Abstract
Background
Detection of concurrent diastolic dysfunction (DD) may be beneficial in patients with persistent and longstanding persistent atrial fibrillation (AF). The role of transthoracic echocardiography (TTE) in assessing DD in patients with AF has not been well characterized. We sought to determine the utility of TTE in detecting elevated left atrial pressure (LAP) in patients with persistent and longstanding persistent non-valvular AF using directly measured LAP as the reference standard.
Methods
We retrospectively studied 157 patients with persistent AF and preserved left ventricular ejection fraction who underwent pulmonary vein isolation (PVI). LAP was determined in conjunction with trans-septal puncture at the time of catheter ablation. TTE was performed 1 day after PVI and included two dimensional, pulse wave spectral Doppler and tissue Doppler assessments.
Results
The clinical parameter that strongly correlated with elevated LAP is longstanding persistent AF. Four strongest TTE parameters identified to moderately correlate with LAP include 1. left atrial minimum volume (LAVmin), 2. peak velocity of early mitral diastolic inflow velocity (E), 3. pulmonary vein systolic flow velocity (PVS), and 4. ratio of early diastolic transmitral inflow velocity to mitral annular velocity at the lateral site (E/E′ lateral).
Conclusion
Accurate assessment of diastolic dysfunction in patients with persistent and longstanding persistent AF is difficult using TTE. A combination of LAVmin, PVS, and E might be helpful to determine elevated LAP.
Abbreviations
AF, atrial fibrillation;HF, heart failure;LAP, left atrial pressure;RFA, radiofrequency catheter ablation;TTE, transthoracic echocardiography;LA, left atrium;LAA, left atrial area;LAV, left atrial volume;LAVi, indexed left atrial volume;E, early transmitral diastolic inflow;DT, deceleration time;PVS, pulmonary vein systolic flow velocity;PVD, pulmonary vein diastolic flow velocity;E/E′, ratio of early peak transmitral inflow to mitral annular motion velocity
Keywords
Persistent atrial fibrillation;Diastolic dysfunction;Echocardiography
1. Introduction
Atrial fibrillation (AF) and heart failure (HF) are among the most common cardiac diseases encountered in clinical practice, and their incidence is rising as the population ages. Patients with concurrent AF and chronic HF, including those with preserved ejection fraction, have worse prognosis than patients with AF alone [1] ; [2]. Accurate diagnosis of coexisting diseases is essential to the initiation of appropriate therapies. Recent studies have demonstrated the importance of early detection of diastolic dysfunction (DD) in patients with persistent and longstanding persistent AF [3]; [4]; [5] ; [6]. Transthoracic echocardiography (TTE) is routinely used in the evaluation of diastolic function, but its utility in detecting DD in patients with persistent and longstanding persistent AF has not been well characterized. The ventricular filling index, or the ratio of early diastolic transmitral velocity E and early diastolic tissue Doppler velocity of the mitral annulus E/E′, is one of the commonly used TTE parameters that is sensitive for detection of elevated left atrial pressure (LAP) in patients with preserved ejection fraction [7] ; [8]. There is work to suggest that E/E′ may also be sensitive for the detection of elevated LAP in a small cohort of 27 non-valvular AF patients; E/E′ septal ≥ 11 predicts elevated LV filling pressure (≥ 15 mm Hg) with sensitivity of 75% and specificity of 93% [9]. E/E′ also correlated with symptomatic HF in AF patients with preserved ejection fraction (EF) and E/E′ decreased with symptomatic improvement [10]. However, the potential role of other routinely used TTE parameters of diastolic function in detecting elevated LAP in patients with AF is not clear. Therefore, we sought to determine the utility of two dimensional (2D), pulsed wave spectral Doppler (PWD), and tissue Doppler imaging (TDI) echocardiographic parameters in detecting elevated LAP in a large cohort of patients with persistent and longstanding persistent non-valvular AF using directly measured LAP as the reference standard.
2. Methods
2.1. Study population
The study protocol was approved by the Institutional Review Board at our institution with waiver of consent for retrospective review of existing clinical images. There were 493 patients with drug refractory persistent and longstanding persistent AF who underwent pulmonary vein isolation (PVI) by radiofrequency catheter ablation at our institution between April 2009 and April 2011. The patients were excluded from analysis if they had previous catheter ablation (251 patients), a history of open heart surgery (17 patients), moderate or severe mitral regurgitation (14 patients), reduced left ventricular (LV) ejection fraction (EF) (< 50%, 30 patients), mitral annular calcification (5 patients), pacemaker dependency (4 patients), were in AF at time of TTE (7 patients), and no left atrial (LA) pressure registration (25 patients). Of the remaining 157 patients with chronic AF, 121 had persistent AF, defined as continuous AF for greater than 7 days or cardioversion after 48 h of continuous AF [11], and 36 had longstanding persistent AF, defined as continuous AF for greater than 12 months [11]. Clinical data were obtained by reviewing medical records.
2.2. Echocardiography
All patients underwent routine clinical TTE examinations including M-mode, 2D, PWD and TDI on the first post-procedural day following PVI using commercially available echocardiographic machines (Vivid 7; GE Healthcare Technologies, Waukesha, Wisconsin, USA or iE33, Philips Health Care, the Netherlands). All studies were analyzed in a blinded fashion on dedicated workstations (ProSolv CardioVascular Client version 4.0.4). LA diameter (LAD) was assessed in the parasternal long-axis view (PLAX). LA area (LAA) and LA length (LAL) were measured in the apical 4-chamber view (4C) and apical 2-chamber view (2C). LA volume (LAV) was derived using the biplane area–length method. Both LAA and LAV were measured at LV end-systole (LA maximum volume (LAVmax); LA maximum area (LAAmax)) and at LV end-diastole (LA minimum volume (LAVmin); LA minimum area (LAAmin)). LA volume index (LAVi) was calculated based on body surface area (BSA). Mitral inflow measurements using PWD included peak early flow velocity (E) and deceleration time of early mitral flow velocity (DT). Peak late mitral inflow velocity was not measured due to the diminutive atrial contraction post ablation. Pulmonary venous flow on PWD was characterized by peak systolic flow velocity (PVS), peak diastolic flow velocity (PVD) and systolic filling fraction or the ratio of PVS to PVD (PVSD). TDI using spectral Doppler including early velocities from the septal and lateral mitral annulus (E′ septal and E′ lat respectively) was obtained from the apical 4C view. E/E′ was calculated for both lateral and septal annular sites (E/E′ lateral and E/E′ septal respectively) and was also averaged between the two sites (E/E′ average).
2.3. Catheter ablation and left atrial pressure recordings
PVI was performed per routine at our institution as previously described [12] ; [13]. Briefly, multipolar catheters were placed in the coronary sinus and posterior right atrium (RA) and a diagnostic intracardiac ultrasound catheter was advanced to the RA. Two trans-septal punctures were performed through which the ablation and circular mapping catheters were advanced into the LA. A bolus of unfractionated heparin was administered prior to the first trans-septal puncture and infusion was titrated to maintain activated clotting time between 350 and 400 s for the duration of the procedure. Immediately after the trans-septal access, the LA pressure was transduced through the trans-septal needle. LA x-wave pressure nadir, LA peak v-wave pressure, and mean LA pressure were recorded using an electrophysiologic recording system (Prucka-GE, Houston, TX, USA). Elevated LAP was defined as mean LAP equal to or greater than 15 mm Hg. All patients underwent antral PVI [13].
2.4. Statistical analysis
Statistical analyses were performed using STATA software (version 10, StataCorp, Texas, USA). Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as percentages. Relationship between mean LAP and TTE parameters were evaluated using univariate linear regression analysis followed by multiple linear regression. Potential determinants of elevated LAP were identified by univariate logistic regression analysis and all identified parameters were entered into multiple logistic regression. Comparison of data between the normal and elevated LAP groups was performed by use of a two-tailed, unpaired Students t test. p values of < 0.05 were considered statistically significant.
2.5. Reproducibility
Fifteen randomly selected studies were read by two independent blinded observers to assessing inter-observer variability. The same studies were re-interpreted by the first reader in a blinded fashion 3 months after the initial reading to assess intra-observer variability.
3. Results
3.1. Baseline clinical characteristics
Baseline patient characteristics are shown in Table 1. The patients' mean age was 60.6 ± 8.7 years (range 36 to 78 years). This group of patients is predominantly male and hypertensive. Seventy-seven percent of patients had persistent AF, whereas 23% had longstanding persistent AF. All patients had preserved LVEF (≥ 50%).
| Characteristics | Total (n = 157) |
|---|---|
| Demographics and comorbidities | |
| Age (years) | 60.6 ± 8.7 |
| BSA (m2) | 2.2 ± 0.2 |
| Gender (% male) | 82 |
| Hypertension (%) | 67 |
| Diabetes mellitus (%) | 17 |
| Stroke (%) | 6 |
| LVEF (%) | 61.4 ± 6.6 |
| Coronary artery disease (%) | 13 |
| History of heart failure (%) | 3 |
| Pacemaker or ICD (%) | 3 |
| Asthma, COPD (%) | 8 |
| Obstructive sleep apnea (%) | 26 |
| Thyroid disease (%) | 7 |
| Atrial fibrillation (%) | |
| Longstanding persistent | 23 |
| Persistent | 77 |
| Baseline medications (%) | |
| ACE Inhibitor | 26 |
| Angiotensin receptor blocker | 12 |
| Diuretic | 26 |
| Inhaled Beta agonist | 3 |
| Beta blocker | 40 |
| AAD Class I. | 31 |
| AAD Class III. | 70 |
| AAD Class IV. | 22 |
| Digoxin | 16 |
Abbreviations: LVEF = left ventricular ejection fraction, ICD = implantable cardioverter-defibrillator, COPD = chronic obstructive pulmonary disease, ACE = angiotensin converting enzyme, ARB = angiotensin receptor blocker, AAD = anti-arrhythmic drug.
3.2. Clinical characteristics and mean left atrial pressure
Clinical characteristics between patients with normal and elevated LAP are shown in Table 2. BSA and longstanding persistent AF demonstrated significant association with elevated LAP.
| Clinical parameters | LAP ≥ 15 mm Hg (n = 84) | LAP < 15 mm Hg (n = 73) | p Value |
|---|---|---|---|
| BSA (m2) | 2.2 ± 0.3 | 2.1 ± 0.2 | 0.030 |
| Age | 60.9 ± 8.4 | 60.5 ± 9.1 | 0.390 |
| CHADS2 score | 1.2 ± 0.9 | 1.0 ± 0.8 | 0.060 |
| Gender (male, %) | 78 | 85 | 0.300 |
| Hypertension (%) | 66.7 | 67.1 | 0.950 |
| Diabetes mellitus (%) | 21.4 | 10.9 | 0.078 |
| Coronary artery disease (%) | 13.1 | 12.3 | 0.886 |
| COPD (%) | 5.9 | 5.5 | 0.890 |
| Obstructive sleep apnea (%) | 27.0 | 23.3 | 0.570 |
| Lone AF (%) | 10.7 | 16.4 | 0.290 |
| Longstanding persistent AF (%) | 30.9 | 13.7 | 0.010 |
Abbreviations as in Table 1. Significance: p < 0.05.
3.3. Echocardiographic parameters and mean left atrial pressure
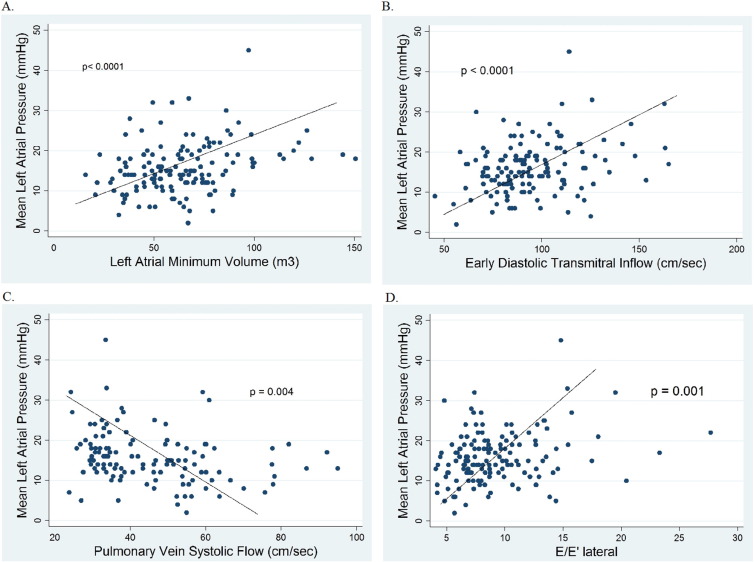
The correlation of mean LAP with various TTE parameters of diastolic function is shown in Table 3 and Fig. 1. Among 2D parameters, LA minimum volume (LAVmin) had the strongest correlation with mean LAP (r = 0.30, p < 0.0001). Among PWD parameters, E (r = 0.31, p < 0.0001), PVS (r = 0.26, p = 0.004) and PVSD (r = 0.24, p = 0.01) demonstrated a significant correlation with mean LAP. Among TDI parameters, E/E′ lateral (r = 0.27, p = 0.001), E/E′ septal (r = 0.20, p = 0.016), and E/E′ average (r = 0.28, p = 0.001) showed significant correlation with LAP. On multiple linear regression analysis, LAVmin (p = 0.002), PVS (p = 0.01), and E/E′ lateral (p = 0.02) were the best independent parameters correlated with LAP (r = 0.46).
| Echocardiographic parameters | Univariate linear regression | Multiple linear regression |
|---|---|---|
| p Value | p Value | |
| 2D | ||
| LA diameter (mm) | 0.14 | |
| LAA maximum (4C, cm2) | 0.01 | |
| LAA minimum (4C, cm2) | 0.008 | |
| LA max. length (4C, cm) | 0.09 | |
| LA min length (4C, cm) | 0.29 | |
| LAA maximum (2C, cm2) | 0.03 | |
| LAA area minimum (2C, cm2) | 0.004 | |
| LA max. length (2C, cm) | 0.02 | |
| LA min length (2C, cm) | 0.01 | |
| LAVmax (cm3) | 0.007 | |
| LAVmin (cm3) | < 0.0001 | 0.002 |
| LAVimax (cm3/m2) | 0.11 | |
| LAVimin (cm3/m2) | 0.03 | |
| LA ejection fraction (%) | 0.02 | |
| PWD | ||
| E (cm/s) | < 0.0001 | |
| Deceleration time (sec) | 0.76 | |
| PVS (cm/s) | 0.004 | 0.012 |
| PVD (cm/s) | 0.97 | |
| PVSD | 0.01 | |
| TDI | ||
| E′ lateral (cm) | 0.11 | |
| E′ septal (cm) | 0.71 | |
| E′ avg. (cm) | 0.16 | |
| E/E′ lateral | 0.001 | 0.016 |
| E/E′ septal | 0.016 | |
| E/E′ average | 0.001 |
Abbreviations: LA = left atrium, 4C = four chamber view, 2C = two chamber view, LAA = LA area, LAV = LA volume, LAVmax = LA maximum volume, LAVmin = LA minimum volume, LAVi = LA volume indexed to body surface area, E = early diastolic transmitral flow velocity, E′ lateral, septal, avg. are mitral annular velocity at lateral, septal side and average of them, respectively, E/E′ = ratio of early diastolic transmitral flow velocity to mitral annular motion velocity, PVS = pulmonary vein systolic flow velocity, PVD = pulmonary vein diastolic flow velocity, PVSD = ratio of PVS to PVD. Significance: p < 0.05.
|
|
|
Fig. 1. Scatter plots of mean left atrial pressure to TTE parameters with the strongest correlation. A. Left atrial minimum volume (p < 0.0001). B. Peak early diastolic transmitral inflow (p = 0.002). C. Pulmonary vein systolic flow (p = 0.006). D. E/E′ lateral (p = 0.008). |
3.4. Echocardiographic determination of elevated mean left atrial pressure
A number of TTE parameters were found to be significantly different in patients with normal and elevated LAP, shown in Table 4. The most robust difference was seen in the same four parameters in linear regression, namely, LAVmin (56 ± 18 vs. 72 ± 27 cm3, p < 0.0001), PVS (49 ± 16 vs. 42 ± 14 cm/s, p = 0.006), E (88 ± 20 vs. 99 ± 23 cm/s, p = 0.002) and E/E′ lateral (8.4 ± 3.1 vs. 9.9 ± 4.2, p = 0.008). The result of simple logistic regression analysis for predictors of elevated LAP is displayed in Table 5. Among 2D TTE parameters, the strongest predictor of LAP ≥ 15 mm Hg was LAVmin (p ≤ 0.0001). The strongest PWD parameter was E (p = 0.01) while PVS (p = 0.02) and PVSD (p = 0.04) were also significant parameters. Among TDI parameters, the strongest predictors of LAP ≥ 15 mm Hg were E/E′ lateral (p = 0.02) and E/E′ average (p = 0.03). Using multiple logistic regression, LAVmin (p = 0.001) and PVS (p = 0.015) were found to be the best independent predictors of elevated LAP with an area under the curve (AUC) of 0.72. The second best logistic model included LAVmin (p < 0.001) and E/E′ lateral (p = 0.048) with an AUC of 0.70. The third best logistic model was LAVmin (p = 0.001) and E (p = 0.035) with an AUC of 0.70.
| Echocardiographic parameters | LAP ≥ 15 mm Hg (n = 84) | LAP < 15 mm Hg (n = 73) | p Value |
|---|---|---|---|
| 2D | |||
| LA diameter (mm) | 42.6 ± 11.8 | 40.0 ± 11.8 | 0.145 |
| LAA maximum (4C, cm2) | 25.5 ± 5.7 | 23.2 ± 4.5 | 0.004 |
| LAA minimum (4C, cm2) | 19 ± 5.4 | 17 ± 3.8 | 0.004 |
| LA max. length (4C, cm) | 5.4 ± 0.6 | 5.3 ± 0.6 | 0.037 |
| LA min length (4C, cm) | 4.6 ± 0.6 | 4.5 ± 0.6 | 0.210 |
| LAA maximum (2C, cm2) | 25.5 ± 5.4 | 23.0 ± 4.2 | 0.001 |
| LAA minimum (2C, cm2) | 19.4 ± 4.7 | 17.0 ± 4.1 | 0.001 |
| LA max. length (2C, cm) | 5.5 ± 0.7 | 5.1 ± 0.6 | < 0.0001 |
| LA min length (2C, cm) | 4.7 ± 0.6 | 4.4 ± 0.6 | < 0.0001 |
| LAVmax (cm3) | 107 ± 36 | 89 ± 23 | < 0.0001 |
| LAVmin (cm3) | 72 ± 27 | 56 ± 18 | < 0.0001 |
| LAVimax (cm3/m2) | 48 ± 16 | 42 ± 11 | 0.008 |
| LAVimin (cm3/m2) | 32 ± 13 | 27 ± 8 | 0.004 |
| LA ejection fraction (%) | 33 ± 11 | 37 ± 13 | 0.010 |
| PWD | |||
| E (cm/s) | 99 ± 23 | 88 ± 20 | 0.002 |
| Deceleration time (sec) | 200 ± 4 | 198 ± 5 | 0.390 |
| PVS (cm/s) | 42 ± 2 | 49 ± 2 | 0.006 |
| PVD (cm/s) | 65 ± 2 | 66 ± 3 | 0.380 |
| PVSD | 0.68 ± 0.03 | 0.79 ± 0.04 | 0.020 |
| TDI | |||
| E′ lateral (cm) | 10.9 ± 2.9 | 11.4 ± 3.1 | 0.150 |
| E′ septal (cm) | 9.5 ± 2.7 | 9.1 ± 2.7 | 0.170 |
| E′ avg. (cm) | 10.0 ± 2.6 | 10.2 ± 2.5 | 0.290 |
| E/E′ lateral | 9.9 ± 4.1 | 8.4 ± 3.1 | 0.008 |
| E/E′ septal | 11.4 ± 4.6 | 10.7 ± 3.8 | 0.180 |
| E/E′ average | 10.8 ± 4.3 | 9.3 ± 3.1 | 0.010 |
Abbreviations as in Table 3. Significance: p < 0.05.
| Echocardiographic parameters | Univariate logistic regression (LAP ≥ 15 mm Hg) | |
|---|---|---|
| p | OR [95% CI] | |
| 2D | ||
| LA diameter | 0.291 | 0.98 [0.96–1.0] |
| LAA maximum (4C) | 0.010 | 1.10 [1.02–1.2] |
| LAA minimum (4C) | 0.010 | 1.10 [1.02–1.2] |
| LA max. length (4C) | 0.076 | 1.60 [0.95–2.7] |
| LA min length (4C) | 0.421 | 1.24 [0.73–2.1] |
| LAA maximum (2C) | 0.004 | 1.11 [1.03–1.2] |
| LAA minimum (2C) | 0.002 | 1.14 [1.05–1.23] |
| LA max. length (2C) | 0.001 | 2.54 [1.45–4.46] |
| LA min length (2C) | 0.001 | 2.78 [1.54–5.03] |
| LAVmax | 0.001 | 1.02 [1.00–1.04] |
| LAVmin | < 0.0001 | 1.03 [1.02–1.05] |
| LAVimax | 0.019 | 1.03 [1.01–1.06] |
| LAVimin | 0.010 | 1.05 [1.01–1.08] |
| LA ejection fraction | 0.031 | 0.97 [0.94–1.00] |
| PWD | ||
| E | 0.005 | 1.02 [1.0–1.04] |
| Deceleration time | 0.794 | 1.00 [1.0–1.01] |
| PVS | 0.016 | 0.97 [0.95–1.0] |
| PVD | 0.756 | 1.00 [0.98–1.02] |
| PVSD | 0.044 | 0.25 [0.06–0.96] |
| TDI | ||
| E′ lateral | 0.307 | 0.94 [0.84–1.05] |
| E′ septal | 0.352 | 1.06 [0.94–1.20] |
| E′ avg. | 0.595 | 0.97 [0.85–1.09] |
| E/E′ lateral | 0.022 | 1.13 [1.02–1.25] |
| E/E′ septal | 0.363 | 1.04 [0.96–1.12] |
| E/E′ average | 0.025 | 1.12 [1.01–1.23] |
Abbreviations as in Table 3. Significance: p < 0.05.
3.5. Reproducibility
Inter- and intra- observer variability for echocardiographic measurements is shown in Table 6. With the exception of the LA diameter measurements, intra-observer variability was minimal with biases of 0.1% to 5.2%. Inter-observer agreement was excellent with biases of 0.4% to 7.7%.
| Parameters | Intra-observer | Inter-observer | ||
|---|---|---|---|---|
| R | Difference (%) | R | Difference (%) | |
| 2D | ||||
| LA diameter | 0.70 | 4.50 | 0.60 | 1.30 |
| LAA maximum (4C) | 0.97 | 5.20 | 0.90 | 6.70 |
| LAA maximum (2C) | 0.90 | 2.50 | 0.80 | 0.40 |
| LAVmax | 0.90 | 0.80 | 0.80 | 2.40 |
| LAVmin | 0.80 | 1.50 | 0.80 | 7.70 |
| PWD | ||||
| E | 0.90 | 2.50 | 0.96 | 1.40 |
| Decel time | 0.90 | 0.10 | 0.90 | 2.50 |
| PVS | 0.90 | 2.70 | 0.95 | 3.30 |
| PVD | 0.95 | 0.96 | 0.97 | 3.70 |
| TDI | ||||
| E′ lateral | 0.98 | 2.60 | 0.98 | 3.60 |
| E′ septal | 0.97 | 1.97 | 0.90 | 3.30 |
Abbreviations as in Table 3.
4. Discussion
We investigated the utility of transthoracic echocardiography in detecting diastolic dysfunction in a large cohort of patients with LVEF > 50% and persistent and longstanding persistent AF using directly measured LAP as the reference standard. We demonstrated that there is a modest but significant correlation between directly measured LAP and a number of TTE parameters including the LAVmin, PVS, E and E/E′ lateral.
4.1. Correlation between elevated left atrial pressure and clinical parameters
We found a significant correlation between body surface area and longstanding persistent AF and elevated LAP. The duration of atrial arrhythmia may contribute to an increase in atrial afterload through decreasing coordinated atrial contractions which leads to atrial fibrosis. Patients with longstanding persistent AF have higher LAP as the result of atrial remodeling. Obesity may also cause elevated LA pressure through atrial remodeling [14].
4.2. LA minimal volume and left atrial pressure
LAVmin was shown to identify the mean pulmonary wedge pressure > 12 mm Hg in patients with coronary artery disease [15]. Our study showed that among 2D TTE parameters, LAVmin had the strongest correlation with LAP. LA size is known to be a predictor of adverse clinical outcomes [16], although LAVmax rather than LAVmin is generally used to assess LA size [17]. However, LAVmax is influenced by atrio-ventricular mechanical coupling. Both mechanical traction of the LV longitudinal fibers and the twisting motion of LV during systole cause stretching of the LA and an active suction of blood into the atrium from the pulmonary veins [18] ; [19]. Conversely, during early diastole, untwisting of the LV contributes to LV filling [20], and in end-diastole when the mitral valve is open, the LA is directly exposed to the LV pressure. Therefore, LA volume at end LV diastole may be the measurement that best reflects LA size independent of atrio-ventricular coupling mechanics. Indeed, recent work has shown that LAVmin as measured by real-time 3-D echocardiography is more strongly correlated to LV diastolic function and LV filling pressure than LAVmax in patients with sinus rhythm [21]. Furthermore, LAVmin was shown to be a robust independent predictor of first AF and first atrial flutter in an elderly cohort [22]. Nevertheless, to date, most studies have focused on LA end-systolic volume. Our study has extended the importance of LAVmin to a group of persistent and longstanding persistent AF patients where LAVmin had a stronger correlation than LAVmax with mean LAP.
4.3. Pulsed wave Doppler parameters (E and PVS) and mean left atrial pressure
Transmitral E velocity is the rapid early diastolic left ventricular inflow velocity that occurs with the mitral valve opening and was shown to have modest correlation with LV filling pressure in AF [23]. High E velocity may be found in either young patients with normal relaxation and low LV filling pressures, or in patients with elevated LV filling pressures in the setting of abnormal relaxation without significant mitral regurgitation [24]. In our study, we found a modest, significant correlation between directly measured LAP and E. We also found that PVS correlated with directly measured LAP in our cohort of patients. Previous studies have demonstrated a correlation between PVS and LAP in patients without AF [25] but the utility of PVS for detecting DD in patients with persistent AF has not been previously reported. Systolic flow from the pulmonary veins into the left atrium is affected by a number of factors including atrial relaxation, atrial compliance, atrial pressure, mitral regurgitation, and LV systolic function. We excluded patients with impaired LV systolic function as well as patients with moderate or severe mitral regurgitation, hence our observation that PVS is correlated with LAP is based primarily on LA diastolic function.
4.4. E/E′ lateral and mean left atrial pressure
E/E′ is useful in assessing elevated LV filling pressure in patients in sinus rhythm [7] ; [8]. It has been shown on small series that E/E′ is prognostically important in patients with non-valvular AF [26] and that it may be useful for the detection of elevated LAP in patients with AF [9] ; [27]. Our study used directly measured LAP as the reference standard and showed that E/E′ was not the best single non-invasive parameter to predict diastolic dysfunction in this large cohort of patients with persistent AF. Together with LAVmin and PVS, E/E′ lateral was the best independent parameters that correlated with LAP, but the cutoff value was difficult to determine. In light of recent data showing that the relationship between non-invasive indices and the pulmonary wedge pressure is highly variable in patients with heart failure with preserved ejection fraction [28], the limited correlation of E/E′ to mean LAP that we observed in this cohort of non heart failure patients with preserved EF raises the question regarding the importance of E/E′ in patients with persistent AF.
5. Study limitations
An important limitation of this study is that the direct LAP measurements were obtained prior to PVI, whereas TTE assessment of diastolic function was performed after PVI when the patients were in sinus rhythm. In this cohort of patients, we had 4 patients who had LAP measured both prior and immediately post ablation and there was no statistical difference (p = 0.49) between the two measurements. In addition, we also had 8 patients who had pre-procedural echocardiogram (the day prior to the procedure) and the first post-procedural day echocardiogram and there was no statistical difference in all measured echocardiographic parameters. It is also reported that atrial electrical–mechanical recoupling and reverse atrial remodeling does not occur immediately after cardioversion or radiofrequency ablation [29]. Therefore it is reasonable to assume that TTE parameters measured on the first day following PVI reflect values similar to what would have been obtained prior to PVI. Our study population was predominantly male and the results may not be applicable on female patients with persistent AF.
6. Conclusion
Assessment of diastolic function in patients with AF is challenging. Patients with longstanding persistent AF are at higher risk of having elevated LAP. LAVmin is the single best echocardiographic parameter to indicate elevated LAP with directly measured LAP as a reference standard in non heart failure patients with persistent and longstanding persistent AF and preserved ejection fraction. Combining 2D, PWD, and TDI parameters, it is possible to have a modest assessment of diastolic function in these patients.
Author contributions
Concept/design: MK, YH, DJC.
Data collection: MK, EZ, LG.
Data analysis/interpretation: MK, YH, DRO.
Drafting article: MK.
Critical revision of article: YH, EZ, FEM, DJC.
Approval of article: MK, YH, EZ, FEM, DJC, LG, DRO.
Statistics: MK, YH.
Funding secured by: MK, FEM, YH.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- [1] E. Braunwald; Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities; N. Engl. J. Med., 337 (1997), pp. 1360–1369
- [2] T.J. Wang, M.G. Larson, D. Levy, R.S. Vasan, E.P. Leip, P.A. Wolf, et al.; Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study; Circulation, 107 (2003), pp. 2920–2925
- [3] A.B. Miller, I.L. Pina; Understanding heart failure with preserved ejection fraction: clinical importance and future outlook; Congest. Heart Fail., 15 (2009), pp. 186–192
- [4] T.S. Tsang, B.J. Gersh, C.P. Appleton, A.J. Tajik, M.E. Barnes, K.R. Bailey, et al.; Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women; J. Am. Coll. Cardiol., 40 (2002), pp. 1636–1644
- [5] R. Nagarakanti, M. Ezekowitz; Diastolic dysfunction and atrial fibrillation; J. Interv. Card. Electrophysiol., 22 (2008), pp. 111–118
- [6] R. Parkash, W.H. Maisel, F.M. Toca, W.G. Stevenson; Atrial fibrillation in heart failure: high mortality risk even if ventricular function is preserved; Am. Heart J., 150 (2005), pp. 701–706
- [7] S.R. Ommen, R.A. Nishimura, C.P. Appleton, F.A. Miller, J.K. Oh, M.M. Redfield, et al.; Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study; Circulation, 102 (2000), pp. 1788–1794
- [8] M. Kasner, D. Westermann, P. Steendijk, R. Gaub, U. Wilkenshoff, K. Weitmann, et al.; Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study; Circulation, 116 (2007), pp. 637–647
- [9] D.W. Sohn, J.M. Song, J.H. Zo, I.H. Chai, H.S. Kim, H.G. Chun, et al.; Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation; J. Am. Soc. Echocardiogr., 12 (1999), pp. 927–931
- [10] T. Watanabe, M. Iwai-Takano, M. Oikawa, T. Yamaki, H. Yaoita, Y. Maruyama; Optimal noninvasive assessment of diastolic heart failure in patients with atrial fibrillation: comparison of tissue Doppler echocardiography, left atrium size, and brain natriuretic peptide; J. Am. Soc. Echocardiogr., 21 (2008), pp. 689–696
- [11] H. Calkins, K.H. Kuck, R. Cappato, J. Brugada, A.J. Camm, S.A. Chen, et al.; 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society; Heart Rhythm., 9 (2012), pp. 632–696 e21
- [12] P. Leong-Sit, E. Zado, D.J. Callans, F. Garcia, D. Lin, S. Dixit, et al.; Efficacy and risk of atrial fibrillation ablation before 45 years of age; Circ. Arrhythm. Electrophysiol., 3 (2010), pp. 452–457
- [13] S. Dixit, F.E. Marchlinski, D. Lin, D.J. Callans, R. Bala, M.P. Riley, et al.; Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study; Circ. Arrhythm. Electrophysiol., 5 (2012), pp. 287–294
- [14] Y.K. Iwasaki, Y. Shi, B. Benito, M.A. Gillis, K. Mizuno, J.C. Tardif, et al.; Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea; Heart Rhythm., 9 (2012), pp. 1409–1416 e1
- [15] C.P. Appleton, J.M. Galloway, M.S. Gonzalez, M. Gaballa, M.A. Basnight; Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction; J. Am. Coll. Cardiol., 22 (1993), pp. 1972–1982
- [16] T.S. Tsang, M.E. Barnes, B.J. Gersh, K.R. Bailey, J.B. Seward; Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden; Am. J. Cardiol., 90 (2002), pp. 1284–1289
- [17] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, et al.; Recommendations for chamber quantification: a report from the American Society of Echocardiographys Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology; J. Am. Soc. Echocardiogr., 18 (2005), pp. 1440–1463
- [18] R. Castello, A.C. Pearson, P. Lenzen, A.J. Labovitz; Evaluation of pulmonary venous flow by transesophageal echocardiography in subjects with a normal heart: comparison with transthoracic echocardiography; J. Am. Coll. Cardiol., 18 (1991), pp. 65–71
- [19] P. Barbier, S.B. Solomon, N.B. Schiller, S.A. Glantz; Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function; Circulation, 100 (1999), pp. 427–436
- [20] S.F. Nagueh, C.P. Appleton, T.C. Gillebert, P.N. Marino, J.K. Oh, O.A. Smiseth, et al.; Recommendations for the evaluation of left ventricular diastolic function by echocardiography; J. Am. Soc. Echocardiogr., 22 (2009), pp. 107–133
- [21] C. Russo, Z. Jin, S. Homma, T. Rundek, M.S. Elkind, R.L. Sacco, et al.; Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function; Heart, 98 (2012), pp. 813–820
- [22] K. Fatema, M.E. Barnes, K.R. Bailey, W.P. Abhayaratna, S. Cha, J.B. Seward, et al.; Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study; Eur. J. Echocardiogr., 10 (2009), pp. 282–286
- [23] S.F. Nagueh, H.A. Kopelen, M.A. Quinones; Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation; Circulation, 94 (1996), pp. 2138–2145
- [24] M.J. Garcia, J.D. Thomas, A.L. Klein; New Doppler echocardiographic applications for the study of diastolic function; J. Am. Coll. Cardiol., 32 (1998), pp. 865–875
- [25] H.F. Kuecherer, I.A. Muhiudeen, F.M. Kusumoto, E. Lee, L.E. Moulinier, M.K. Cahalan, et al.; Estimation of mean left atrial pressure from transesophageal pulsed Doppler echocardiography of pulmonary venous flow; Circulation, 82 (1990), pp. 1127–1139
- [26] H. Okura, Y. Takada, T. Kubo, K. Iwata, S. Mizoguchi, H. Taguchi, et al.; Tissue Doppler-derived index of left ventricular filling pressure, E/E′, predicts survival of patients with non-valvular atrial fibrillation; Heart, 92 (2006), pp. 1248–1252
- [27] K. Kusunose, H. Yamada, S. Nishio, N. Tomita, T. Niki, K. Yamaguchi, et al.; Clinical utility of single-beat E/e′ obtained by simultaneous recording of flow and tissue Doppler velocities in atrial fibrillation with preserved systolic function; JACC Cardiovasc. Imaging, 2 (2009), pp. 1147–1156
- [28] P.S. Bhella, E.L. Pacini, A. Prasad, J.L. Hastings, B. Adams-Huet, J.D. Thomas, et al.; Echocardiographic indices do not reliably track changes in left-sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction; Circ. Cardiovasc. Imaging, 4 (2011), pp. 482–489
- [29] P. Reant, S. Lafitte, P. Jais, K. Serri, R. Weerasooriya, M. Hocini, et al.; Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation; Circulation, 112 (2005), pp. 2896–2903
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?