Abstract
Objective
The aim of our study was to define predictors of cardiac compression development including clinical, electrocardiographic, echocardiographic, chest-X-ray and perioperative parameters and their diagnostic value.
Methods
Overall 243 patients with pericardial disease, among them 123 with compression (tamponade, constriction) and 120 without signs of compression were included in the study. Clinical, laboratory, electrocardiographic, chest-X-Ray, echocardiographic and perioperative data were included in the logistic regression analysis to define predictors of tamponade/constriction development.
Results
Logistic regression analysis demonstrated large effusion (> 20 mm) (OR 5.393, 95%CI 1.202–24.199, p = 0.028), cardiac chamber collapse (OR 31.426, 95%CI 1.609–613-914, p = 0.023) and NYHA class > 3 (OR 8.671, 95%CI 1.730–43.451, p = 0.009) were multivariable predictors of compression development. The model including these three variables allowed predicting compression in 91.7% of cases.
ROC analyses demonstrated that all three variables had significant diagnostic value with sensitivity of 75.6% and specificity of 74.2% for large effusion, low sensitivity and high specificity for cardiac chamber collapse (35% and 92%) and NYHA class (32.5% and 94.2%).
Conclusion
The independent predictors of compression development are presence of large effusion > 20 mm, cardiac chamber collapse and high NYHA class. The model including all three parameters allows correctly predicting compression in 91.4% of cases. The diagnostic accuracy of each parameter is characterized by high sensitivity and specificity of large effusion, high specificity of cardiac chamber collapse and NYHA class.
Keywords
Pericardial disease;Compression;Tamponade;Constriction;Predictors;Diagnostic accuracy
1. Introduction
Tamponade and constrictive pericarditis (CP) are complications of pericardial diseases (PD), causing heart compression and accompanied by perioperative mortality in 4–14% of cases [1]; [2]; [3]; [4] ; [5]. Several studies [2]; [4]; [5]; [6]; [7] ; [8] reported that in patients with CP referred for pericardiectomy along with tuberculosis as the main etiological cause, there is an increase of idiopathic, postradiotherapy pericarditis and postpericardiotomy syndrome (PPTS). Therefore, early detection of compression and risk stratification seems to be important in prevention of constriction and tamponade.
Clinical tamponade signs like jugular vein distention, hepatomegaly, hypotension and paradoxical pulse, might not be evident in cases of localized effusion or low-pressure tamponade [1]; [9] ; [10]. Several studies also demonstrated, that clinical signs were not suspected in > 50% of cases with echocardiographic (echo) and cardiac catheterization signs of tamponade [11] ; [12].
Imaging methods allow accurately diagnose tamponade and constriction, though several parameters need to be combined to identify correctly compression, as they differ by accuracy and some signs can accompany other structural heart diseases [13]; [14]; [15] ; [18].
However, studies on predictors of tamponade and constriction development in PD are limited and few is known on factors determining development of compression. It is known that risk of tamponade increases three-fold when paradoxical pulse exceeds 10 mmHg [19], and in presence of low voltage QRS, accompanying large effusion [20]. Chronic large effusion might progress to tamponade in about 1/3 of cases, while in subacute large effusions risk of tamponade is high in cases unresponsive to medical treatment [13]; [21] ; [22]. Constriction develops in tuberculous pericarditis accompanied by tamponade, despite full resolution of effusion after pericardiocentesis and in cases of acute pericarditis resistant to treatment with acetylsalicylic acid [6] ; [23].
It was demonstrated that cardiac chamber collapse (CCol) and inferior vena cava (IVC) plethora lacked value in prediction of tamponade, while large pericardial effusion increased risk 78-fold [24]. Large effusion size (> 500 ml) was shown to predict tamponade development with accuracy of 100% [25]. It should be also noted, that CCol could be observed in 1/3 of cases without clinical signs of tamponade [26].
Thus, evidence on factors predictive for development of compression in PD is limited, few is known whether combination of clinical, hemodynamic, X-Ray, ECG, and echo parameters may improve early identification of patients at risk of constriction and tamponade.
The aim of our study was to define predictors of cardiac compression development including clinical, electrocardiographic (ECG), echo, chest-X-ray and perioperative parameters and their diagnostic value.
2. Methods
We retrospectively analyzed records included in the prospective database of patients with PD admitted to the Scientific Research Institute of Heart Surgery and Organ Transplantation between 1997 and 2014.
2.1. Patients
Overall, 243 consecutive patients with PD referred for treatment in our center, were included in the study. All patients were divided into 2 groups according to presence of tamponade and constriction signs [1]: 123 patients with syndrome of compression and 120 patients without signs of compression.
2.2. Clinical variables
The following demographic and clinical data were included in the analysis: age, gender, etiology of PD, NYHA class, duration of hospitalization; presence of inflammation, laboratory analysis; hemodynamic status — heart rate (HR), systolic (SBP) and diastolic (DBP) blood pressure, central venous pressure (CVP) where appropriate; cardiothoracic index (CTI), presence of pleural effusion and superior vena cava (SVC) dilatation/compression on chest X-Ray.
2.3. Echocardiography
Cardiac chambers` size and pericardial involvement (thickening, calcification, effusion size, tamponade and constriction signs) were estimated according to recommendations of American Society of Echocardiography and European Association of Cardiovascular Imaging [27] ; [28] using transthoracic 2-dimensional (2D), 2D-guided M-mode and Doppler echocardiography (echo). The following echo data were included in analysis: left atrial (LA) size, right atrial (RA) enlargement, left ventricular (LV) end-systolic and end-diastolic dimensions, LV ejection fraction (LVEF, estimated by Simpson rule), RV dimension, mean pulmonary arterial pressure, signs of LA, RA, RV and LV collapse [29]; [30] ; [31], IVC plethora [14], respiratory variations of tricuspid and mitral flows` [32], interventricular septal (IVS) and inter-atrial septal paradoxical movement, flat atrioventricular (AV) groove [16], constrictive E/a pattern of mitral flow [15]; [17] ; [33], hepatic vein dilatation and flow reversal [15], and presence of fibrin detachments in pericardial cavity. We also measured presence and extent of pericardial thickening and calcification, extent and size of effusion. Based on above-mentioned echo data, pericardial compression was defined as tamponade and constriction [13]; [15]; [27]; [28]; [29]; [30]; [31]; [32]; [33] ; [34].
2.4. Electrocardiography
ECGs were scanned and digitally analyzed as described previously [35] for presence of PR segment depression, low voltage QRS, QRS alternans, ST junction elevation/depression, and P-wave morphology abnormalities (notched P-wave, changes in amplitude — P mitrale and pulmonale patterns) [20]; [36] ; [37]. Arrhythmias and conduction defects [38] were evaluated from ECGs, and whenever available Holter monitoring and telemetry records.
2.5. Pericardiocentesis and pericardiectomy
Pericardiocentesis with and without drainage, subxiphoid pericardiostomy and pericardiectomy were performed by standard technique [1]; [9]; [39] ; [40]. During interventions and surgery, we assessed size of effusion obtained by drainage and intraoperatively, and its extent; during surgery we also evaluated extent and size of adhesions and calcifications.
2.6. Definitions
Based on above-mentioned data, pericardial involvement was classified as effusion, effusion with compression (tamponade), constriction and constriction with effusion, adhesive, adhesive with effusion and adhesive-effusive with signs of compression, as well presence or absence of compression (tamponade or constriction).
Extent and size of pericardial effusion, thickening and calcification of pericardium were classified based on echo and drainage (effusion)/intraoperative data. Effusions were categorized by extent as localized and diffuse; and by size as small (< 100 ml by drainage/intraoperatively, < 10 mm by echo for diffuse effusion and < 5 mm for localized effusion), moderate (100–400 ml by drainage/intraoperatively, 10–20 mm by echo for diffuse effusion and < 5–10 mm for localized effusion), and large (> 400 ml drainage, > 20 mm by echo for diffuse effusion and > 10 mm for localized effusion), [1]; [34]; [41] ; [42]. Pericardial thickening and calcifications were graded as localized and diffuse.
Presence of inflammation was judged on increased levels of C-reactive protein (CRP), sedimentation rate, and immune markers where applicable.
2.7. Outcomes
Outcomes were recorded based on hospital records and follow-up of patients. The following outcomes were included in the analysis: recurrent PD, heart failure, death and composite outcome.
2.8. Statistical analysis
Statistical analyses were performed using SPSS for Windows software (IBM, New York). Categorical variables are presented as number (percentage) and continuous variables as mean (SD). The normality of data distribution was assessed by Kolmogorov–Smirnov test. Comparisons between groups were performed using Chi-square test for categorical variables and unpaired t test for independent samples for normally distributed data and Mann–Whitney U test for abnormally distributed data. Logistic regression analysis (LRA) was performed to identify predictors of compression syndrome. The dependent variable was binary-presence or absence of cardiac compression; for selection of independent variables — variables with significance value < 0.1 on univariate analysis were included in the model. p value < 0.05 was accepted as a significant value for all tests. Diagnostic value of multivariable predictors of cardiac compression was defined using ROC analysis with assessment of area under the curve (AUC), 95% CI, p values, sensitivity and specificity.
3. Results
3.1. Clinical characteristics, chest X-ray and ECG (Table 1)
As can be seen from Table 1, patients' groups did not differ by age and duration of hospitalization. There were significantly more males (p = 0.004); patients with neoplastic, infectious PD, heart failure and trauma (p < 0.0001), high NYHA class, high CVP, tachycardia, low SBP and high CRP values (p < 0.0001, p = 0.001, p = 0.007, p = 0.001, and p = 0.04, respectively) in group of compression as compared to control group.
| Variables | Compression + (n = 123) | Compression − (n = 120) | p |
|---|---|---|---|
| Age, yearsa | 43.42 (17.92) | 44.12 (16.74) | 0.75 |
| Sex, n(%) | |||
| Female | 49 (39.8) | 70(58.3) | 0.004 |
| Male | 74(60.2) | 50(41.7) | |
| Etiology, n(%) | |||
| Neoplastic | 20(16.3) | 5(4.2) | < 0.0001 |
| Idiopathic | 34(27.6) | 31(25.8) | |
| Infectious | 35(28.5) | 24(20) | |
| Immune | 2(1.6) | 12(10) | |
| Heart failure | 16(13) | 5(4.2) | |
| Metabolic | 4(3.3) | 1(0.8) | |
| Trauma | 4(3.3) | – | |
| Postpericardiotomy syndrome | 7(5.7) | 42(35) | |
| Pericardial tumor | 1(0.8) | – | |
| NYHA classa | 3.27 (0.56) | 2.63(0.61) | < 0.0001 |
| Duration of hospitalizationa | 13.42 (10.14) | 14.52(8.91) | 0.38 |
| CVP, mma | 168.07(77.84) | 84.62(32.56) | 0.001 |
| HR, beats/mina | 94.18(17.95) | 86.07(20.33) | 0.001 |
| SBP, mmHga | 108.25(18.14) | 115.48(21.63) | 0.007 |
| DBP, mmHga | 68.82(12.84) | 71.30(14.00) | 0.16 |
| C-reactive protein, mg/dLa | 27.35(50.68) | 5.37(3.34) | 0.04 |
| Hemoglobin, mg/dLa | 121.68(20.21) | 124.13(22.99) | 0.39 |
| Red blood cells, × 1012a | 4.07(0.67) | 4.25(0.68) | 0.04 |
| SR, mm/ha | 18.52(16.76) | 17.58(15.27) | 0.66 |
| Chest X-ray | |||
| CTI,%a | 59.31(11.11) | 60.70(9.19) | 0.54 |
| Pleural effusion, n(%) | 63(54.8) | 33(28.9) | < 0.0001 |
| Superior vena cava, n(%) | |||
| Dilatation | 33(26.8) | 2(1.7) | < 0.0001 |
| Compression | 8(6.5) | 0(0) | |
| Electrocardiography | |||
| P “pulmonale” pattern n(%) | 9(17.6) | 3(5.7) | 0.026 |
| Notched P wave, n(%) | 26(47.3) | 10(24.4) | 0.032 |
| PR segment depression, n(%) | 34(60.7) | 3(7.1) | < 0.0001 |
| STj elevation, n(%) | 26(49.1) | 14(26.4) | 0.027 |
| STj depression, n(%) | 10(28.6) | 2(6.5) | 0.026 |
| Low voltage QRS, n(%) | 59(57.8) | 17(20.0) | < 0.0001 |
| QRS alternans, n(%) | 6(6.0) | 0(0) | 0.032 |
| Arrhythmias and conduction disturbances, n(%) | |||
| Sinus tachycardia > 100 beats/min | 45(36.5) | 21(17.5) | 0.013 |
| Sinus bradycardia < 60 beats/min | 1(0.8) | 6(5.0) | |
| SVT | 4(3.2) | 1(0.8) | |
| AF/Atrial flutter | 14(11.38) | 29(24.1) | |
| Sinoatrial block | 2(1.6) | 1(0.8) | |
| AV block (I and II degree) | 3(2.4) | 1(0.8) | |
| BBB | 3(2.4) | 3(2.5) | |
AF — atrial fibrillation, AV — atrioventricular, BBB — bundle branch block, CVP — central venous pressure, DBP — diastolic blood pressure, HR — heart rate, SBP — systolic blood pressure, SR — sedimentation rate, SVT — supraventricular tachycardia.
a. Continuous variables presented as mean(SD).
Chest X-ray showed higher proportion of patients with pleural effusion and dilatation/compression of SVC (p < 0.0001 for both) in group of compression, though groups did not differ by CTI.
ECG analysis revealed association of compression with high frequency of P “pulmonale” pattern and notched P wave (p = 0.026 and p = 0.032), PR-segment depression (p < 0.0001), STj elevation or depression (p = 0.027 and p = 0.026, respectively), low voltage QRS and QRS alternans (p < 0.0001 and p = 0.032). Arrhythmias and conduction disturbances more often accompanied compression (p = 0.01), with high occurrence of sinus tachycardia.
3.2. Echocardiographic data
Analysis of echo data (Table 2) showed no differences between groups in cardiac chambers' size and mean pulmonary arterial pressure (p > 0.05). Patients with compression had low LVEF (p = 0.027); more often RA dilatation (p = 0.014), larger effusion amount (p < 0.0001); high proportion of large localized and diffuse effusions (p < 0.0001, p < 0.0001, p = 0.004), diffuse pericardial thickening and diffuse pericardial calcification (p < 0.0001 for both), and fibrin detachments (p < 0.0001) as compared to those without compression. Compression was associated with high proportion of IVS paradoxical movement, CCol, IVC plethora, flat AV groove, respiratory changes in the mitral and tricuspid flow patterns, reversal of flow in hepatic veins and their dilatation (p < 0.0001 for all).
| Variables | Compression + (n = 123) | Compression − (n = 120) | p |
|---|---|---|---|
| LA, mm | 33.78(8.75) | 35.89(10.43) | 0.10 |
| LVEDD, mm | 45.04(6.98) | 46.46(6.45) | 0.11 |
| LVESD, mm | 28.51(5.74) | 28.88(5.71) | 0.63 |
| LVEF,% | 63.43(9.50) | 66.05(8.03) | 0.027 |
| RV, mm | 20.55(5.40) | 19.67(7.00) | 0.30 |
| Mean PAP, mmHg | 29.92(12.81) | 30.31(14.09) | 0.85 |
| RA dilatation, n(%) | 32(27.6) | 16(13.9) | 0.014 |
| LA dilatation, n(%) | 26(22.8) | 41(34.5) | 0.06 |
| Effusion extent, n(%) | |||
| Localized | 15(12.2) | 58(48.3) | < 0.0001 |
| Diffuse | 88(71.5) | 32(26.7) | |
| Size of localized effusion, n(%) | |||
| Small < 5 mm | 2(5.4) | 12(20.7) | < 0.0001 |
| Moderate 5–9 mm | 1(7.7) | 35(60.3) | |
| Large ≥ 10 mm | 10(76.9) | 11(19) | |
| Size of diffuse effusion, n(%) | |||
| Small < 10 mm | 1(1.2) | 0(0) | 0.004 |
| Moderate 10–20 mm | 3(3.6) | 6(24) | |
| Large > 20 mm | 80 (95.2) | 19(76) | |
| Mean pericardial thickening, mm* | 1.20(1.85) | 0.30(0.30) | 0.48 |
| Pericardial thickening, n(%) | 60(48.8) | 37(30.8) | 0.006 |
| Extent of pericardial thickening, n(%) | |||
| Localized | 16 (13) | 34(28.3) | < 0.0001 |
| Diffuse | 44 (35.8) | 3(2.5) | |
| Calcification of pericardium, n(%) | 31(25.2) | 2(1.7) | < 0.0001 |
| Extent of pericardial calcification, n(%) | |||
| Localized | 12(9.8) | 2(1.7) | < 0.0001 |
| Diffuse | 19(15.4) | 0(0) | |
| Fibrin detachments, n(%) | 46(37.4) | 13(10.8) | < 0.0001 |
| IVS paradoxical movement, n(%) | 19(15.4) | 1(0.8) | < 0.0001 |
| IAS paradoxical movement, n(%) | 3(2.4) | 0(0) | 0.247 |
| Flat AV groove | 13(10.6) | 0(0) | < 0.0001 |
| IVC plethora, n(%) | 37(30.1) | 0(0) | < 0.0001 |
| Cardiac chamber collapse, n(%) | 43(35) | 1(0.8) | < 0.0001 |
| Mitral flow respiratory changes, n(%) | 88(71.5) | 24(20) | < 0.0001 |
| Tricuspid flow respiratory changes, n(%) | 89(72.4) | 17(14.2) | < 0.0001 |
| Hepatic veins dilatation, n(%) | 23(18.7) | 1(0.8) | < 0.0001 |
| Hepatic vein flow reversal, n(%) | 19(15.4) | 0(0) | < 0.0001 |
| Constriction, n(%) | 44(35.8) | 0(0) | < 0.0001 |
| Tamponade, n(%) | 53(43.1) | 0(0) | < 0.0001 |
AV — atrioventricular, IVC — inferior vena cava, IVS — interventricular septum, IAS — interatrial septum, LA — left atrium, LVEDD — left ventricular end-diastolic dimension, LVEF — left ventricular ejection fraction, LVESD — left ventricular end-systolic dimension, PAP — pulmonary arterial pressure, RA — right atrium, RV — right ventricle.
3.3. Type of intervention, perioperative data and outcomes (Table 3)
Majority of patients with compression underwent pericardiocentesis (54.5%) and pericardiectomy (27.6%), while controls received mostly medical therapy (56.7%) (p < 0.0001). Peri-interventional data demonstrated significantly larger mean effusion size with 80.6% of large effusions (> 400 ml) of mostly fibrinous and hemorrhagic nature (p < 0.0001, p = 0.02, p < 0.0001), as well as high proportion of diffuse adhesions and diffuse calcifications of pericardium (p < 0.0001, p < 0.0001) in compression group as compared to control group.
| Variables | Compression + (n = 123) | Compression − (n = 120) | p |
|---|---|---|---|
| Types of treatment, n(%) | |||
| Medical | 11(8.9) | 68 (56.7) | < 0.0001 |
| Pericardiocentesis | 67(54.5) | 10 (8.3) | |
| Pericardioectomy | 34 (27.6) | 0(0) | |
| Pericardial intervention along with other types of cardiac surgery | 8(6.5) | 40(33.3) | |
| Subxiphoid pericardiostomy | 3(2.4) | 2(1.7) | |
| Mean effusion size, ml (intraop., intervention, drainage)a | 1295.67(1023.53) | 290.68(461.94) | < 0.0001 |
| Effusion size by drainage/intraop., n(%) | |||
| Small < 100 ml | 6(8.3) | 1(6.7) | 0.02 |
| Moderate 100–400 ml | 8(11.1) | 6(40) | |
| Large > 400 ml | 58(80.6) | 8(53.3) | |
| Extent of effusion echo/drainage/intraop., n(%) | |||
| Small | 6(4.9) | 12(10) | < 0.0001 |
| Moderate | 5(4.1) | 47(39.2) | |
| Large | 93(75.6) | 31(25.8) | |
| Effusion type, n(%) | |||
| Serous | 52 (42.3) | 85 (70.8) | < 0.0001 |
| Purulent | 9(7.3) | 4(3.3) | |
| Hemorrhagic | 30(24.4) | 4(3.3) | |
| Fibrinous | 15(12.2) | 7(5.8) | |
| Adhesion and calcifications echo/intraop, n(%) | |||
| Adhesions | 27(22) | 55(45.8) | < 0.0001 |
| Adhesions and calcifications | 35(28.5) | 2(1.7) | |
| Extent of adhesions & calcifications echo/intraop, n(%) | |||
| Localized | 18(14.6) | 54(25) | < 0.00001 |
| Diffuse | 44(35.8) | 3(2.5) | |
| Type of pericardial involvement, n(%) | |||
| Effusive | 7(5.7) | 66(55) | < 0.0001 |
| Effusion with compression (tamponade) | 60(48.8) | 0(0) | |
| Constriction | 22(17.9) | 0(0) | |
| Constrictive-effusive | 23(18.7) | 0(0) | |
| Adhesive | 0(0) | 26(21.7) | |
| Adhesive-effusive | 2(1.6) | 28(23.3) | |
| Adhesive-effusive with compression | 9(7.3) | 0(0) | |
| Outcomes | |||
| Recurrence of PD | 16 (13) | 9 (7.5) | 0.017 |
| Death | 23 (18.7) | 1 (0.8) | |
| Rehospitalization due to HF | 1 (0.8) | 0 (0) | |
| Composite outcome n(%) | 45 (36.6) | 11 (9.2) | < 0.0001 |
Echo — echocardiography, HF — heart failure, intraop. — intraoperative, PD — pericardial disease.
a. Continuous variables presented as mean(SD).
Overall, in compression group 47.8% of patients had signs of tamponade and 17.9%/18.7% patients had constriction/constrictive–effusive involvement, while in control group patients had predominantly effusive, adhesive and adhesive/effusive involvement without signs of compression (p < 0.0001).
Analysis of outcomes revealed that 36.6% patients with compression and 9.2% of patients without compression had developed adverse outcomes (p < 0.0001). The difference between groups in outcomes was mostly due to higher mortality rate in patients with compression (18.7% vs. 0.8%, p = 0.017).
3.4. Predictors of compression syndrome development and diagnostic accuracy of markers of compression syndrome
Logistic regression analysis (Table 4) demonstrated male sex, NYHA class, tachycardia, low voltage QRS, presence of pleural effusion, SVC compression, extent of effusion and extent of pericardial thickening, paradoxical movement of IVS septum, flat AV groove, CCol, IVC plethora, respiratory changes in mitral and tricuspid flow patterns, and dilatation of and flow reversal in hepatic veins as univariate predictors of compression in patients with PD. However, only large effusion (> 20 mm) (OR 5.393, 95%CI 1.202–24.199, p = 0.028), CCol (OR 31.426, 95%CI 1.609–613-914, p = 0.023) and NYHA class > 3 (OR 8.671, 95%CI 1.730–43.451, p = 0.009) were multivariable predictors of compression development. The model including these three variables allowed predicting compression in 91.7% of cases.
| Variables | Univariate | Multivariable | |||
|---|---|---|---|---|---|
| Score | p | OR | 95%CI | p | |
| Age | 0.069 | 0.792 | |||
| NYHA class | 36.329 | < 0.0001 | 8.671 | 1.730–43.451 | 0.009 |
| Sex | 7.001 | 0.008 | |||
| HR | 12.217 | < 0.0001 | |||
| SBP | 2.464 | 0.117 | |||
| DBP | 0.065 | 0.799 | |||
| Active inflammation | 0.243 | 0.622 | |||
| Pleural effusion | 15.366 | < 0.0001 | |||
| SVC compression | 26.981 | < 0.0001 | |||
| Effusion | 25.599 | < 0.0001 | |||
| Large effusion | 22.236 | < 0.0001 | 5.393 | 1.202–24.199 | 0.028 |
| Pericardial thickening | 6.741 | 0.009 | |||
| Diffuse pericardial thickening | 11.057 | 0.001 | |||
| Flat AV groove | 8.171 | 0.004 | |||
| Paradoxical movement of IAS | 2.347 | 0.126 | |||
| Paradoxical movement of IVS | 11.736 | 0.001 | |||
| Cardiac chamber collapse | 26.163 | < 0.0001 | 31.426 | 1.609–613-914 | 0.023 |
| Tricuspid flow respiratory changes | 46.627 | < 0.0001 | |||
| Mitral flow respiratory changes | 39.778 | < 0.0001 | |||
| IVC plethora | 23.637 | < 0.0001 | |||
| Dilatation of hepatic veins | 12.656 | < 0.0001 | |||
| Hepatic vein flow reversal | 9.930 | 0.002 | |||
| Low voltage QRS | 28.191 | < 0.0001 | |||
| Arrhythmias&conduction disturbances | 0.839 | 0.360 | |||
Cox & Snell R Square—0.649 Nagelkerke R Square—0.871.
Predicted 91.7% correct.
AV — atrioventricular, CI — confidence interval, DBP — diastolic blood pressure, HR — heart rate, IAS — interatrial septum, IVC — inferior vena cava, IVS — interventricular septum, OR — odds ratio, PD — pericardial disease, SBP — systolic blood pressure, SVC — superior vena cava.
- OR, 95%CI and p values selected in bold reflect significance of association.
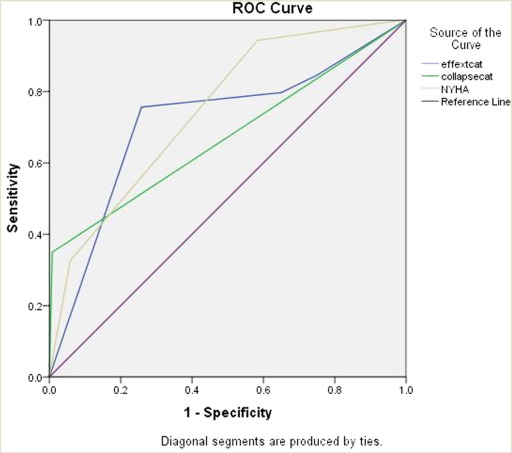
ROC analyses (Fig. 1) demonstrated that all three variables had significant diagnostic value with sensitivity of 75.6% and specificity of 74.2% for large effusion, low sensitivity and high specificity for CCol (35% and 92%) and NYHA class (32.5% and 94.2%).
Fig. 1.
Diagnostic value of large effusion, cardiac chamber collapse and NYHA class in prediction of compression: ROC analysis.
| AUC | 95%CI | p | Cut-off | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Large effusion | 0.71 | 0.647-0.782 | < 0.0001 | Large effusion > 20mm | 75.6% | 74.2% |
| Cardiac chamber collapse | 0.67 | 0.607-0.739 | < 0.0001 | Presence of collapse | 35% | 92% |
| NYHA class | 0.74 | 0.687-0.808 | < 0.0001 | NYHA 3 and higher | 32.5% | 94.2% |
| AUC – area under the curve, CI – confidence interval, ROC – receiver operator curve | ||||||
4. Discussion
Our study demonstrated that compression in PD was characterized by large diffuse effusion and diffuse calcification associated with tamponade and constriction physiology, accompanied by severe clinical manifestations, hemodynamic compromise, high occurrence of pleural effusion and compression of vena cava; high incidence of arrhythmias, and high rate of adverse outcomes. We also showed that patients with NYHA class > 3, signs of large effusion on echo > 20 mm and CCol were 5.3–31.4 times more likely to develop compression syndrome. These 3 predictors of tamponade and constriction were independent of sex, tachycardia, pleural effusion and SVC compression, low voltage QRS, diffuse thickening and calcification of pericardium, paradoxical movement of IVS and flat AV groove, plethora of IVC, constrictive pattern of mitral flow, respiratory changes of mitral and tricuspid flows, dilatation of hepatic veins and their flow reversal. Analysis of diagnostic accuracy of these parameters showed that presence of large effusion alone might identify correctly compression in 3/4 of cases, absence of CCol and absence of NYHA class > 3 might allow to withdraw diagnosis of compression in 92% of cases.
The results on clinical, echo, perioperative features of tamponade and constriction are in agreement with previous studies [1]; [2]; [3]; [5]; [7]; [9]; [13] ; [15]. Our data are also in concordance with previous investigations on prognostic significance of large effusion in pericarditis [24] ; [25], and pleural effusion in PPTS [43], diagnostic value of low voltage QRS in cardiac tamponade [20], as well as high diagnostic value of echo signs of tamponade [13] ; [14] and constriction [15]; [16] ; [17] in patients with PD.
We extended previous investigations [24]; [25] ; [26] demonstrating combined prognostic value and diagnostic accuracy of large effusion, CCol and high NYHA class in prediction of tamponade and constriction development. Though large effusion has been shown to have prognostic value in development of tamponade, we for the first time demonstrated the diagnostic accuracy of large effusion > 20 mm, CCol and NYHA class in prediction of compression development.
So far, only few studies evaluated the prognostic value of large effusion, CCol and IVC plethora in prediction of tamponade development [24] ; [25]. It has been shown that CCol and IVC plethora had no prognostic value, while size of pericardial effusion alone or when used in combination with other clinical parameters could predict tamponade development [24] ; [25]. Eisenberg et al. [24] in a study of 187 patients with pericardial effusion, among them 6% with large, 21% with moderate and 73% with small effusion, demonstrated RA collapse in 12% and RV collapse in 7% of patients and IVC plethora in 35% of patients. During the period of hospitalization 5% developed tamponade signs and 9% had tamponade signs treated by pericardiocentesis or surgery. Authors demonstrated, that among all above mentioned echo parameters, only effusion size had predictive value for tamponade development (OR 78, 95%CI 14–421, p = 0.0001). In a recent study [25] of 44 patients with uremic pericardial effusion, large effusions > 500 ml were detected in 30% and tamponade developed in 16% of patients. The predictive value of large effusion for tamponade and drainage was 100%. In our study, the predictive value of large effusion (OR 5.3) was lower than in study by Eisenberg et al. [24] and can be explained by differences in study population and design, in our study we included both patients with effusions and adhesions/calcifications. Yet, we found that in our heterogeneous population large effusion > 20 mm had reasonably high sensitivity of 75.6% and specificity of 74.2%, meaning when using this parameter alone compression might be predicted in 3/4 of patients with large effusion, though 1/4 of them may not develop compression.
In opposite to previous study [24], we demonstrated that presence of CCol increased by 31.496-fold risk of compression/tamponade development with only 8% of patients without compression syndrome would have tamponade development. It is known that about 1/3 of patients without tamponade might have any CCol [26]. Merce et al. [26] in a study of 110 patients with pericardial effusion demonstrated that among 38 patients with clinical signs of tamponade 90% had CCol, while in patients without tamponade collapse were registered in 34% of patents, yielding 90% sensitivity and 65% specificity for any chamber collapse. Our study is distinctive by the fact that we demonstrated diagnostic value of CCol in prediction of compression development. Based on our and Merce et al. [26] study results, we can suppose that in about 1/3 of those patients with effusion and CCol but without tamponade signs risk of further tamponade development is 31-fold high.
We also found that our patients with NYHA class > 3 had 8.681-fold high risk of compression development. Previous studies [2]; [3]; [5] ; [7], demonstrated NYHA class as a predictor of adverse outcomes in patients with constrictive pericarditis and tamponade, undergoing surgery. High NYHA class had low sensitivity and high specificity in our study and it might have not value when used alone, as it may accompany advanced structural heart disease.
Overall, results on diagnostic accuracy of 3 predictors of compression, suggest their complementary diagnostic and predictive value, model including all three parameters allowed to correctly predict syndrome of compression in 91.4% of patients with signs of tamponade and constriction. This relatively simple model will allow to rise suspicion of compression syndrome in patients with PD, even without specific clinical signs, using bedside echo or in facilities without advanced diagnostic methods and refer early patients for evaluation and proper management.
We should also emphasize that multivariable predictors of compression were only those characteristic for tamponade: CCol and large effusion, this can be explained by the fact that compression by tamponade in our patients was due to relatively faster accumulation of effusion as compared to development of thickening and calcification of pericardium in constriction. We also cannot exclude the factor of heterogeneity of the patient group with compression, including majority with effusion/tamponade and only 1/3 with constriction.
5. Study limitations
The potential limitations of the study are the relatively small sample, retrospective analysis, and inclusion patients with tamponade and constrictive pericarditis. This requires further study on predictors of compression syndrome separately in patients with tamponade and constrictive pericarditis.
6. Conclusion
The independent predictors of compression development are presence of large effusion > 20 mm, cardiac chamber collapse and high NYHA class. The model including all three parameters allows correctly predicting compression in 91.4% of cases. The diagnostic accuracy of each parameter is characterized by high sensitivity and specificity of large effusion, high specificity of cardiac chamber collapse and NYHA class.
Conflict of interest
There is no conflict of interest to declare.
Funding
The study was not supported by any material or monetary means, grants of government funding.
Appendix A. List of abbreviations used in manuscript, tables and figure
| AF | atrial fibrillation |
| AUC | area under the curve |
| AV | atrioventricular |
| BBB | bundle branch block |
| CCol | cardiac chamber collapse |
| CI | confidence interval |
| CP | constrictive pericarditis |
| CRP | C-reactive protein |
| CTI | cardio-thoracic index |
| CVP | central venous pressure |
| DBP | diastolic blood pressure |
| ECG | electrocardiogram |
| echo | echocardiography |
| HF | heart failure |
| HR | heart rate |
| IAS | interatrial septum |
| intraop. | intraoperative |
| IVC | inferior vena cava |
| IVS | interventricular septum |
| LA | left atrium |
| LRA | logistic regression analysis |
| LV | left ventricle |
| LVEDD | left ventricular end diastolic dimension |
| LVEF | left ventricular ejection fraction |
| LVESD | left ventricular end systolic dimension |
| NYHA | New York Heart Association |
| OR | odds ratio |
| PAP | pulmonary arterial pressure |
| PD | pericardial disease |
| PPTS | postpericardiotomy syndrome |
| RA | right atrium |
| ROC | receiver operator curve |
| RV | right ventricle |
| SBP | systolic blood pressure |
| SD | standard deviation |
| SR | sedimentation rate |
| SVC | superior vena cava |
| SVT | supraventricular tachycardia |
References
- [1] Y. Adler, P. Charron, M. Imazio, L. Badano, G. Barón-Esquivias, J. Bogaert, et al.; 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS); Eur. Heart J., 36 (2015), pp. 2921–2964
- [2] S.C. Bertog, S.K. Thambidorai, K. Parakh, P. Schoenhagen, V. Ozduran, P.L. Houghtaling, et al.; Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy; J. Am. Coll. Cardiol., 43 (2004), pp. 1445–1452
- [3] P.A. DeValeria, W.A. Baumgartner, A.S. Casale, P.S. Greene, D.E. Cameron, T.J. Gardner, et al.; Current indications, risks, and outcome after pericardiectomy; Ann. Thorac. Surg., 52 (1991), pp. 219–224
- [4] A.K. Mutyaba, S. Balkaran, R. Cloete, N. du Plessis, M. Badri, J. Brink, B.M. Mayosi; Constrictive pericarditis requiring pericardiectomy at Groote Schuur Hospital, Cape Town, South Africa: causes and perioperative outcomes in the HIV era (1990–2012); J. Thorac. Cardiovasc. Surg., 148 (2014), pp. 3058–3065 (e1)
- [5] A. Porta-Sánchez, J. Sagristà-Sauleda, I. Ferreira-González, A. Torrents-Fernández, I. Roca-Luque, D. García-Dorado; Constrictive pericarditis: etiologic spectrum, patterns of clinical presentation, prognostic factors, and long-term follow-up; Rev. Esp. Cardiol., 68 (2015), pp. 1092–1100
- [6] M. Imazio, B. Demichelis, I. Parrini, M. Giuggia, E. Cecchi, G. Gaschino, et al.; Day-hospital treatment of acute pericarditis: a management program for outpatient therapy; J. Am. Coll. Cardiol., 43 (2004), pp. 1042–1046
- [7] L.H. Ling, J.K. Oh, H.V. Schaff, G.K. Danielson, D.W. Mahoney, J.B. Seward, et al.; Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy; Circulation, 100 (1999), pp. 1380–1386
- [8] R.B.H. Myers, D.H. Spodick; Constrictive pericarditis: clinical and pathophysiologic characteristics; Am. Heart J., 138 (1999), pp. 219–232
- [9] B. Maisch, P.M. Seferovic, A.D. Ristic, R. Erbel, R. Rienmuller, Y. Adler, et al.; Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary: the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology; Eur. Heart J., 25 (2004), pp. 587–610
- [10] E. Argulian, F. Messerli; Misconceptions and facts about pericardial effusion and tamponade; Am. J. Med., 126 (2013), pp. 858–861
- [11] B.A. Guberman, N.O. Fowler, P.J. Engel, M. Gueron, J.M. Allen; Cardiac tamponade in medical patients; Circulation, 64 (1981), pp. 633–640
- [12] M.J. Levine, B.H. Lorell, D.J. Diver, P.C. Come; Implications of echocardiographically assisted diagnosis of pericardial tamponade in contemporary medical patients: detection before hemodynamic embarrassment; J. Am. Coll. Cardiol., 17 (1991), pp. 59–65
- [13] M. Imazio, Y. Adler; Management of pericardial effusion; Eur. Heart J., 34 (2013), pp. 1186–1197
- [14] R. Himmelman, B. Kircher, D. Rockey, N. Schiller; Inferior vena cava plethora with blunted respiratory response: a sensitive echocardiographic sign of cardiac tamponade; J. Am. Coll. Cardiol., 12 (1988), pp. 1470–1477
- [15] J.P. Dal Bianco, P.P. Sengupta, F. Mookadam, K. Chandrasekaran, A.J. Tajik, B.K. Khanderia; Role of echocardiography in the diagnosis of constrictive pericarditis; J. Am. Soc. Echocardiogr., 22 (2009), pp. 24–33
- [16] P.J. Engel, N.O. Fowler, C.W. Tei, P.M. Shah, H.J. Driedger, R. Shabetai, et al.; M-mode echocardiography in constrictive pericarditis; J. Am. Coll. Cardiol., 6 (1985), pp. 471–474
- [17] L.K. Hatle, C.P. Appleton, R.L. Popp; Differentiation of constrictive pericarditis and restrictive cardiomyopathy by Doppler echocardiography; Circulation, 79 (1989), pp. 357–370
- [18] Y. Ohta, F. Miyoshi, T. Kaminou, Y. Kaetsu, T. Ogawa; The evaluation of cardiac tamponade risk in patients with pericardial effusion detected by non-gated chest CT; Acta Radiol., 57 (2016), pp. 538–546
- [19] C.L. Roy, M.F. Minor, M.A. Brookhart, N.K. Choudry; Does this patient with pericardial effusion have cardiac tamponade; JAMA, 297 (2007), pp. 810–818
- [20] M.J. Eisenberg, L.M. Romeral, P.A. Heidenrich, N.B. Schiller, G.T. Evans; The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG. A technology assessment; Chest, 110 (1996), pp. 318–324
- [21] J. Sagrista-Salueda, J. Angel, G. Permanyer-Miralda, J. Soler-Soler; Long-term follow-up of idiopathic chronic pericardial effusion; N. Engl. J. Med., 341 (1999), pp. 2054–2059
- [22] W.C. Little, G.L. Freeman; Pericardial disease; Circulation, 113 (2006), pp. 1622–1632
- [23] P.K. Suwan, S. Potjalongsilp; Predictors of constrictive pericarditis after tuberculous pericarditis; Br. Heart J., 73 (1995), pp. 187–189
- [24] M.J. Eisenberg, K. Oken, O. Guerrero, M.A. Saniei, N.B. Schiller; Prognostic value of echocardiography in hospitalized patients with pericardial effusion; Am. J. Cardiol., 70 (1992), pp. 934–939
- [25] S. Battaile, P. Brunet, A. Decourt, G. Bonnet, A. Loundou, S. Berland, et al.; Serum albumin and size of pericardial effusion predict drainage necessity; J. Nephrol., 28 (2015), pp. 97–104
- [26] J. Merce, J. Sagrista-Salueda, G. Permanyer-Miralda, A. Evangelista, J. Soler-Soler; Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusions: implementations for diagnosis of cardiac tamponade; Am. Heart J., 138 (1999), pp. 759–764
- [27] R.M. Lang, L.P. Badano, V. Mor-Avi, J. Afilalo, A. Armstrong, L. Ernande, et al.; Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging; J. Am. Soc. Echocardiogr., 28 (2015), pp. 1–39
- [28] A.L. Klein, S. Abbara, D.A. Agler, C.P. Appleton, C.R. Asher, B. Hoit, et al.; American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease endorsed by the society for cardiovascular magnetic resonance and society of cardiovascular computed tomography; J. Am. Soc. Echocardiogr., 26 (2013), pp. 965–1012
- [29] S. Singh, L.S. Wann, G.H. Schuchard, H.S. Klopfenstein, P.P. Leimgruber, M.H. Keelan Jr., et al.; Right ventricular and right atrial collapse in patients with cardiac tamponade—a combined echocardiographic and hemodynamic study; Circulation, 70 (1984), pp. 966–971
- [30] W. Armstrong, B. Schilt, D. Helper, J.C. Dillon, H. Feigenbaum; Diastolic collapse of the right ventricle with cardiac tamponade: an echocardiographic study; Circulation, 65 (1982), pp. 1491–1496
- [31] K. Chuttani, N.G. Pandian, P.K. Mohanty, K. Rosenfield, S.L. Schwartz, J.E. Udelson, et al.; Left ventricular diastolic collapse. An echocardiographic sign of regional cardiac tamponade; Circulation, 83 (1991), pp. 1999–2006
- [32] C.P. Appleton, L.K. Hatle, R. Popp; Cardiac tamponade and pericardial effusion: respiratory variation in transvalvular flow velocities studied by Doppler echocardiography; J. Am. Coll. Cardiol., 11 (1988), pp. 1020–1030
- [33] J.K. Oh, H. Lk, J.B. Seward, G.K. Danielson, H.V. Schaff, G.S. Reeder, et al.; Diagnostic role of Doppler echocardiography in constrictive pericarditis; J. Am. Coll. Cardiol., 23 (1994), pp. 154–162
- [34] M.S. Horowitz, C.S. Schultz, E.B. Stinson, D.C. Harrison, R.L. Popp; Sensitivity and specificity of echocardiographic diagnosis of pericardial effusion; Circulation, 50 (1974), pp. 239–247
- [35] T. Kudaiberdiev, A. Dzhumagulova, S. Joshibayev, K. Tilemanbetova, G. Imanalieva; Electrocardiographic abnormalities in patients with pericardial disease — association of PR segment depression with arrhythmias and clinical signs: experience of cardiac surgery center; J. Electrocardiol., 49 (2016), pp. 29–36
- [36] D.H. Spodick; Diagnostic electrocardiographic sequences in acute pericarditis. Significance of PR segment and PR vector changes; Circulation, 48 (1973), pp. 575–580
- [37] D. Avgoustakis, D. Lazarides, D. Athanasiades, G. Michaelides; Electrocardiogram in constrictive P before and after radical pericardiectomy; Chest, 57 (1970), pp. 460–467
- [38] D. Spodick; Frequency of arrhythmias in acute pericarditis determined by Holter monitoring; Am. J. Cardiol., 53 (1984), pp. 842–845
- [39] B. Maisch, A.D. Ristić, P.M. Seferović, T.S.M. Tsang (Eds.), Interventional Pericardiology: Pericardiocentesis, Pericardioscopy, Pericardial Biopsy, Balloon Pericardiotomy and Intrapericardial Therapy, Springer Medizin Verlag, Heidelberg, Germany (2011), p. 184
- [40] J.W. Kirklin, B.G. Barrat-Boyes; Cardiac Surgery: Morphology, Diagnostic Criteria, Natural History, Techniques, Results, and Indications; (second ed.)Churchill Livingstone, New York (1999), p. 1779
- [41] M. Pepi, M. Muratori, P. Barbier, E. Doria, V. Arena, M. Berti, et al.; Pericardial effusion after cardiac surgery: incidence, site, size and hemodynamic consequences; Br. Heart J., 72 (1994), pp. 327–331
- [42] T.S. Tsang, M.E. Barnes, B.J. Gersh, K.R. Bailey, J.B. Seward; Outcomes of clinically significant idiopathic pericardial effusion requiring intervention; Am. J. Cardiol., 91 (2003), pp. 704–707
- [43] J. Lehto, J. Gunn, P. Karjalainen, J. Airaksinen, T. Kiviniemi; Incidence and risk factors of postpericardiotomy syndrome requiring medical attention: the Finland postpericardiotomy syndrome study; J. Thorac. Cardiovasc. Surg., 149 (2015), pp. 1324–1329
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?