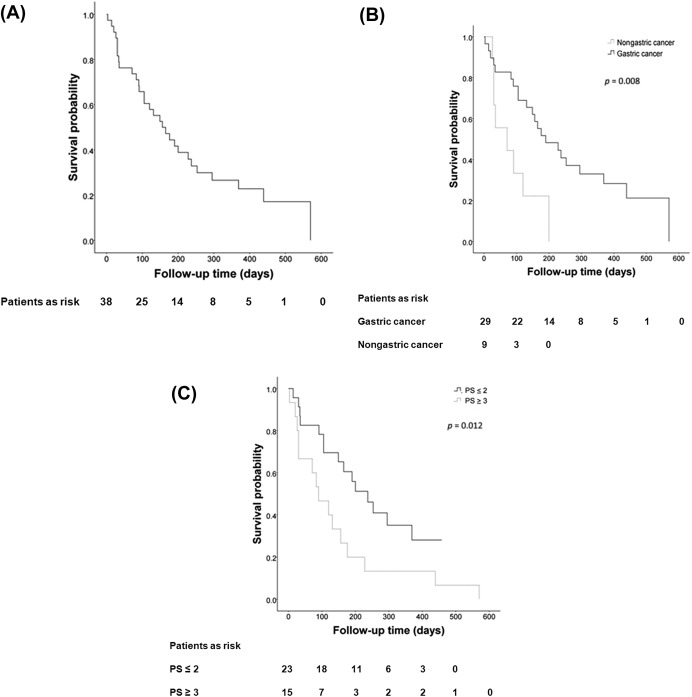
Thirty patients died during the follow-up period and the median survival after the initial procedure was 156 days (range, 3–570 days; Fig. 4 ). Using univariate and multivariate analyses, worse performance status (Eastern Cooperative Oncology Group score ≥3) and cancer nongastric in origin were the significant risk factors of mortality after stent placement (Table 5 ; Fig. 4 ).
|
|
|
Figure 4. Survival curves after stent placement in: (A) all patients with malignant gastric outlet obstruction; (B) patients with cancer gastric or nongastric in origin; and (C) patients with high or low performance status. APC = argon plasma coagulation; NJ = nasojejunal; PS = performance status. |
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| n | Death (%) | p | HR | 95% CI | p | |
| Age (y) | 0.531 | |||||
| <70 | 18 | 77.8 | ||||
| ≥70 | 20 | 80 | ||||
| Sex | 0.055 | |||||
| Male | 23 | 73.9 | ||||
| Female | 15 | 86.7 | ||||
| Performance status | 0.023 | 2.647 | 1.240–5.561 | 0.012 | ||
| ≥3 | 15 | 100 | ||||
| ≤2 | 23 | 65.2 | ||||
| Tumor origin | 0.010 | 3.466 | 1.381–8.698 | 0.008 | ||
| Nongastric cancer | 9 | 88.9 | ||||
| Gastric cancer | 29 | 75.9 | ||||
| Stage | 0.238 | |||||
| III | 2 | 100 | ||||
| IV | 36 | 28 | ||||
| Location of obstruction | 0.082 | |||||
| Antrum | 11 | 100 | ||||
| Pylorus | 19 | 68.4 | ||||
| Duodenum | 7 | 85.7 | ||||
| Length of stenosis (cm) | 0.433 | |||||
| <5 | 10 | 80 | ||||
| ≥5 | 12 | 66.7 | ||||
| Length of stent (cm) | 0.814 | |||||
| <9 | 6 | 83.3 | ||||
| ≥9 | 32 | 78.1 | ||||
| Previous RT | 0.446 | |||||
| Yes | 2 | 100 | ||||
| No | 36 | 77.8 | ||||
| Post-procedure RT | 0.511 | |||||
| Yes | 1 | 100 | ||||
| No | 37 | 78.4 | ||||
| Previous CT | 0.713 | |||||
| Yes | 23 | 78.3 | ||||
| No | 15 | 80 | ||||
| Post-procedure CT | 0.158 | |||||
| Yes | 14 | 78.6 | ||||
| No | 24 | 79.2 | ||||
| Peritoneal carcinomatosis | 0.763 | |||||
| Yes | 16 | 75 | ||||
| No | 22 | 81.8 | ||||
CI = confidence interval; CT = chemotherapy; HR = hazard ratio; RT = radiotherapy.
Discussion
In this retrospective study, we found that the technical and clinical success rates of stent placement were high and the rate of major complications was low. Our results are comparable to those of other studies [6] ; [7] ; [8] ; [9] . Stent placement helped most patients to take in food and helped half of the patients to receive further chemotherapy. At present, commonly suggested treatments for MGOO are surgical gastrojejunostomy (GJ) and stent placement [3] . GJ has better long-term outcomes than stent placement, which has better short-term outcomes [4] . Further, GJ has a higher mortality rate, morbidity risk, prolonged hospital stay, and delayed symptom relief compared with metallic stents. Stent placement has a higher occlusion rate [4] . Therefore surgery is more suitable for patients in a better condition and with a longer life expectancy, whereas a metallic stent is better for those in a poorer condition. In our study, none of our patients were suitable for surgery as a result of advanced disease and its associated malnutrition and poor performance status.
However, metallic stents are very expensive and many patients cannot afford to use them. In such cases, tube feeding can be considered [10] . The cost of an NJ tube is less than that of a metallic stent. The fees for an NJ tube or metallic stent in MGOO are not covered by the Bureau of National Health Insurance in Taiwan. The price of an NJ tube and metallic stent are around NT$3000 and NT$70,000 in Taiwan, respectively. Although a higher dysfunction rate is associated with an NJ tube, the use of an NJ tube could still provide nutrition and achieve a similar survival rate to a metallic stent [10] . Therefore the patients with a lower economic status may choose an NJ tube placement to improve their nutrition. Adequate selection and evaluation of patients with MGOO are important when considering a palliative treatment modality for these patients.
The major procedure-related complication in our patients was aspiration pneumonia. One patient died, despite adequate treatment. Much food is retained in the stomach as a result of obstruction of the gastric outlet. These food remnants are hard to remove by suction because they often clog the orifices of nasogastric tubes or endoscopes. Further, food aspiration can develop without any obvious vomiting, particularly in those patients with poor mental or performance status [11] . Aspiration could occur prior to or during stent placement. Our patients were mostly in the late stages of their disease with generalized cachexia and were at a particularly high risk of developing aspiration pneumonia. We need to fully explain this risk to patients and take care with aspiration during stent placement.
Minor complications such as abdominal pain and vomiting occurred in one-third of our patients after receiving the procedures. Although these discomforts can be treated conservatively, they may be associated with a longer stay in hospital [4] . The pain and vomiting may be the result of stimulation of the stent on the mucosa of the tumor. In addition, long-term food remnants and poor gastric peristalsis due to neoplastic involvement of the celiac plexus also can lead to nausea and vomiting. In some cases, poor gastric peristalsis may explain the lack of improvement in the GOOSS score [5] .
The common causes of malfunction of the stent after placement are tumor ingrowth or outgrowth, stent migration, food impaction, and biliary obstruction [5] . Laasch et al [12] reported that 20–25% of patients needed endoscopic re-intervention after stent placement; this was 28.9% in our series of patients. The reasons for re-intervention in our patients were mostly tumor ingrowth causing stent restenosis. In our current study, pre-existing peritoneal carcinomatosis was the only significant risk factor for stent restenosis. This implies that patients with peritoneal carcinomatosis might have a tendency for rapid tumor growth to cause tumor ingrowth. By contrast, another study showed that patients with MGOO and peritoneal carcinomatosis had similar rates of early and late stent failure to those without peritoneal carcinomatosis [13] . However, in that study, the patients with carcinomatosis were younger and had more favorable cancer types [13] . Post-stent chemotherapy did not contribute significantly to stent patency in our patients, although previous studies have shown that chemotherapy after stent placement significantly increased the maintenance of stent patency [14] ; [15] . Different sample sizes, cohorts, and chemotherapy regimens may explain this discrepancy. In fact, one patient in our study who experienced stent migration had an improvement in pyloric obstruction after chemotherapy. Therefore further large prospective trials are needed to evaluate which chemotherapy regimens, with or without radiotherapy, could further prolong stent patency.
Four of our patients received placement of another new metallic stent. No further obstruction episodes occurred in these patients prior to death or the end of follow-up. Jang et al [16] showed that 19 of 20 patients (95%) had an improvement in symptoms after a second stent placement, but 1 of 20 patients (5%) developed duodenal perforation. Gutzeit et al [17] found that half of their patients (3/6) receiving a second stent developed stent dysfunction at 4 days, 47 days, and 240 days after the procedure, respectively. Thus the occluded stent could be corrected by using a stent-in-stent procedure. However, the safety and long-term patency should be evaluated by further prospective studies.
The median survival after stent placement in our study was 156 days compared with other studies that ranged from 7 days to 152 days [5] ; [6] ; [18] ; [19] ; [20] ; [21] ; [22] ; [23] ; [24] . We found that poor performance status and cancer nongastric in origin were the significant predictors of shorter survival. Poor performance status has been known to be associated with poor survival in cancer patients [25] . Jemal et al [26] reported that patients with a primary pancreatic carcinoma had a shorter survival time than those with a gastric or duodenal carcinoma. Kim et al [27] further showed that the survival rate was higher in patients with gastric cancer than in patients with pancreatic cancer with gastric outlet obstruction, but the stent patency did not differ between the two groups. Our data are in agreement with the study of Kim et al [27] .
In conclusion, despite the limitations of this retrospective study and the small sample size, our study suggests that metallic stent placement is an effective and relatively safe method for patients with MGOO. Metallic stent placement should be considered as a first line of treatment in patients who are able to afford the related costs.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
The authors thank Liao Dong Ming for his excellent technical assistance.
References
- [1] K.D. Lillemoe, H.A. Pitt; Palliation. Surgical and otherwise; Cancer, 78 (1996), pp. 605–614
- [2] D.G. Adler, T.H. Baron; Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients; Am J Gastroenterol, 97 (2002), pp. 72–78
- [3] J.E. Lopera, A. Brazzini, A. Gonzales, W.R. Castaneda-Zuniga; Gastroduodenal stent placement: current status; Radiographics, 24 (2004), pp. 1561–1573
- [4] S.M. Jeurnink, E.W. Steyerberg, Hof Gv, C.H. van Eijck, E.J. Kuipers, P.D. Siersema; Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients; J Surg Oncol, 96 (2007), pp. 389–396
- [5] I. Boskoski, A. Tringali, P. Familiari, M. Mutignani, G. Costamagna; Self-expandable metallic stents for malignant gastric outlet obstruction; Adv Ther, 27 (2010), pp. 691–703
- [6] M. Piesman, R.A. Kozarek, J.J. Brandabur, D.K. Pleskow, R. Chuttani, V.E. Eysselein, et al.; Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial; Am J Gastroenterol, 104 (2009), pp. 2404–2411
- [7] T. Sasaki, H. Isayama, I. Maetani, Y. Nakai, H. Kogure, K. Kawakubo, et al.; Japanese multicenter estimation of wallflex duodenal stent for unresectable malignant gastric outlet obstruction; Dig Endosc, 25 (2013), pp. 1–6
- [8] J.M. Canena, A.C. Lagos, I.N. Marques, S.D. Patrocinio, M.G. Tomé, M.A. Liberato, et al.; Oral intake throughout the patients' lives after palliative metallic stent placement for malignant gastroduodenal obstruction: a retrospective multicentre study; Eur J Gastroenterol Hepatol, 24 (2012), pp. 47–755
- [9] H.T. Cheng, Y.K. Tsou, C.H. Lin, C.L. Cheng, J.H. Tang, C.S. Lee, et al.; Endoscopic metal stents for the palliation of malignant upper gastroduodenal obstruction; Hepatogastroenterology, 58 (2011), pp. 1998–2002
- [10] C.L. Lin, C.L. Perng, Y. Chao, C.P. Li, M.C. Hou, H.S. Tseng, et al.; Application of stent placement or nasojejunal feeding tube placement in patients with malignant gastric outlet obstruction: a retrospective series of 38 cases; J Chin Med Assoc, 75 (2012), pp. 624–629
- [11] C.B. Pearce, H.D. Duncan; Enteral feeding. Nasogastric, nasojejunal, percutaneous endoscopic gastrostomy, or jejunostomy: its indications and limitations; Postgrad Med J, 78 (2002), pp. 198–204
- [12] H.U. Laasch, D.F. Martin, I. Maetani; Enteral stents in the gastric outlet and duodenum; Endoscopy, 37 (2005), pp. 74–81
- [13] R.B. Mendelsohn, H. Gerdes, A.J. Markowitz, C.J. DiMaio, M.A. Schattner; Carcinomatosis is not a contraindication to enteral stenting in selected patients with malignant gastric outlet obstruction; Gastrointest Endosc, 73 (2011), pp. 1135–1140
- [14] J.H. Kim, H.Y. Song, J.H. Shin, E. Choi, T.W. Kim, H.Y. Jung, et al.; Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients; Gastrointest Endosc, 66 (2007), pp. 256–264
- [15] C.G. Kim, S.R. Park, I.J. Choi, J.Y. Lee, S.J. Cho, Y.I. Park, et al.; Effect of chemotherapy on the outcome of self-expandable metallic stents in gastric cancer patients with malignant outlet obstruction; Endoscopy, 44 (2012), pp. 807–812
- [16] J.K. Jang, H.Y. Song, J.H. Kim, M. Song, J.H. Park, E.Y. Kim; Tumor overgrowth after expandable metallic stent placement: experience in 583 patients with malignant gastroduodenal obstruction; AJR Am J Roentgenol, 196 (2011), pp. W831–W836
- [17] A. Gutzeit, C.A. Binkert, E. Schoch, T. Sautter, R. Jost, C.L. Zollikofer; Malignant gastroduodenal obstruction: treatment with self-expanding uncovered wallstent; Cardiovasc Intervent Radiol, 32 (2009), pp. 97–105
- [18] M. Del Piano, M. Ballare, F. Montino, A. Todesco, M. Orsello, C. Magnani, et al.; Endoscopy or surgery for malignant GI outlet obstruction?; Gastrointest Endosc, 61 (2005), pp. 421–426
- [19] J. Espinel, S. Vivas, F. Munoz, F. Jorquera, J.L. Olcoz; Palliative treatment of malignant obstruction of gastric outlet using an endoscopically placed enteral wallstent; Dig Dis Sci, 46 (2001), pp. 2322–2324
- [20] I. Graber, R. Dumas, B. Filoche, J. Boyer, D. Coumaros, H. Lamouliatte, et al.; The efficacy and safety of duodenal stenting: a prospective multicenter study; Endoscopy, 39 (2007), pp. 784–787
- [21] I. Keränen, A. Lepistö, M. Udd, J. Halttunen, L. Kylänpää; Stenting for malignant colorectal obstruction: a single-center experience with 101 patients; Surg Endosc, 26 (2012), pp. 423–430
- [22] M.S. Phillips, S. Gosain, H. Bonatti, C.M. Friel, K. Ellen, P.G. Northup, et al.; Enteral stents for malignancy: a report of 46 consecutive cases over 10 years, with critical review of complications; J Gastrointest Surg, 12 (2008), pp. 2045–2050
- [23] I.T. Pinto; Malignant gastric and duodenal stenosis: palliation by peroral implantation of a self-expanding metallic stent; Cardiovasc Intervent Radiol, 20 (1997), pp. 431–434
- [24] R. Razzaq, H.U. Laasch, R. England, A. Marriott, D. Martin; Expandable metal stents for the palliation of malignant gastroduodenal obstruction; Cardiovasc Intervent Radiol, 24 (2001), pp. 313–318
- [25] I. Chau, A.R. Norman, D. Cunningham, J.S. Waters, J. Oates, P.J. Ross; Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data; J Clin Oncol, 22 (2004), pp. 2395–2403
- [26] A. Jemal, T. Murray, E. Ward, A. Samuels, R.C. Tiwari, A. Ghafoor, et al.; Cancer statistics; CA Cancer J, 2005 (55) (2005), pp. 10–30
- [27] J.H. Kim, H.Y. Song, J.H. Shin, H.T. Hu, S.K. Lee, H.Y. Jung, et al.; Metallic stent placement in the palliative treatment of malignant gastric outlet obstructions: primary gastric carcinoma versus pancreatic carcinoma; AJR Am J Roentgenol, 193 (2009), pp. 241–247
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?