Abstract
Introduction
Percutaneous transvenous mitral commissurotomy (PTMC) is one of the nonsurgical commissurotomy in patients with hemodynamically significant mitral stenosis. The aim of the present study is to assess the immediate, intermediate and long term outcomes of PTMC in relation to initial mitral valve score and to assess its impact on mitral valve area (MVA), clinical and hemodynamic parameters.
Methods
It is a retrospective study on a total of 303 patients who underwent successful PTMC between 1994 and 2001, were called back and their preprocedural, immediate post and follow-up (4, 7 and 10 year.) data were analyzed. Echo was performed in patients before and after PTMC. The patients were divided into two groups, group-I with Wilkins score of ≤ 8 and group-II with Wilkins score between 8 and 12.
Results
PTMC patients who have completed 4, 7 and 10 years of follow up revealed the mitral valve area, mean transmitral gradient and pulmonary artery pressures to be significantly different in both group-I and group-II. At all follow-up periods group-II showed higher restenosis than group-I, but its distribution between the groups was not statistically significant (χ2 = 0.029; p = 0.986). Furthermore, losses of the MVA during different periods of follow-up revealed a gradual increase in attrition.
Conclusions
MV score, Mitral valve area, mitral gradient and pulmonary artery pressures appeared to influence the outcome of PTMC. A clear-cut prospective assessment of individual components of the mitral valve apparatus using 3-D echocardiographic images may provide a more precise prediction of the PTMC outcome based on its morphological abnormalities.
Keywords
Rheumatic heart;Valve area;Mitral stenosis
1. Introduction
Percutaneous transvenous mitral commissurotomy (PTMC) is one of the nonsurgical commissurotomy in patients with hemodynamically significant mitral stenosis [1]. The important steps in performing PTMC are atrial septal puncture and crossing the mitral valve [2] ; [3]. This procedure is cost effective, less invasive and free from complications related to surgery and very rarely leads to pericardial tamponade [4] ; [5]. The assessment of the mitral valve morphology is very important because the success of PTMC is grater in patients with thin, pliable valve leaflets and little subvalvular disease [6] ; [7]. Although PTMC procedure increases the mitral valve area (MVA), in some patients this procedure is unable to obtain an optimal MVA, indicating the need for clinical evaluation before selecting the patient for the procedure [8]; [9] ; [10]. Furthermore, other PTMC related complications such as cardiac perforation, embolic stroke and mitral regurgitation limit the procedure. Sometimes, mitral regurgitation and mitral restenosis warrant emergency mitral valve replacement or a redo of PTMC [11] ; [12].
The Wilkins score is one of the most widely used echocardiographic scoring systems that is used to assess the suitability of the mitral valves morphology for PTMC [13] ; [14]. The Wilkins score assigns approximate measurements to assess mitral leaflets thickening, mobility, calcification, and the degree of the subvalvular apparatus disease. Several immediate, short and long term follow-up studies revealed that patients with a score of less than or equal to 8 showed better outcome than those with a score of more than 8 of Wilkins score [15]. The present study is aimed to assess the immediate, short and long term outcomes of PTMC in patients with severe mitral stenosis at Department of Cardiology, Sri Ramachandra Medical College & Research Institute, Chennai, India.
2. Materials and methods
2.1. Subjects
This retrospective observational study was carried out on 303 patients of mitral stenosis who underwent successful PTMC between 1994 and 2001 at Sri Ramachandra Medical College and Research Institute, Chennai. Patients of either sex, aged between 7 and 58 years having symptomatic mitral stenosis with mitral valve area < 1.5 cm2 were included in the study. Patients suffering from mild mitral stenosis (MVA > 1.5 cm2) with grade II mitral regurgitation (≥ 2/4), left atrial thrombus, congestive heart failure (NYHA class IV), Wilkins score > 12, patients who needed open heart surgery or other valves or coronary artery diseases/ascending aortas were excluded from the study. Principles outlined in the Declaration of Helsinki were strictly followed during data collection.
2.2. Pre-procedural evaluation
Patients were subjected to an initial evaluation where physical examination and detailed functional disability were assessed by New York Heart Association classification; chest X-ray and surface electrocardiogram were recorded in all patients. A complete echo Doppler study was performed in all patients 24 to 48 h before PTMC. All hemodynamic measurements were recorded.
2.3. Procedure
The self-positioning single balloon (Inoue balloon) was used for the commissurotomy in all patients. The upper limit of the balloon dilating diameter was chosen according to the patients body weight (26 mm/< 50 kg, 28 mm/50–60 kg, and 30 mm/> 60 kg). PTMC was performed with all aseptic precautions through femoral vein approach. Inflation was started at 1–2 mm less than the maximum diameter. If the hemodynamic results were suboptimal, the procedure was repeated by increasing the balloon diameter to the predetermined level. If optimal hemodynamic results were not obtained at the balloons maximum diameter, additional inflation was not attempted as a general rule. Once the balloon catheters tip traversed the interatrial septum, the stiffening cannula for the central lumen was used to facilitate the passage of the balloon portion into the left atrium. Once in the left atrium the balloon was maneuvered into the left ventricle. The balloon was then inflated with diluted contrast material until the waist of the balloon disappeared.
2.4. Post-procedural evaluation
Immediately after completing the procedure, all hemodynamic measurements were repeated. To evaluate the severity of the resultant mitral regurgitation, if any, cine left ventriculography in the right anterior oblique view was performed in all patients. The severity of mitral regurgitation was graded by the Sellers classification from 0 to 4 +. A complete echo Doppler study was repeated again 24 h after the procedure by both transthoracic and multiplane transesophageal echocardiography using HP sonos 2500 imaging. Valve morphology was assessed using Wilkins score. Mitral regurgitation was quantified and left atrial clots were excluded. 2D views of the mitral valve were obtained from parasternal windows and planimetery was performed. Continuous wave Doppler recordings through the mitral valve were obtained from the apical four chamber window and mitral valve area was estimated by using pressure half time principle. Three cardiac cycles were recorded and their results were averaged for every patient. The peak and mean transmitral gradient and pulmonary artery pressures were also measured. Follow up was viewed at 4, 7 and 10 years. For analysis patients who underwent the procedure in 1994, 1995, and 1996 were grouped into the 10 year segment, 1997, 1998, and 1999 into the 7 year segment. Both immediately after PTMC and at follow up the mitral Valve Area (MVA), mean gradient (MG), pulmonary artery pressure (PAP) and functional class of the patients were recorded. The patients were divided into two prominent groups depending on their mitral valve score. Group-I consisted of patients with mitral valve score of ≤ 8 and group-II with mitral valve score between 8 and 12.
2.5. Statistical analysis
The data were analyzed using the SPSS software (version 11.0, Chicago, IL). Categorical variables were summarized as number (percent), and continuous variables as either median (range) or mean ± standard deviation. Comparisons of immediate-PTMC versus follow-up measures were performed using the paired t test.
3. Results
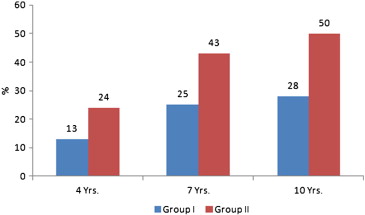
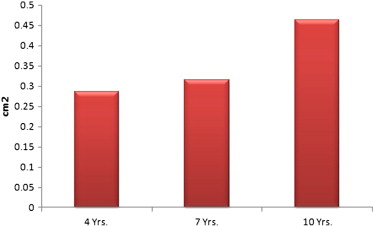
Our study included 303 patients who returned for follow up after successful PTMC. About three-fourth of patients (72%) were 21–40 years old followed by 13.2% 41–50 years and 11.6% were below 20 years. Only 3.2% were > 50 years old. The mean age of the patients was 32.05 ± 9 years. A female predominance was observed in the series with female to male ratio of 3:1. The duration of follow-up was 4, 7 and 10 years respectively for 27.4%, 19.8% and 52.8% of patients. Out of 303 patients, 184 (60.7%) showed a Wilkins score of less than or equal to 8 and 119 (39.3%) showed more than 8. About 59.4% of the patients were categorized as NYHA functional class-III, 38% as class-II and 2.6% as class-IV. Variations in the echocardiographic variables at pre-PTMC between group-I and group-II were presented in Table 1. The distribution of NYHA functional classes was significantly different between group-I and group-II patients (p < 0.001). Mitral valve area was not significantly different between group-I and group-II patients (p = 0.452). Both group-I and group-II patients showed significant differences in pulmonary artery pressure (p < 0.001) and mean transmitral pressure gradients (p < 0.001). Mitral valve area significantly increased following PTMC in both group-I (p < .001) and II (p < 0.001) patients. Both pulmonary artery pressure and mean transmittal pressure gradients were significantly reduced following PTMC (Table 2). Comparison of mean MVA values of immediate post-PTMC and different follow-up periods showed significant reduction in MVA over a period of time and the trend was similar in both group-I and group-II (Table 3). Both pulmonary artery pressure (PAP) and mean gradient (MG) were significantly increased during follow-up in group-I and group-II (Table 3). Distribution of these echocardiographic variables revealed that the MVA, MG and PAP were significantly different in different follow-up periods when compared to baseline (Table 4). The percentage of restenosis during different periods of follow-up in both group-I and group-II was depicted in Fig. 1. At all follow-up periods group-II restenosis was higher compared to group-I, but its distribution between the groups was not statistically significant (χ2 = 0.029; p = 0.986). Losses of the MVA during different periods of follow-up revealed a gradual increase in the attrition (Fig. 2). The NHYA functional class category distribution between group-I and group-II was not statistically significant for 4 and 7 years of follow-up. However, classes III and IV showed significant functional impairment (OR = 4.61; p < 0.001) compared to classes I and II after 10 years of follow-up (Table 5).
| Group-I (Wilkins score ≤ 8) | Group-II (Wilkins score > 8) | P value | |
|---|---|---|---|
| NYHA: Class-I & Class-II | 86 (28.4) | 37 (12.2) | |
| NYHA: Class-III | 90 (29.7) | 90 (29.7) | < 0.001 |
| MVA | 0.99 ± 0.13 | 0.98 ± 0.13 | 0.452 |
| MG | 12.45 ± 3.47 | 14.12 ± 3.27 | < 0.001 |
| PAP | 55.98 ± 14.5 | 65.97 ± 12.43 | < 0.001 |
| Pre-PTMC | Post-PTMC | P value | ||
|---|---|---|---|---|
| MVA | Group-I | 0.99 ± 0.13 | 2.08 ± 0.27 | < 0.001 |
| Group-II | 0.98 ± 0.13 | 2.0 ± 0.0 | < 0.001 | |
| MG | Group-I | 12.45 ± 3.47 | 6.41 ± 2.62 | < 0.001 |
| Group-II | 14.12 ± 3.27 | 8.74 ± 2.83 | < 0.001 | |
| PAP | Group-I | 55.98 ± 14.48 | 35.66 ± 12.49 | < 0.001 |
| Group-II | 65.97 ± 12.43 | 48.07 ± 11.72 | < 0.001 |
| Parameters | Group I (Mean ± SD) | Group II (Mean ± SD) | P value |
|---|---|---|---|
| Mitral valve area | |||
| Immediate | 2.10 ± 0.322 | 1.850 ± 0.271 | < 0.001 |
| 4 years | 1.821 ± 0.379 | 1.603 ± 0.265 | < 0.001 |
| 7 years | 1.627 ± 0.423 | 1.476 ± 0.388 | < 0.001 |
| 10 years | 1.715 ± 0.528 | 1.354 ± 0.478 | < 0.001 |
| Mean gradient | |||
| Immediate | 5.88 ± 2.17 | 7.93 ± 2.77 | < 0.001 |
| 4 years | 7.89 ± 3.81 | 9.24 ± 3.89 | < 0.05 |
| 7 years | 9.19 ± 3.77 | 9.93 ± 1.13 | < 0.001 |
| 10 years | 8.01 ± 4.16 | 11.45 ± 4.32 | < 0.001 |
| PAP | |||
| Immediate | 36.16 ± 12.59 | 47.45 ± 13.23 | < 0.001 |
| 4 years | 39.70 ± 11.03 | 52.79 ± 15.47 | < 0.001 |
| 7 years | 42.07 ± 14.28 | 50.62 ± 23.62 | < 0.001 |
| 10 years | 40.41 ± 20.92 | 60.69 ± 20.63 | < 0.001 |
| Duration | N | Variable (Mean ± SD) | P value |
|---|---|---|---|
| MVA | |||
| Baseline | 303 | 0.99 ± 0.13 | – |
| Immediate | 303 | 2.05 ± 0.21 | < 0.001 |
| 4 years | 83 | 1.75 ± 0.46 | < 0.001 |
| 7 years | 60 | 1.78 ± 1.18 | < 0.001 |
| 10 years | 160 | 1.64 ± 0.49 | < 0.001 |
| MG | |||
| Baseline | 303 | 13.11 ± 3.48 | – |
| Immediate | 303 | 7.32 ± 2.93 | < 0.001 |
| 4 years | 83 | 9.17 ± 4.11 | < 0.001 |
| 7 years | 60 | 10.37 ± 5.18 | < 0.001 |
| 10 years | 160 | 9.62 ± 4.59 | < 0.001 |
| PAP | |||
| Baseline | 303 | 59.9 ± 14.64 | – |
| Immediate | 303 | 40.46 ± 13.68 | < 0.001 |
| 4 years | 83 | 45.57 ± 14.24 | < 0.001 |
| 7 years | 60 | 50.4 ± 23.26 | < 0.001 |
| 10 years | 160 | 48.2 ± 21.23 | < 0.001 |
|
|
|
Fig. 1. Restenosis of mitral valve during different periods of follow-up. |
|
|
|
Fig. 2. Loss of the mitral valve area during different periods of follow-up. |
| NYHA | Group-I | Group-II | OR (95% CI) | P value |
|---|---|---|---|---|
| Classes I & II | 74 (24.4) | 27 (8.9) | Reference | |
| Classes III & IV | 22 (7.3) | 37 (12.2) | 4.61 (2.32–9.17) | < 0.001 |
4. Discussion
Analysis of 303 successful PTMC patients who have completed 4, 7 and 10 years of follow up revealed the mitral valve area, mean transmitral gradient and pulmonary artery pressures to be significantly different in both groups I and II. At all follow-up periods group-II showed higher restenosis than group-I. Furthermore, losses of the MVA during different periods of follow-up revealed a gradual increase in the attrition.
The higher MVA gain in group-I than in group-II indicated the intervention to be effective in patients with Wilkins score of less than 8. These findings were consistent with a previous study that showed a significant favorable impact on immediate post PTMC in patients with < 8 Wilkins score [16]. Several others also found mitral anatomy to be the best predictor of mitral opening though a good result could also be obtained in cases with a high score as well [17].
Rheumatic process and/or abnormal turbulences are generated by the already deformed valve. Both mechanisms might contribute to further commissural fusion, thickening, and calcification of valvular and subvalvular structures both in natural and previously commissurotomized valves. In this study series we found that the mean MVA loss progressively increased with time in both the groups. Previous studies demonstrated the rapid progression of valve disease in those subjects with a greater mitral valve echocardiographic score and higher peak and mean transmitral gradients [18]. Long-term clinical and echocardiographic follow-up after PTMC in Spanish patients revealed a mild mitral valve area loss (0.13 ± 0.21 cm2) and it gradually increased with time. Follow-up of 7 years revealed a MVA decrease of 0.2 cm2 irrespective of score, which is in fact smaller than that reported in untreated valves [19]. In contrast to this, Spanish study groups findings showed an accelerated MVA loss in valves with a higher score [20].
Restenosis is an ambiguous term that includes a mixture of poor result, inaccuracies in MVA determination, true restenosis, and disease progression. Its definition could be made on a clinical basis or in terms of mitral area, absolute area loss, % of area loss, or loss of gain. With all these confounding factors, it is not surprising that restenosis rate after PTMC has ranged from 3% to 70% at 1 to 3 years [21]. When restenosis is defined as a 50% loss of area gain, patients with a poor initial result meet restenosis criteria with only a mild area loss. This fact explains the apparent contradictory finding that patients with a high score had a smaller mitral area loss but a higher restenosis rate than those with a lower score. The percentage of restenosis is different at different follow-up periods in different studies. In Spanish patients, the restenosis rate was 10% at 4 years, 18% at 5 years and 39% at the end of 7 years [19]. In American patients restenosis rate was 40% at the end of 6 years [22]. Though the percentages for restenosis in groups A and B were divergent across different follow-up periods we could establish that a low pre-procedure Wilkins score resulted in lesser restenosis rates only in the 10 year segment. Post-PTMC Cox regression analysis however identified mitral echocardiographic score of > 8 as a predictor of restenosis [23].
The mean mitral diastolic gradient in our series reduced significantly in both the groups. A similar trend in mean transmitral gradient fall was reported in two independent studies [24] ; [25]. We could establish no statistically significant difference in mean transmitral reduction between group-I and group-II either immediately after the procedure or at different follow-up periods. From this it is evident that though the diastolic gradient dropped significantly after PTMC in both the groups it gradually increased, and also worsened with time. Extrapolation to establish a connection between MVA and symptomatic worsening however would require further study.
The immediate results obtained with the pulmonary artery systolic pressures were not dissimilar from the transmitral mean with a drop in PA pressures. This was consistent with the findings of Chen et al. [26] who reported a reduction in PA pressures from 51.2 ± 14.8 to 33.9 ± 8.8 mm Hg. In contrast to the mean mitral gradient the reduction in PA pressure was significantly lower in group-I as in group-II immediately after PTMC. Also, while we found an insignificant difference in pulmonary artery pressure increase at 4, and 7 years follow up, the pulmonary pressures were significantly higher in group-II patients at the end of 10 years. Although numerous studies have implicated higher post-PTMC pulmonary artery pressure as independent predictor of combined events at long-term follow-up, clear cut distinction with respect to valve score is yet to be defined [10].
Clinical improvement assessed by the NYHA functional class revealed the absence of statistically significant difference in the achieved clinical improvement between the groups immediately after PTMC. A significant difference in functional impairment was only established at 10 years of follow-up, between groups-I and II with a lesser number of group-I patients exhibiting impairment. In patients with significant MVA loss, the functional impairment is consistent with increased attrition and time. In a multivariate analysis, a high NYHA in pre-PTMC class as an independent predictor of poor functional results as well as combined events at long term follow up was noticed [27] ; [28].
The study done by Mahfouz et al. showed significant correlation between the posterior mitral valve leaflet to anterior mitral valve leaflet length ratio (PMVL/AMVL length ratio) and post- balloon mitral valvuloplasty mitral valve area and the cardiac events [29]. Unfortunately, PMVL/AMVL length ratio was not analyzed in our study. The present study suffers from a number of the following limitations. Firstly, data were collected from only one clinical setting, thus generalizability of these findings might be limited. Secondly, the convenient sampling strategy adopted in this study limits the extrapolation of results to all PTMC procedures. Lastly, this particular study underutilised the potential of the posterior mitral valve leaflet to anterior mitral valve leaflet length ratio (PMVL/AMVL length ratio). However, in summary, MV score, Mitral valve area, mitral gradient and pulmonary pressures with isolated symptomatic worsening needs further insight and larger studies not only to improve event free survival but also to improve the symptom free survival. A clear-cut prospective assessment of individual components of the mitral valve apparatus using 3-D echocardiographic images may provide more precise prediction of the PTMC outcome based on its morphological abnormalities.
Conflict of interest
There are no conflicts of interests.
Acknowledgments
The authors would like to thank Sri Ramachandra University for providing the necessary facilities and the subjects.
References
- [1] A. Vahanian, P. Luxereau, E. Brochet, B. Cormier, B. Iung; Percutaneous mitral commissurotomy: technique, results, and selection of patients; Przegl Lek, 61 (6) (2004), pp. 543–546
- [2] A. Vahanian; Percutaneous mitral commissurotomy: an effective treatment in ‘ideal’ candidates whatever the approach; Eur Heart J, 18 (11) (1997), pp. 1689–1690
- [3] A. Vahanian, B. Iung; Percutaneous mitral balloon commissurotomy: a useful and necessary treatment for the western population; Eur Heart J, 21 (20) (2000), pp. 1651–1652
- [4] T. Thomas, R. Ananthakrishna, N.A. Chikkabasavaiah, R. Basavappa; Application of a novel percutaneous transluminal mitral commissurotomy technique in deformed mitral valve; BMJ Case Rep, 2011 (2011)
- [5] Y. Ootaki, S. Kozawa, T. Asada, N. Mukohara, T. Higami, K. Iwahashi; Rupture of the papillary muscle after percutaneous transvenous mitral commissurotomy (PTMC)—a case report; Nihon Kyobu Geka Gakkai Zasshi, 45 (10) (1997), pp. 1738–1742
- [6] T. Feldman, J.D. Carroll; Percutaneous transvenous balloon mitral commissurotomy: when? For whom? An alternative to surgery in symptomatic mitral stenosis; J Crit Illn, 6 (10) (1991), pp. 1009–1027
- [7] G.T. Wilkins, A.E. Weyman, V.M. Abascal, P.C. Block, I.F. Palacios; Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation; Br Heart J, 60 (4) (1988), pp. 299–308
- [8] A. Drighil, D. Ghellab, J.W. Mathewson, L. Ouarga, H. Alalou, L. Azzouzi; Immediate impact of successful percutaneous mitral valve commissurotomy on echocardiographic measures of right ventricular contractility; J Am Soc Echocardiogr, 25 (11) (2012), pp. 1245–1250
- [9] H. Hasan-Ali, H. Shams-Eddin, A.A. Abd-Elsayed, M.H. Maghraby; Echocardiographic assessment of mitral valve morphology after Percutaneous Transvenous Mitral Commissurotomy (PTMC); Cardiovasc Ultrasound, 5 (48) (2007)
- [10] H. Sadeghian, M. Salarifar, M. Rezvanfard, E. Nematipour, M. Lotfi Tokaldany, A. Safir Mardanloo, et al.; Percutaneous transvenous mitral commissurotomy: significance of echocardiographic assessment in prediction of immediate result; Arch Iran Med, 15 (10) (2012), pp. 629–634
- [11] F. Rahman, N. Akhter, K. Anam, M.A. Rashid, M.J. Uddin, C.M. Ahmed, et al.; Balloon mitral valvuloplasty: immediate and short term haemodynamic and clinical outcome; Mymensingh Med J, 19 (2) (2010), pp. 199–207
- [12] J.K. Harrison, J.S. Wilson, S.E. Hearne, T.M. Bashore; Complications related to percutaneous transvenous mitral commissurotomy; Cathet Cardiovasc Diagn (Suppl. 2) (1994), pp. 52–60
- [13] C.N. Manjunath, A. Panneerselvam, K.H. Srinivasa, B. Prabhavathi, K. Rangan, C. Dhanalakshmi, et al.; Incidence and predictors of atrial septal defect after percutaneous transvenous mitral commissurotomy—a transesophageal echocardiographic study of 209 cases; Echocardiography, 30 (2) (2013), pp. 127–130
- [14] T. Tsuji, Y. Ikari, T. Tamura, Y. Wanibuchi, K. Hara; Pathologic analysis of restenosis following percutaneous transluminal mitral commissurotomy; Catheter Cardiovasc Interv, 57 (2) (2002), pp. 205–210
- [15] D. Sarath Babu, K.P. Ranganayakulu, D. Rajasekhar, V. Vanajakshamma, T. Pramod Kumar; Assessment of mitral valve commissural morphology by transoesophageal echocardiography predicts outcome after balloon mitral valvotomy; Indian Heart J, 65 (3) (2013), pp. 269–275
- [16] I.F. Palacios, P.L. Sanchez, L.C. Harrell, A.E. Weyman, P.C. Block; Which patients benefit from percutaneous mitral balloon valvuloplasty? Prevalvuloplasty and postvalvuloplasty variables that predict long-term outcome; Circulation, 105 (12) (2002), pp. 1465–1471
- [17] L. Brunton; Preliminary note on the possibility of treating mitral stenosis by surgical methods; Lancet, 159 (4093) (1902), p. 352
- [18] S.P. Gordon, P.S. Douglas, P.C. Come, W.J. Manning; Two-dimensional and Doppler echocardiographic determinants of the natural history of mitral valve narrowing in patients with rheumatic mitral stenosis: implications for follow-up; J Am Coll Cardiol, 19 (5) (1992), pp. 968–973
- [19] R. Hernandez, C. Banuelos, F. Alfonso, J. Goicolea, A. Fernandez-Ortiz, J. Escaned, et al.; Long-term clinical and echocardiographic follow-up after percutaneous mitral valvuloplasty with the Inoue balloon; Circulation, 99 (12) (1999), pp. 1580–1586
- [20] E.C. Cutler, C.S. Beck; The present status of the surgical procedures in chronic valvular disease of the heart: final report of all surgical cases; Arch Surg, 18 (1_PART_II) (1929), pp. 403–416
- [21] H.S. Souttar; The surgical treatment of mitral stenosis; Br Med J, 2 (3379) (1925), pp. 603–606
- [22] A. Wang, R.A. Krasuski, J.J. Warner, K. Pieper, K.B. Kisslo, T.M. Bashore, et al.; Serial echocardiographic evaluation of restenosis after successful percutaneous mitral commissurotomy; J Am Coll Cardiol, 39 (2) (2002), pp. 328–334
- [23] M.E. Fawzy, H. Hegazy, M. Shoukri, F. El Shaer, A. ElDali, M. Al-Amri; Long-term clinical and echocardiographic results after successful mitral balloon valvotomy and predictors of long-term outcome; Eur Heart J, 26 (16) (2005), pp. 1647–1652
- [24] C.R. Chen, T.O. Cheng, J.Y. Chen, Y.G. Huang, T. Huang, B. Zhang; Long-term results of percutaneous balloon mitral valvuloplasty for mitral stenosis: a follow-up study to 11 years in 202 patients; Cathet Cardiovasc Diagn, 43 (2) (1998), pp. 132–139
- [25] M. Nobuyoshi, N. Hamasaki, T. Kimura, H. Nosaka, H. Yokoi, H. Yasumoto, et al.; Indications, complications, and short-term clinical outcome of percutaneous transvenous mitral commissurotomy; Circulation, 80 (4) (1989), pp. 782–792
- [26] C.R. Chen, T.O. Cheng, J.Y. Chen, Y.L. Zhou, J. Mei, T.Z. Ma; Long-term results of percutaneous mitral valvuloplasty with the Inoue balloon catheter; Am J Cardiol, 70 (18) (1992), pp. 1445–1448
- [27] S. Lunghetti, E. Palmerini, R. Urselli, S. Maffei, E. Guarino, M. Focardi, et al.; Effects of levosimendan without loading dose on systolic and diastolic function in patients with end-stage heart failure; Cardiol J, 18 (5) (2011), pp. 532–537
- [28] I.F. Palacios, P.C. Block, G.T. Wilkins, A.E. Weyman; Follow-up of patients undergoing percutaneous mitral balloon valvotomy. Analysis of factors determining restenosis; Circulation, 79 (3) (1989), pp. 573–579
- [29] R.A. Mahfouz; Utility of the posterior to anterior mitral valve leaflets length ratio in prediction of outcome of percutaneous balloon mitral valvuloplasty; Echocardiography, 28 (10) (2011), pp. 1068–1073
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?