Summary
Primary gastrointestinal (GI) lymphoma accounts for 30–50% of all extranodal non-Hodgkin’s lymphomas, making the GI tract the most common site of extranodal non-Hodgkin’s lymphomas. Most GI lymphomas belong to the B cell lineage. Burkitt lymphoma (BL) is a highly aggressive mature B cell neoplasm that occurs in three forms: endemic, sporadic, and immunodeficiency-associated. Sporadic BL accounts for 1–2% of all adult lymphomas and usually presents as an abdominal manifestation of extranodal disease involving the distal ileum or cecum. Primary BL of the duodenum is rare. However, this report emphasizes the importance of awareness of the malignancy potential of duodenal polyps. We report the case of a 70-year-old woman admitted to our ward with upper GI bleeding due to duodenal polyps. An upper GI endoscopic examination showed button-like polyps with central depression, and an immunohistochemical study of the polyps revealed a high-grade B cell malignancy (BL). Consequently, the patient was treated with aggressive chemotherapy. The tumors regressed after chemotherapy. Although primary duodenal Burkitt lymphoma is very rare, the possibility of malignancy should be considered if a patient presents with duodenal button-like polyps with a central depressed surface.
Keywords
Burkitt lymphoma ; Duodenal polyp ; Non-Hodgkins lymphoma
Introduction
Lymphomas are solid malignancies of the lymphoid system and can be classified into Hodgkins and non-Hodgkins lymphoma (NHL). Almost all lymphomas involving the gastrointestinal (GI) tract are NHLs. Moreover, the GI tract is the most common site of extranodal NHLs, accounting for 30–50% of all cases. Small intestinal NHLs can be subdivided into B cell and T cell lymphomas; however, the latter are less common [1] .
Burkitt lymphoma (BL) is a highly aggressive mature B cell neoplasm that occurs in three forms: endemic, sporadic, and immunodeficiency-associated. Sporadic BL accounts for 1–2% of all adult lymphomas and usually presents with abdominal disease involving the sites of the distal ileum, cecum, mesentery, or cecum and mesentery sites [2] . Primary BL of the small intestine involving the duodenum is rare, and only a few cases have been documented in the English literature. Here, we report the case of a 70-year-old woman with rare sessile, button-like duodenal polyps, who initially presented with upper GI bleeding but was finally diagnosed with primary duodenal BL.
Case report
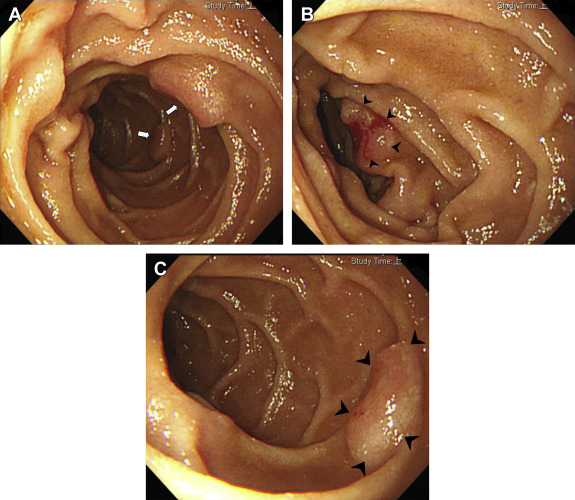
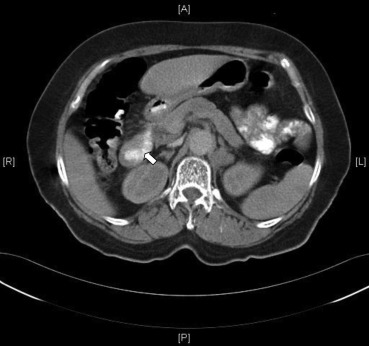
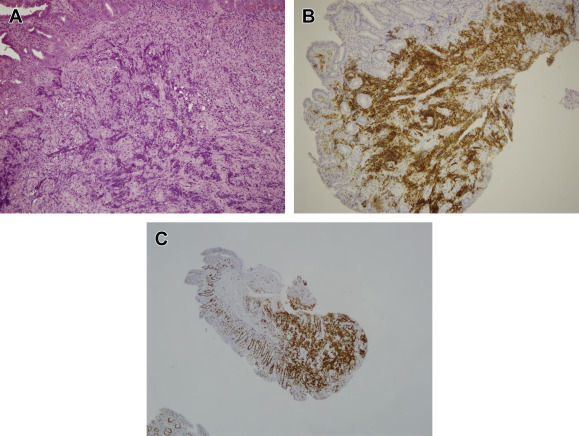
A 70-year-old woman was admitted to our hospital because of dyspnea on exertion and diffuse abdominal discomfort. The patient had noted one episode of melana prior to her admission. Her physical examination results showed a systolic and diastolic pressure of 110/70 mmHg, a heart rate of 106 beats/min, clear breathing sounds, and no abdominal rebounding pain, but slight tenderness over the epigastric area. Her laboratory test results revealed the following: hemoglobin level, 7.1 g/dL; mean cell volume, 66.7%; red cell distribution width, 18.8%; reticulocyte volume, 0.5%; creatinine level, 0.7 mg/dL; serum iron level, 13 μg/dL; total iron-binding capacity, 409 μg/dL; ferritin level, 5.2 ng/mL; and a positive stool occult blood test. Because of suspected iron deficiency anemia due to GI blood loss, an upper GI endoscopy was performed to identify the bleeder. Multiple round, sessile, button-like polyp lesions with a slight central depression and ulceration over the top were discovered, measuring approximately 1 cm in diameter, in the second and third portions of the duodenum (Figs. 1 A–1C). The barium study of jejunum and ileum and colonoscopy both showed negative finding. A contrast-enhanced abdominal computed tomography scan showed focal wall thickening of the duodenum (Fig. 2 ) and lymphadenopathy of the para-aortic area. A histopathological examination of the duodenal biopsy specimens from the polyp lesion showed sheets of neoplastic lymphoid infiltrate in the mucosal layer (Fig. 3 A). The immunohistochemical staining showed that the cells were positive for leukocyte common antigen, CD20 (Fig. 3 B), CD10, and nuclear B cell lymphoma (BCL-6), and were negative for CD3, CD56, CD5, CD23, chromogranin, and B cell leukemia/lymphoma-2 (BCL-2). A very high Ki-67 index (>90%; Fig. 3 C) was also observed in the tumor cells. A bone marrow biopsy was negative for tumor involvement. On the basis of these findings, the patient was diagnosed with primary duodenal BL, stage IIE, using the modified Ann Arbor staging system. Consequently, this patient received systemic chemotherapy with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) every 3 weeks for 10 courses. She achieved complete remission after aggressive treatment and was still well 36 months after initial diagnosis.
|
|
|
Figure 1. (A) Multiple round, sessile, button-like polyp lesions with a slight central depression and ulceration in the second and third portions of the duodenum (white arrows). (B) A button-like polyp (arrowheads) with bleeding in the second portion of the duodenum. (C) Closer view of a round, button-like duodenal polyp lesion (arrowheads). |
|
|
|
Figure 2. Focal wall thickening in the second portion of the duodenum (white arrow). |
|
|
|
Figure 3. (A) Diffuse neoplastic lymphoid cells infiltrated in the mucosa of the duodenal polyp (hematoxylin and eosin staining, ×100 original magnification). (B) Positive CD20 immunoreaction in neoplastic lymphoid cells, suggesting that the tumor was of B cell origin (CD20, ×100 original magnification). (C) High Ki-67 index in the neoplastic lymphoid cells (Ki-67, ×40 original magnification). |
Discussion
BL, as first described by Dennis Burkitt in 1958, is a highly aggressive NHL presenting frequently at extranodal sites or as an acute leukemia. According to the World Health Organization Classification of Lymphoid Neoplasms, BL is classified into mature B cell neoplasm and characterized by the translocation and deregulation of the c-myc gene on chromosome 8 [2] . Three distinct clinical forms of BL are recognized: endemic, sporadic, and immunodeficiency-associated. The sporadic type of BL accounts for 1–2% of all adult lymphomas in Western Europe and the United States.
Adult sporadic or immunodeficiency-associated BL with extranodal disease usually involves the abdomen and affects the bowel or intra-abdominal lymph nodes. Sometimes, the liver, spleen, kidney, and ovary may be involved. Symptoms may include abdominal pain, nausea, vomiting, bowel obstruction, GI bleeding, acute appendicitis, biliary obstruction, or intussusceptions [1] ; [2] ; [3] . In this study, our patient initially presented with symptoms of anemia because of tumor bleeding and diffuse abdominal discomfort. According to Kohno et als [4] study, primary NHL of the duodenum is rare. The ileocecal region is reported to be the most common site involved in the GI tract [5] . If the BL is found in the GI tract other than the ileocecal region such as duodenum or stomach, a survey of other parts of the small bowel or colon should be performed. Gloria Silva et al [6] reported the case of a 16-year-old male teenager who presented with BL and acute pancreatitis caused by an infiltrative tumor of the gastric mucosa and duodenum with giant ulcers. Liou et al [1] documented a gastric BL case with endoscopic findings of bleeding from multiple donut-like tumors in the body and the antrum of the stomach. Jeen et al [7] reported an Epstein–Barr virus-related BL in the terminal ileum with a diffusely infiltrative mass encircling the ileal wall, which was stenotic with shallow ulcers, mucosal nodularity, and blood clots. Endoscopic findings such as those in our study have not been reported previously. Here, we have reported the first case of BL with an endoscopic finding of round button-like polypoid lesions in the duodenum.
BL cells express surface immunoglobulin M and B cell-associated antigens (CD19, CD20, CD22, and CD79a) in addition to CD10, HLA-DR, and CD43 [2] . The biopsied tissue stained positive for CD10, CD20 (indicating B cell lineage), and BCL-6 markers, which are also present on BL cells, and stained negative for CD5, BCL-2, and CD23, which are typically absent on BL cells [8] . A high proliferation fraction and Ki-67 fraction of > 90% were noted. The results of the standard immunohistochemical staining satisfied the diagnostic criteria for BL.
BL progresses rapidly and is fatal if not treated; however, it responds well to aggressive chemotherapy, and the cure rates range from 50% to 90%, depending on the extent of the disease [9] . Thus, early diagnosis is important for a better clinical result. Surgery is seldom used in BL except under emergent conditions including obstruction, perforation, and hemorrhage. Because the tumor responds dramatically to chemotherapy, it can result in a rapid regression of the tumors with full thickness involvement of the bowel wall and subsequent perforation. According to Vaidya et als [10] study, 5% of patients developed a perforation after chemotherapy [10] . Bowel perforation in GI lymphomas significantly contributes to the morbidity and mortality, so early identification of patients with impending perforation may permit early surgical intervention. In conclusion, although primary duodenal BL is rare, the possibility of malignancy should be strongly suspected if a patient presents with button-like polyps and central depressed surface in the duodenum.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] J.M. Liou, H.P. Wang, B.S. Ko, T.Y. Cheng, M.S. Wu, J.T. Lin; Images of interest. Gastrointestinal: Burkitts lymphoma; J Gastroenterol Hepatol, 20 (2005), p. 1616
- [2] K.A. Blum, G. Lozanski, J.C. Byrd; Adult Burkitt leukemia and lymphoma; Blood, 104 (2004), pp. 3009–3020
- [3] N. Gupta, J.G. Wright, C.A. Bowman; An unusual cause of acute obstructive jaundice in an HIV-infected patient; Int J STD AIDS, 22 (2011), pp. 110–111
- [4] S. Kohno, K. Ohshima, S. Yoneda, T. Kodama, T. Shirakusa, M. Kikuchi; Clinicopathological analysis of 143 primary malignant lymphomas in the small and large intestines based on the new WHO classification; Histopathology, 43 (2003), pp. 135–143
- [5] C.A. Bethel, N. Bhattacharyya, C. Hutchinson, F. Ruymann, D.R. Cooney; Alimentary tract malignancies in children; J Pediatr Surg, 32 (1997), pp. 1004–1008
- [6] F. Gloria Silva, M. Paiva, A. Tavares, A. Lacerda, G. Pereira, A. Marques, et al.; Paediatric Burkitt lymphoma presenting as acute pancreatitis; Acta Med Port, 21 (2008), pp. 515–520
- [7] Y.T. Jeen, R.S. Chung, H.J. Chun, C.D. Kim, H.S. Ryu, J.H. Hyun; Small intestine Burkitts lymphoma; Gastrointest Endosc, 56 (2002), p. 731
- [8] C.F. Garcia, L.M. Weiss, R.A. Warnke; Small noncleaved cell lymphoma: an immunophenotypic study of 18 cases and comparison with large cell lymphoma; Hum Pathol, 17 (1986), pp. 454–461
- [9] D.A. Thomas, S. Faderl, S. O'Brien, C. Bueso-Ramos, J. Cortes, G. Garcia-Manero, et al.; Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia; Cancer, 106 (2006), pp. 1569–1580
- [10] R. Vaidya, T.M. Habermann, J.H. Donohue, K.M. Ristow, M.J. Maurer, W.R. Macon, et al.; Bowel perforation in intestinal lymphoma: incidence and clinical features; Ann Oncol, 24 (2013), pp. 2439–2443
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?