Summary
Objective
This study aims to establish a noninvasive scoring system to predict the risk of erosive esophagitis (EE).
Methods
From 2002 to 2009, a total of 34,346 consecutive adults who underwent health check-ups and upper gastrointestinal endoscopy were retrospectively enrolled. Of the participants, 22,892 in the earlier two-thirds period of examination were defined as the training set and the remaining 11,454 as the validation set. EE was diagnosed by upper gastrointestinal endoscopy. Independent risk factors associated with EE were analyzed by multivariate analysis using a logistic regression model with the forward stepwise selection procedure in the training set. Subsequently, an EE risk scoring system was established and weighted by β coefficient. This risk scoring system was further validated in the validation set.
Results
In the training set, older age, male gender, higher body mass index, higher waist circumference, higher serum triglyceride, and lower high-density lipid cholesterol levels were independent risk factors for predicting EE. According to the β coefficient value of each independent risk factor, the total score ranging from 0 to 10 was established, and then low- (0–3), moderate- (4–6), and high-risk (7–10) groups were identified. In the validation set, the prevalence rates of EE in the low-, moderate-, and high-risk groups were 5.15%, 15.76% and 26.11%, respectively (p < 0.001).
Conclusion
This simple noninvasive risk scoring system, including factors of age, gender, body mass index, waist circumference, triglyceride, and high-density lipid cholesterol, effectively predicted EE and stratified its incidence.
Keywords
Epidemiology ; Erosive esophagitis ; Noninvasive marker ; Risk score
Introduction
The incidence of gastroesophageal reflux esophagitis (GERD) is increasing worldwide [1] ; [2] . The symptoms of GERD include heart burn, regurgitation, and abdominal pain with a decreased quality of life [3] , which result in a growing burden for health care systems and employers [1] . Moreover, it may develop serious complications such as esophageal adenocarcinoma [1] . Consequently, GERD has become an important health care challenge.
The risk factors of GERD are not fully identified till now. Recent studies demonstrate that GERD and erosive esophagitis (EE) are associated with metabolic syndromes, including central obesity and increased waist circumference (WC) [4] ; [5] . Moreover, lipid profiles of the metabolic syndromes have also been demonstrated as independent risk factors for GERD [4] . Some studies have proposed that the increasing trend of GERD in the recent decades may be partly explained by an increasing body mass index (BMI) of the general population and a higher prevalence of metabolic syndrome worldwide [6] ; [7] . Besides metabolic syndromes, whether other epidemiologic risk factors are associated with GERD and EE is still under debate [2] ; [8] . For example, the impact of age and sex on GERD has been shown to be different between Japan and Western countries [9] .
It is crucial to identify the risk factors for predicting GERD and EE, which may be benefit for disease control, and for the target of lifestyle modification and medical therapies. The aim of the present study, therefore, was to investigate the risk factors for EE and establish a noninvasive scoring system to predict its incidence.
Materials and methods
Patients
Patients who completed the health check-up service at the Health Management Center of Taipei Veterans General Hospital, Taipei, Taiwan from 2002 to 2009 were considered for enrollment. As gastric cancer is an important cause of cancer mortality in Taiwan, esophagogastroduodenoscopy (EGD) is a routine examination in our physical check-up service. The demographic data including age, sex, BMI, WC, and blood pressure (BP) were recorded. Patients who did not have the data of EGD and all the parameters for the study during health check-up were excluded. Finally, 34,346 consecutive and eligible patients were enrolled for analysis.
According to the revised National Cholesterol Education Program-Adult Treatment Panel III criteria, BMI was calculated by dividing the body weight (in kilograms) by the square of the patient’s height (in meters), and obesity was defined as BMI ≥ 25 kg/m2[10] ; [11] . The upper limits of WC were 90 cm for men and 80 cm for women. BP was measured after the examinees had been seated for > 5 minutes. Systolic BP (SBP) and diastolic BP (DBP) were recorded as the means of three consecutive readings with a difference in the SBP of < 10 mmHg. The upper limits of SBP and DBP were 130 mmHg and 85 mmHg, respectively.
This study complied with the standards of the Declaration of Helsinki and current ethical guidelines. It was approved by the Institutional Review Board of Taipei Veterans General Hospital (No. 2011-08-010IC).
Biochemical and serologic markers
Venous blood samples were collected after overnight fasting. Serum biochemical tests were measured using Roche/Hitachi Modular Analytics Systems (Roche Diagnostics GmbH, Mannheim, Germany). The reference limits of these tests were as follows: alanine transaminase (ALT) level, 40 IU/L; total cholesterol level, 200 mg/dL; high-density lipoprotein-cholesterol (HDL-C) level, 40 mg/dL in men and 50 mg/dL in women; low-density lipoprotein-cholesterol level, 130 mg/dL; triglyceride (TG) level, 150 mg/dL; fasting glucose level, 100 mg/dL; and 2-hour postload plasma glucose level, 150 mg/dL.
Endoscopic findings
Eleven experienced endoscopists performed the EGD procedures and recorded the findings on a digital file system. EE was diagnosed according to the Los Angles criteria by two senior endoscopists (Y.-J.W. and J.-C.L., both had performed more than 5000 EGD procedures) [12] . If the diagnosis for the same patient was inconsistent between these two doctors, the digital file was reviewed again to reach a consensus.
Statistical analysis
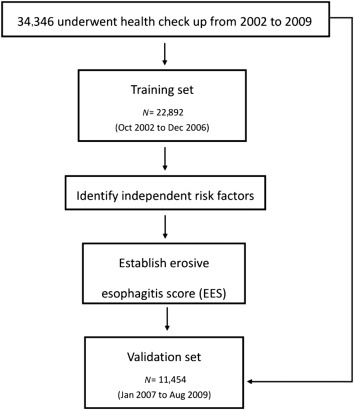
To establish and validate a risk scoring system to predict EE, we divided our study cohort into model derivation (training) and validation sets in a 2:1 ratio. Therefore, we selected 22,892 patients who underwent physical check-ups in the initial two-thirds of the study period (from October 2002 to December 2006) as the training set to generate the risk scoring system for EE, and the remaining 11,454 patients (from January 2007 to August 2009) as the validation set (Figure 1 ).
|
|
|
Figure 1. Study flow chart. EES = erosive esophagitis score. |
In the training set, Chi-square analysis was used to compare categorical variables, and the Student t test was used to compare continuous variables. Variables with statistical significance (p < 0.05) or proximate to it (p < 0.1) in univariate analysis were included in multivariate analysis using a logistic regression model with the forward stepwise selection procedure. Subsequently, the independent risk factors were scored and weighted by β coefficient, and the risk scoring system for EE was established. Low-, moderate-, and high-risk groups for EE were stratified according to the risk scores. We then validated this scoring system in the validation set and all the enrollees for its discriminative ability to predict EE among different risk groups.
A two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline clinical characteristics and prevalence of EE
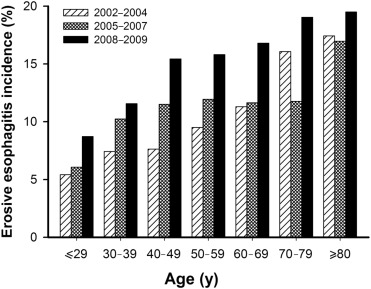
A total of 34,346 patients who underwent health check-ups were enrolled. EE was identified in 4044 (11.78%) patients by EGD (EE group), and the remainder were defined as the normal group. The incidence of EE, stratified by age and time (per 3 years), had an increasing tendency (Figure 2 ).
|
|
|
Figure 2. Incidence of erosive esophagitis stratified by age and time (every 3 years). |
Patients in the EE group were significantly older than those in the normal group (p < 0.001; Table 1 ). There was male predominance in both groups; however, the male-to-female ratio was higher in the EE group (81.2% vs. 50.8%, p < 0.001). BMI, WC, SBP, and DBP were significantly higher in the EE group. Lower HDL-C, higher TG, and low-density lipoprotein-cholesterol were also found in the EE group. Patients in the EE group also had higher fasting glucose and ALT levels than those in the normal group ( Table 1 ).
| Mean ± SD or n (%) | Hazard ratio (95% CI) | p | ||
|---|---|---|---|---|
| Normal group (n = 30,302) | EE group (n = 4044) | |||
| Age (y) | 51.8 ± 13.1 | 54.1 ± 13.0 | 1.014 (1.011–1.016) | <0.0001 |
| Male, n (%) | 15401 (50.8) | 3285 (81.2) | 1.014 (1.011–1.016) | <0.0001 |
| BMI (kg/m2 ) | 23.6 ± 3.6 | 25.1 ± 3.4 | 1.114 (1.104–1.124) | <0.0001 |
| WC (cm) | 83.1 ± 10.2 | 88.4 ± 9.4 | 1.051 (1.047–1.054) | <0.0001 |
| Systolic BP (mmHg) | 123.7 ± 18.7 | 127.3 ± 17.9 | 1.010 (1.008–1.012) | <0.0001 |
| Diastolic BP (mmHg) | 77.2 ± 13.6 | 79.8 ± 19.1 | 1.012 (1.010–1.015) | <0.0001 |
| Fasting glucose (mg/dL) | 95.1 ± 24.4 | 98.6 ± 27.4 | 1.005 (1.004–1.006) | <0.0001 |
| Cholesterol (mg/dL) | 198.3 ± 37.2 | 198.5 ± 36.4 | 1.000 (0.999–1.001) | 0.7577 |
| HDL cholesterol (mg/dL) | 54.2 ± 15.2 | 49.3 ± 13.5 | 0.976 (0.974–0.978) | <0.0001 |
| LDL cholesterol (mg/dL) | 124.5 ± 33.0 | 126.2 ± 32.3 | 1.002 (1.001–1.002) | 0.0028 |
| Triglycerides (mg/dL) | 125.5 ± 85.1 | 152.2 ± 100.2 | 1.003 (1.002–1.003) | <0.0001 |
| ALT (U/L) | 28.0 ± 25.4 | 33.7 ± 39.2 | 1.006 (1.005–1.007) | <0.0001 |
ALT = alanine transaminase; BMI = body mass index; BP = blood pressure; CI = confidence interval; EE = erosive esophagitis; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SD = standard deviation; WC = waist circumference.
Factors associated with EE in the training set
In the training set, univariate analysis showed that older age, male gender, higher BMI, WC, SBP, DBP, fasting glucose, ALT, TG, and lower HDL-C were significantly associated with EE (Table 2 ).
| Mean ± SD or n (%) | Hazard ratio (95% CI) | p | ||
|---|---|---|---|---|
| Normal (n = 20,523) | Erosive esophagitis (n = 2369) | |||
| Age (y) | 52.1 ± 13.1 | 54.7 ± 13.2 | 1.015 (1.012–1.018) | <0.0001 |
| Male, n (%) | 10691 (52.1) | 1945 (82.1) | 4.219 (3.785–4.702) | <0.0001 |
| BMI (kg/m2 ) | 23.7 ± 3.5 | 25.0 ± 3.2 | 1.109 (1.096–1.122) | <0.0001 |
| WC (cm) | 83.4 ± 10.1 | 88.4 ± 9.1 | 1.050 (1.045–1.054) | <0.0001 |
| Systolic BP (mmHg) | 125.0 ± 18.7 | 128.1 ± 18.0 | 1.009 (1.006–1.011) | <0.0001 |
| Diastolic BP (mmHg) | 77.9 ± 13.7 | 79.8 ± 11.6 | 1.009 (1.006–1.013) | <0.0001 |
| Fasting glucose (mg/dL) | 96.4 ± 25.0 | 100.3 ± 29.3 | 1.005 (1.004–1.006) | <0.0001 |
| Cholesterol (mg/dL) | 199.2 ± 37.2 | 200.1 ± 36.6 | 1.001 (1.000–1.002) | 0.2656 |
| HDL cholesterol (mg/dL) | 54.8 ± 14.9 | 50.4 ± 12.9 | 0.978 (0.975–0.981) | <0.0001 |
| LDL cholesterol (mg/dL) | 124.6 ± 32.9 | 126.5 ± 32.6 | 1.002 (1.000–1.003) | 0.0071 |
| Triglycerides (mg/dL) | 124.8 ± 79.5 | 148.6 ± 92.8 | 1.003 (1.002–1.003) | <0.0001 |
| ALT (U/L) | 27.6 ± 24.2 | 32.5 ± 40.0 | 1.005 (1.004–1.006) | <0.0001 |
ALT = alanine transaminase; BMI = body mass index; BP = blood pressure; CI = confidence interval; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SD = standard deviation; WC = waist circumference.
In multivariate analysis, older age was not only an independent risk factor for EE, but also associated with a tendency of increased risk. Male gender, BMI, WC, HDL-C, and TG were independent risk factors for EE (Table 3 ).
| Parameter | B | SE | HR | 95.0% CI for HR | p | |
|---|---|---|---|---|---|---|
| Age 40–60/<40 (y) | 0.148 | 0.069 | 1.159 | 1.013 | 1.327 | 0.032 |
| Age ≥60/<40 (y) | 0.330 | 0.079 | 1.391 | 1.191 | 1.625 | <0.001 |
| Sex (male/female) | 1.386 | 0.059 | 4.000 | 3.560 | 4.494 | <0.001 |
| BMI | 0.215 | 0.057 | 1.240 | 1.109 | 1.387 | <0.001 |
| WC | 0.338 | 0.056 | 1.402 | 1.256 | 1.565 | <0.001 |
| HDL cholesterol | −0.131 | 0.056 | 0.877 | 0.786 | 0.980 | 0.020 |
| TG | 0.184 | 0.051 | 1.202 | 1.088 | 1.327 | <0.001 |
B = β coefficient; BMI = body mass index; CI = confidence interval; HDL = high-density lipoprotein; HR = hazard ratio; SE = standard error; TG = triglyceride; WC = waist circumference.
Establishing a risk score for predicting EE in the training set and validation of the EE score
According to the β coefficient values in Table 3 , each independent risk factor was weighted by different scores and the risk scoring system for predicting EE was established (Table 4 ). The total score ranged from 0 to 10. Low-, moderate-, and high-risk groups were defined by scores of 0–3, 4–6, and 7–10, respectively.
| Parameter | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Age (y) | <40 | 40–60 | >60 | |
| Sex | Female | — | — | Male |
| BMI (kg/m2 ) | <25 | ≥25 | — | — |
| WCb | Normal | — | Abnormal | — |
| HDL cholesterolc | Normal | Abnormal | — | — |
| TGd | Normal | Abnormal | — | — |
BMI = body mass index; HDL = high-density lipoprotein; TG = triglyceride; WC = waist circumference; — = not applicable.
a. Total score range: 0–10; 0–3: low-risk group; 4–6: moderate-risk group; 7–10: high-risk group.
b. Normal WC: Male < 90 cm; female < 80 cm; abnormal means: male ≥ 90 cm, female ≥ 80 cm.
c. Normal HDL: male > 40 mg/dL; female > 50 mg/dL; abnormal means: male ≤ 40 mg/dL, female ≤ 50 mg/dL.
d. Normal TG: < 150 mg/dL; abnormal means TG ≥ 150 mg/dL.
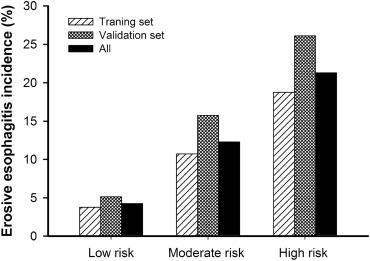
In the validation set, the prevalence rates of EE in the low-, moderate-, and high-risk groups were 5.15%, 15.76%, and 26.11%, respectively (p < 0.001). In all the enrollees (training and validation sets), the prevalence rates of EE in the low-, moderate-, and high-risk groups were 4.26%, 12.31%, and 21.3%, respectively (p < 0.001). This scoring system showed good discriminability for EE prediction by logistic regression ( Figure 3 ).
|
|
|
Figure 3. Incidence of erosive esophagitis stratified by risk score. By logistic regression p < 0.001. The erosive esophagitis score could discriminate between different risk groups in all EE patients (p < 0.001). |
Discussion
Although previous studies have evaluated and established the risk factors for EE, this study is the first large-scale study using a health check-up population to establish a simple noninvasive scoring system, weighted by the risk factors of age, gender, BMI, WC, TG, and HDL-C, to effectively predict the occurrence of EE and stratify its incidence. Initially, two-thirds of the participants were enrolled as the training set to identify the independent risk factors for EE after multivariate regression analysis. These independent risk factors, including increasing age, male gender, higher BMI, WC, TG, and lower HDL-C, were then weighted according to the β coefficient in the multivariate analysis and used in the noninvasive scoring system. The remaining one-third of the enrollees were used to validate and test the discriminability of the scoring system.
The increasing incidence of endoscopic EE in our study coincides with other physical check-up studies in Taiwan, which have revealed an increasing incidence of EE in the past 10 years [13] ; [14] . Our findings are also consistent with other data from Asia, including China, Japan, Singapore, Korea, as well as from Europe and America [1] ; [8] ; [9] ; [15] .
The impact of aging on the incidence of EE is unclear [2] ; [9] ; [16] . No relationship between increasing age and GERD symptoms was found in the Olmsted County study [17] ; [18] . However, an increasing number of recent studies have suggested that increasing age may be correlated with the incidence of EE [9] ; [16] ; [19] ; [20] . de Vries et al [21] proposed that increasing age was correlated with decreased intraesophageal pressure and increased gastroesophageal pressure gradient, which can contribute to the development of hiatus hernia. In this study, we also found a good correlation between the age and the incidence of hiatus hernia (data not shown), and a good correlation between the incidence of hiatus hernia and EE, which is consistent with the finding of previous studies [8] ; [13] ; [22] . However, the aim of this study was to establish a simple noninvasive method to predict the occurrence of EE prior to the application of EGD; thus, hiatus hernia was not included in the analysis.
The association between GERD and sex is also under debate [2] ; [8] ; [9] ; [16] ; [17] ; [23] . In Japan, GERD occurs predominantly in females [9] . The explanation for this may be multifactorial, including a longer life span and higher incidence of hiatus hernia in females in Japan [9] ; [24] . Although several studies have concluded that there is no significant association between gender and GERD [2] ; [17] , recent studies have found male gender to be an independent factor for EE [8] ; [16] ; [23] . The mechanisms may be partly explained by insulin resistance, which is more prominent in males than in females [16] .
There is a strong positive correlation between BMI and endoscopic evidence of EE [25] and long-term complications of GERD, such as Barrett’s esophagus and esophageal adenocarcinoma [26] ; [27] . Therefore, previous studies have proposed that the increasing trend of GERD in recent decades may partly be explained by increasing BMI [6] ; [7] . The mechanism and associations between higher BMI and increasing EE may largely be explained by increased intragastric and intra-abdominal pressure due to external compression of the surrounding adipose tissue in obesity, resultant frequent relaxation of the lower esophageal sphincter, and hence, the development of mucosal injury in the esophagus [28] ; [29] . In addition, an abdominal belt study reproduced the manometric characteristics linking BMI with reflux [7] and was consistent with the association between WC and acid reflux [28] . Although both BMI > 25 kg/m2 and WC were independent risk factors for EE in the present study, WC was a more dominant predictor than BMI.
Biochemical abnormalities of metabolic syndrome components, including elevated TG and lower HDL-C, are associated with an increased prevalence of both EE and GERD symptoms [4] ; [15] ; [16] . In addition, metabolic syndrome is associated with accelerated progression to or attenuated regression from an erosive status [23] . In our study, higher TG and low HDL-C were both independent risk factors for EE. However, the mechanism between abnormal lipid profiles and EE was unclear and remains to be clarified.
There are some limitations to this study. First, it was a retrospective study and lacked qualified questionnaires to identify the history and symptoms of GERD. Second, factors such as clinical symptoms and smoking history would be helpful in further stratifying the patients and in the identification of the high-risk group [23] . In addition, the medication history, such as treatment with antacids, H2-blockers, and proton pump inhibitors, may also potentially affect the results of endoscopic findings in patients with EE [23] . Third, although the study cohort was based on a health check-up population, the enrollees cannot completely represent the normal population. In addition, this was a single-center study and may not reflect the whole picture of GERD in Taiwan. Application of the risk scoring system to a community-based population may be needed to validate its good discriminability for EE prediction.
In conclusion, older age, male gender, higher BMI, WC, TG, and lower HDL-C levels were important and independent predictors of EE. This simple noninvasive risk scoring system, weighting by these factors, effectively predicted EE and stratified its incidence.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] H.B. El-Serag; Time trends of gastroesophageal reflux disease: a systematic review; Clin Gastroenterol Hepatol, 5 (2007), pp. 17–26
- [2] J. Dent, H.B. El-Serag, M.A. Wallander, S. Johansson; Epidemiology of gastro-oesophageal reflux disease: a systematic review; Gut, 54 (2005), pp. 710–717
- [3] M. Camilleri, D. Dubois, B. Coulie, M. Jones, P.J. Kahrilas, A.M. Rentz, et al.; Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study; Clin Gastroenterol Hepatol, 3 (2005), pp. 543–552
- [4] S.J. Chung, D. Kim, M.J. Park, Y.S. Kim, J.S. Kim, H.C. Jung, et al.; Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups; Gut, 57 (2008), pp. 1360–1365
- [5] H. Hampel, N.S. Abraham, H.B. El-Serag; Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications; Ann Intern Med, 143 (2005), pp. 199–211
- [6] N. Kim, S.W. Lee, S.I. Cho, C.G. Park, C.H. Yang, H.S. Kim, et al.; The prevalence of and risk factors for erosive oesophagitis and non-erosive reflux disease: a nationwide multicentre prospective study in Korea; Aliment Pharmacol Ther, 27 (2008), pp. 173–185
- [7] M.H. Derakhshan, E.V. Robertson, J. Fletcher, G.R. Jones, Y.Y. Lee, A.A. Wirz, et al.; Mechanism of association between BMI and dysfunction of the gastro-oesophageal barrier in patients with normal endoscopy; Gut, 61 (2012), pp. 337–343
- [8] P.C. Wang, C.S. Hsu, T.C. Tseng, T.C. Hsieh, C.H. Chen, W.C. Su, et al.; Male sex, hiatus hernia and Helicobacter pylori infection associated with asymptomatic erosive esophagitis ; J Gastroenterol Hepatol, 27 (2012), pp. 586–591
- [9] Y. Fujiwara, T. Arakawa; Epidemiology and clinical characteristics of GERD in the Japanese population; J Gastroenterol, 44 (2009), pp. 518–534
- [10] S.M. Grundy, J.I. Cleeman, S.R. Daniels, K.A. Donato, R.H. Eckel, B.A. Franklin, et al.; Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement; Circulation, 112 (2005), pp. 2735–2752
- [11] W.H. Pan, W.T. Yeh, L.C. Weng; Epidemiology of metabolic syndrome in Asia; Asia Pac J Clin Nutr, 17 (Suppl. 1) (2008), pp. 37–42
- [12] L.R. Lundell, J. Dent, J.R. Bennett, A.L. Blum, D. Armstrong, J.P. Galmiche, et al.; Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification; Gut, 45 (1999), pp. 172–180
- [13] T.S. Chen, F.Y. Chang; The prevalence and risk factors of reflux esophagitis among adult Chinese population in Taiwan; J Clin Gastroenterol, 41 (2007), pp. 819–822
- [14] P.H. Tseng, Y.C. Lee, H.M. Chiu, S.P. Huang, W.C. Liao, C.C. Chen, et al.; Prevalence and clinical characteristics of Barretts esophagus in a Chinese general population; J Clin Gastroenterol, 42 (2008), pp. 1074–1079
- [15] H.J. Song, K.N. Shim, S.J. Yoon, S.E. Kim, H.J. Oh, K.H. Ryu, et al.; The prevalence and clinical characteristics of reflux esophagitis in Koreans and its possible relation to metabolic syndrome; J Korean Med Sci, 24 (2009), pp. 197–202
- [16] C.S. Hsu, P.C. Wang, J.H. Chen, W.C. Su, T.C. Tseng, H.D. Chen, et al.; Increasing insulin resistance is associated with increased severity and prevalence of gastro-oesophageal reflux disease; Aliment Pharmacol Ther, 34 (2011), pp. 994–1004
- [17] G.R. Locke 3rd, N.J. Talley, S.L. Fett, A.R. Zinsmeister, L.J. Melton 3rd; Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota; Gastroenterology, 112 (1997), pp. 1448–1456
- [18] G.R. Locke 3rd, N.J. Talley, S.L. Fett, A.R. Zinsmeister, L.J. Melton 3rd; Risk factors associated with symptoms of gastroesophageal reflux; Am J Med, 106 (1999), pp. 642–649
- [19] A. Ruigomez, L.A. Garcia Rodriguez, M.A. Wallander, S. Johansson, H. Graffner, J. Dent; Natural history of gastro-oesophageal reflux disease diagnosed in general practice; Aliment Pharmacol Ther, 20 (2004), pp. 751–760
- [20] J.H. Cho, H.M. Kim, G.J. Ko, M.L. Woo, C.M. Moon, Y.J. Kim, et al.; Old age and male sex are associated with increased risk of asymptomatic erosive esophagitis: analysis of data from local health examinations by the Korean National Health Insurance Corporation; J Gastroenterol Hepatol, 26 (2011), pp. 1034–1038
- [21] D.R. de Vries, M.A. van Herwaarden, A.J. Smout, M. Samsom; Gastroesophageal pressure gradients in gastroesophageal reflux disease: relations with hiatal hernia, body mass index, and esophageal acid exposure; Am J Gastroenterol, 103 (2008), pp. 1349–1354
- [22] S. Menon, H. Jayasena, P. Nightingale, N.J. Trudgill; Influence of age and sex on endoscopic findings of gastrooesophageal reflux disease: an endoscopy database study; Eur J Gastroenterol Hepatol, 23 (2011), pp. 389–395
- [23] Y.C. Lee, A.M. Yen, J.J. Tai, S.H. Chang, J.T. Lin, H.M. Chiu, et al.; The effect of metabolic risk factors on the natural course of gastro-oesophageal reflux disease; Gut, 58 (2009), pp. 174–181
- [24] N. Furukawa, R. Iwakiri, T. Koyama, K. Okamoto, T. Yoshida, Y. Kashiwagi, et al.; Proportion of reflux esophagitis in 6010 Japanese adults: prospective evaluation by endoscopy; J Gastroenterol, 34 (1999), pp. 441–444
- [25] S.Y. Nam, I.J. Choi, K.H. Ryu, B.J. Park, H.B. Kim, B.H. Nam; Abdominal visceral adipose tissue volume is associated with increased risk of erosive esophagitis in men and women; Gastroenterology, 139 (2010), pp. 1902–1911 e1902
- [26] P. Kamat, S. Wen, J. Morris, S. Anandasabapathy; Exploring the association between elevated body mass index and Barretts esophagus: a systematic review and meta-analysis; Ann Thorac Surg, 87 (2009), pp. 655–662
- [27] M. Lindblad, L.A. Rodriguez, J. Lagergren; Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case–control study; Cancer Causes Control, 16 (2005), pp. 285–294
- [28] H.B. El-Serag, G.A. Ergun, J. Pandolfino, S. Fitzgerald, T. Tran, J.R. Kramer; Obesity increases oesophageal acid exposure; Gut, 56 (2007), pp. 749–755
- [29] C.M. Tai, Y.C. Lee, H.P. Tu, C.K. Huang, M.T. Wu, C.Y. Chang, et al.; The relationship between visceral adiposity and the risk of erosive esophagitis in severely obese Chinese patients; Obesity (Silver Spring), 18 (2010), pp. 2165–2169
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?