Summary
Background/Objective
To evaluate the clinical results of patients with infective endocarditis (IE) complicated by acute cerebrovascular accidents (CVAs).
Methods
A total of 44 patients with IE complicated by CVA at admission were retrospectively analyzed in a single medical institute from 2005 to 2011. At the time of admission, 18 patients were diagnosed with hemorrhagic stroke, and 26 patients were diagnosed with ischemic stroke. Fifteen patients received surgical intervention during hospitalization.
Results
The hospital mortality rate was 38.9% for the hemorrhagic stroke group and 42.3% for the ischemic stroke group (p = 0.821). The mortality rate was 33.3% for the surgical group and 44.8% for the nonsurgical group (p = 0.531). At 30 days of hospitalization, 45.8% of the patients experienced an adverse event (defined as death due to organ failure, restroke, cardiogenic shock, or septic shock during the treatment period), and the attrition rate was 1.5% per day. Surgery performed after the adverse events increased mortality (80.0%) compared with surgery performed on patients with no adverse events (10.0%; p = 0.017). A Cox regression analysis revealed that creatinine > 2 mg/dL, diabetes, and staphylococcal infection were the risk factors of the adverse events.
Conclusion
Early surgical intervention for IE with ischemic stroke may prevent adverse events, particularly in patients with impaired renal function, diabetes, or staphylococcal infection. A delay in operation of > 30 days is recommended after hemorrhagic stroke.
Keywords
infective endocarditis;stroke;valve replacement
1. Introduction
Over the past decade, infective endocarditis (IE) has caused significant morbidity and mortality despite the evolution of antimicrobial therapy and the refinement of surgical techniques.1 Infections involving the valve leaflets, endocardium, chordae, tendineae, and even artificial intracardiac materials lead to numerous complications, such as valvular incompetence, embolization, congestive heart failure, and cerebrovascular accidents. Among the various detrimental complications, neurologic complications in patients with endocarditis are the most challenging problems to address2 ; 3 because of the conflicting nature of their pathophysiology (i.e., an embolic event with hemorrhagic transformation) and the potentially fatal outcome of cardiac surgery under cardiopulmonary bypass.4 Recent studies have shown that early surgical intervention may prevent further complications.4; 5; 6 ; 7 Although emergent surgical indications for IE patients with repeated cerebrovascular accidents (CVAs) have been well established, the timing of the surgery and its prognostic impact on IE patients are still controversial. In this study, we analyzed 44 patients with active IE combined with new-onset CVA admitted to one medical institute over the past 6 years during hospitalization to evaluate the clinical outcomes and the complication rate during the waiting period.
2. Methods
Between January 1, 2005, and May 31, 2011, a total of 525 diagnosed IE cases were admitted to one single medical institute. Among them, 44 patients with active IE and new-onset CVA for possible surgical intervention were enrolled. All patients met the modified Duke criteria for definite or possible endocarditis.8 No patient who was initially included was excluded from the analyses after the initial assessment.
2.1. Clinical data
The following clinical and biological parameters were prospectively collected at the time of diagnosis and during hospitalization: age, gender, comorbidity, diabetes, serum creatinine level, location of involved valves, pre-existing prosthetic valve, presence of hemorrhagic stroke, and initial Glasgow Coma Scale (GCS). GCS impairment was defined by a GCS score ≤ 7 for those with endotracheal intubation or a GCS score ≤ 12 for those without. The diagnosis of CVA was based on clinical diagnoses and confirmed through computed tomography (CT). CVA was classified as ischemic or hemorrhagic according to the initial CT scan. Ischemic stroke was defined as the persistence of a focal neurologic deficit caused by altered circulation in the cerebral hemispheres, brain stem, or cerebellum for 24 hours with or without CT or magnetic resonance imaging (MRI) documentation. Hemorrhagic strokes included primary intracerebral hemorrhage, hemorrhagic infarction, and subarachnoid hemorrhage. Any hemorrhagic transformation noted during admission was recorded and placed the patient in the hemorrhagic group. The diagnosis of CVA was confirmed during the same hospitalization period by an experienced neurologist based on clinical signs or imaging evidence. Transthoracic or transesophageal echocardiography was performed in all cases to evaluate cardiac function and determine the location of the involved heart valves. The treatment was at the discretion of the patients treating physician. Adverse events included clinical situations, such as death, restroke, and shock (either cardiogenic or septic). Death due to chronic CVA sequelae was counted as “censor” instead of “death.” Restroke was defined as a new focal neurologic deficit with focal or lateralizing signs on physical examination and with CT confirmation during admission. Cardiogenic shock was defined as needing inotropic medication to maintain the mean arterial pressure above 65 mmHg, oliguria, or lung congestion requiring an oxygen mask to maintain SaO2 over 90%. Septic shock was defined as the presence of systemic inflammatory response syndrome plus signs of end-organ dysfunction. For patients who had undergone surgery for either ischemic or hemorrhagic stroke, days to operation were summarized and compared.
2.2. Statistical analysis
Numeric data were expressed as the mean ± standard deviation. The frequencies of simple events were reported as simple percentages. Differences between groups were compared using Students t test, the Mann–Whitney U test, or the Chi-square test according to the data type. Follow-up events of death and adverse events were analyzed using a Kaplan–Meier survival curve and compared using Cox regression tests. Variables with p < 0.1 according to univariate analysis were included in the multivariate analysis, which was performed with a backward Wald test with an entry probability of 0.1 and a removal probability of 0.1. For all statistical evaluations, differences in data with p < 0.05 were considered significant, and p < 0.1 was considered a trend. All statistical analyses were performed using MedCalc version 12 (MedCalc Software, Ostend, Belgium).
3. Results
A total of 44 patients who met the Duke criteria for IE combined with CVA at the time of admission were enrolled in the present study, including 26 patients with ischemic stroke and 18 patients with hemorrhagic stroke. Three cases of hemorrhagic transformation were included during the same admission. All three transformations happened within 36 hours of admission. The baseline demographic and clinical characteristics of the patients are summarized in Table 1. In brief, diabetes was present in 22.2% and 23.1% of patients in the hemorrhagic and ischemic groups, respectively (p = 0.973). Staphylococcal infections were identified in 38.9% and 34.6% of patients in the hemorrhagic and ischemic groups, respectively (p = 0.772).
| Hemorrhagic (18) | Ischemic (26) | p | |
|---|---|---|---|
| Age (y) | 54.6 ± 12.1 | 57.4 ± 19.1 | 0.484 |
| Male gender | 12 (66.7) | 16 (61.5) | 0.728 |
| Previous prosthetic valve | 4 (22.2) | 6 (23.1) | 0.973 |
| Diabetes | 4 (22.2) | 6 (23.1) | 0.973 |
| Creatinine > 2 mg/dL | 2 (11.1) | 5 (19.2) | 0.469 |
| Aortic valve involved | 5 (27.8) | 7 (26.9) | 0.950 |
| Mitral valve involved | 13 (72.2) | 19 (73.1) | 0.950 |
| Bacteria | |||
| Streptococcus | 8 (44.4) | 8 (30.8) | 0.354 |
| Staphylococcus | 7 (38.9) | 9 (34.6) | 0.772 |
| Other G(+) bacteria | 2 (11.1) | 0 (0.0) | 0.082 |
| G(−) bacteria | 0 (0.0) | 3 (11.5) | 0.135 |
| Fungus | 0 (0.0) | 0 (0.0) | 1.00 |
| Culture negative | 1 (5.6) | 6 (23.1) | 0.118 |
| GCS impairment | 6 (33.3) | 7 (26.9) | 0.647 |
| Hemiplegia | 14 (77.8) | 19 (73.1) | 0.723 |
Data in are presented as mean ± standard deviation or n (%).
GCS = Glasgow coma scale; G(−) = Gram negative; G(+) = Gram positive.
The clinical outcomes are summarized in Table 2. Adverse events during hospitalization were also recorded. Restroke occurred in nine of 44 cases (20.5%), septic shock in seven of 44 cases (15.9%), and cardiogenic shock in two of 44 cases (11.4%). Operations were performed for seven patients in the hemorrhagic group (38.9%) and eight patients in the ischemic group (30.8%; p = 0.576). Overall in-hospital mortality was six (33.3%) in the hemorrhagic group and nine (34.6%) in the ischemic group. The surgical outcomes are summarized in Table 3. The number of days to operation was higher in the hemorrhagic group (32–165 days, median of 60 days) than in the ischemic group (2–37 days, median of 15 days; p = 0.04). The total length of hospital stay was greater in the hemorrhagic group (56.0 ± 51.0 days) than in the ischemic group (25.8 ± 10.3 days; p < 0.01). Neurologic deterioration, defined as worsening GCS after surgical intervention, new-onset hemiplegia, or any clinically significant neurologic deficit, happened post-operatively in one case in the hemorrhagic group and in four cases in the ischemic group (p = 0.598). No paravalvular leakage or conduction block was found in the patients after operation. The number of surgical operations declined mostly because of families' hesitation about operative or neurological risk.
| Hemorrhagic (18) | Ischemic (26) | p | |
|---|---|---|---|
| Restroke a | 4 (22.8) | 5 (19.2) | 0.890 |
| Cardiogenic shock | 2 (11.1) | 3 (11.5) | 0.661 |
| Septic shock a | 3 (16.7) | 4 (15.4) | 0.909 |
| Received operation | 7 (38.9) | 8 (30.8) | 0.576 |
| Hospital stay (d) | 87.6 ± 63.4 | 54.1 ± 31.2 | 0.025 |
| Hospital mortality | 6 (33.3) | 9 (34.6) | 0.814 |
Data are presented as mean ± standard deviation or n (%).
a. Two patients in the hemorrhagic group and two in the ischemic group experienced simultaneous restroke and septic shock, possibly because of multiple septic emboli.
| Hemorrhagic (18) | Ischemic (26) | p | |
|---|---|---|---|
| Surgical treatment | 7 (38.9) | 8 (30.8) | — |
| Time to operation (d) | 32, 33, 59, 60, 63, 121, 165 | 2, 7, 8, 11, 19, 25, 35, 37 | — |
| Mean ± SD (d) | 76.1 ± 45.4 | 18.0 ± 12.4 | 0.04 |
| Median (d) | 60 | 15 | 0.04 |
| Post-operation hospital stay (d) | 56.0 ± 51.0 | 25.8 ± 10.3 | <0.01 |
| Neurologic status deterioration at dischargeb | 1 (5.6) | 4 (15.4) | 0.598 |
Data are presented as mean ± standard deviation (SD) or n (%).
GCS = Glasgow Coma Scale.
a. GCS impairment was defined by a GCS score ≤ 7 for those with endotracheal intubation or a GCS score ≤ 12 for those without.
b. Defined as worsening of GCS impairment after operation, new onset of hemiplegia, or any clinically significant dysfunction of neurologic performance.
3.1. Impact of adverse events
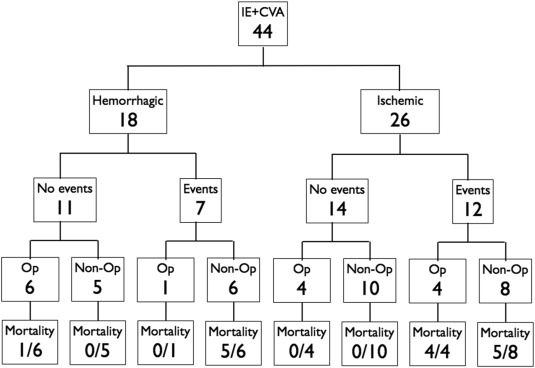
When the patients were grouped by the occurrence of adverse events during the 60-day follow up (Figure 1), the mortality rate was higher among patients who experienced adverse events (14/19, 73.7%) than among those who did not (4/25, 16%; p < 0.001). Furthermore, operation performed on survivors after the occurrence of an adverse event while awaiting surgery was associated with increased mortality (80.0%) compared to surgery performed on patients who did not experience adverse events (10.0%; p = 0.017).
|
|
|
Figure 1. 60-day outcome of patients grouped by CVA and adverse events. Adverse events are defined as death, restroke, cardiogenic shock, or septic shock during the treatment period. CVA = cerebrovascular accident; IE = infective endocarditis; Op: open-heart operation. |
3.2. Risk factor analysis of the adverse event rate
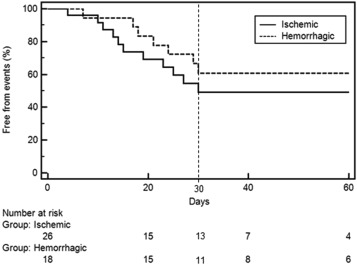
A Kaplan–Meier survival analysis was performed to evaluate the rate of adverse events during hospitalization for the study patients (Figure 2). At 30 days of hospitalization, 54.2% of the patients had not experienced an adverse event, and the attrition rate was 1.5% per day during the treatment period. The incidence of adverse events did not differ significantly between the hemorrhagic and ischemic groups (p = 0.287). Univariate analysis ( Table 4) revealed that diabetes [odds ratio (OR) = 2.65, p = 0.056], creatinine > 2 mg/dL (OR = 3.35, p = 0.018), and staphylococcal infection (OR = 2.29, p = 0.063) were potential risk factors for adverse events, while age, gender, valve involvement, prosthetic valve, stroke type, and admission GCS impairment were not. Multivariate analysis revealed p = 0.008 and p = 0.087 for creatinine > 2 mg/dL and staphylococcal infection, respectively. For the study group, diabetes was highly correlated with creatinine > 2 mg/dL, with a Pearson correlation coefficient of 0.505 (p < 0.001).
|
|
|
Figure 2. Proportion of infective endocarditis patients without adverse events during hospitalization. At 30 days of hospitalization, 54.2% of the patients had not experienced an adverse event, and the attrition rate was 1.5% per day during the treatment period. * p = 0.287. |
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Age (y) | 1.01 (0.98–1.04) | 0.471 | — | — |
| Male gender | 0.92 (0.38–2.38) | 0.916 | — | — |
| Diabetes | 2.65 (1.04–6.74) | 0.056 | — | 0.313 a |
| Creatinine > 2 mg/dL | 3.35 (1.33–8.44) | 0.018 | 3.90 (1.42–10.68) | 0.008 |
| Aortic valve involved | 0.95 (0.34–2.62) | 0.922 | — | — |
| Double valve involved | 0.55 (0.07–4.15) | 0.566 | — | — |
| Prosthetic IE | 1.28 (0.42–3.90) | 0.662 | — | — |
| Hemorrhagic stroke | 0.59 (0.24–1.48) | 0.263 | — | — |
| GCS impairment | 1.65 (0.63–4.36) | 0.313 | — | — |
| Staphylococcus | 2.29 (0.97–5.65) | 0.063 | 2.27 (0.90–5.80) | 0.087 b |
CI = confidence interval; GCS = Glasgow Coma Scale; IE = infective endocarditis.
a. Diabetes was highly correlated with creatinine > 2 mg/dL, with a Pearson correlation coefficient of 0.505 (p < 0.001).
b. With a trend.
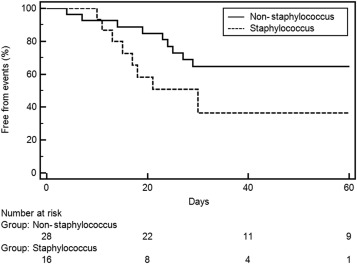
We further performed an event-free analysis according to the presence of staphylococcus infection among the 44 patients (Figure 3). Patients with staphylococcal infection had a high risk for adverse events during the waiting period. At 30 days of hospitalization, 36.4% of the patients in the staphylococcal infection group had experienced an adverse event compared with the 64.6% for the non-staphylococcal infection group (p = 0.084, trend). The attrition rates were roughly 2% per day for the staphylococcal group and 1% per day for the nonstaphylococcal group.
|
|
|
Figure 3. Proportion of infective endocarditis patients with and without staphylococcus infection who experienced no adverse events during hospitalization. * p = 0.084. |
4. Discussion
For the patients with IE and CVA, the adverse event rate during the first 30 days of hospitalization was high, 1.5% per day. The clinical outcome deteriorated following adverse events regardless of whether the patients had received surgical or medical treatment. Based on these data, we suggest early surgical intervention for IE with CVA unless the patient presents with a serious neurological outcome. According to the available clinical guidelines, prompt operation after an ischemic stroke and operation within 30 days after a hemorrhagic stroke are recommended to prevent adverse events, particularly for patients with impaired renal function (creatinine > 2 mg/dL), diabetes, or staphylococcal infection.
IE is still considered a high-risk condition, with overall mortality rates as high as 20–25%. Hemodynamic deterioration and central nervous system embolic events are the primary causes of death.3 Systemic embolism occurs in 22–50% of patients with IE,9 but the true incidence of acute brain embolization in left-sided IE may be significantly higher than indicated by clinical data or CT, as shown by one study.10 According to the current consensus, IE complicated by acute decompensated congestive heart failure is a well-established surgical indication.8; 11 ; 12 However, controversies have persisted over the past decades in regard to the surgical intervention of IE with cerebrovascular events, either ischemic or hemorrhagic. The “treatment dilemma” is due to the surgery itself. First, previously ischemic blood vessels are extremely vulnerable to hemorrhagic transformation during anticoagulation therapy, which is required during a cardiopulmonary bypass to prevent embolus formation in the circuit. Second, tissue perfusion to the brain may be compromised because of hypotensive episodes during cardiac surgery.4 In the early 1990s, the notion of delaying surgery with conservative antibiotic treatment for at least 4 weeks was widely accepted. A multicenter retrospective study conducted in Japan by Eoshi and colleagues13 suggested that cardiovascular surgery should be postponed to at least 4 weeks after the onset of cerebral infarction, yet the progression of cerebral damage is significant and persists even 4 weeks after a cerebral hemorrhage. Another study, conducted from 1983 to 1995,14 suggested that valve replacement should be delayed 2–3 weeks for embolic cerebrovascular events and 4 weeks for embolic cerebrovascular events with hemorrhage. Early surgery is recommended only for certain conditions, including annular or aortic abscesses, heart block, recurrent emboli, resistant infection, fungal endocarditis, Staphylococcus aureus endocarditis, or valvular vegetation. 8 However, several studies support early surgical intervention for IE patients. Gammie and colleagues15 reported that early mitral valve surgery can be safely performed for IE cases with silent ischemic stroke observed using brain CT or MRI. Thuny et al4 also demonstrated that the risk of postoperative neurologic exacerbation is low for IE cases after a silent cerebral embolism, transient ischemic attack, or nonmassive ischemic stroke. Several studies have also reported favorable clinical outcomes of early surgery for IE patients. 7; 8 ; 16 A prospective, randomized study (the Early surgery versus conventional treatment for infective endocarditis (EASE) trial) comparing the clinical outcomes of early surgery and conventional treatment for patients with IE demonstrated that early surgery significantly reduces the composite end point of death from any cause or embolic event by effectively reducing the risk of systemic embolism.17 The revised 2011 Society of Thoracic Surgeons Clinical Practice Guidelines3 state that early surgery performed at < 4 weeks may be reasonable, particularly in patients with small areas of brain infarction with a decline in cardiac function, recurrent stroke, or systemic embolism (Class IIb). Otherwise, valve replacement surgery may be delayed for weeks, unless a second embolic event occurs despite adequate antibiotic therapy.
The data obtained in the present study indicate that the most significant difference in prognosis was the occurrence of adverse events (including restroke, cardiogenic shock, or septic shock) rather than hemorrhagic-type or surgical intervention. The mortality rate greatly increased in patients who had experienced adverse events compared to the rate in those who had not (73.7% vs. 16%, respectively; p < 0.001). For patients with IE and CVA, the present study demonstrates that the adverse event rate during the first 30 days of hospitalization was high, 1.5% per day. The clinical outcome deteriorated following an adverse event, and our result clearly suggests that the operative outcomes became more serious after the adverse event. Our results are in accordance with those of previous studies. In another large prospective cohort study (n = 291), Thuny et al 7 demonstrated that very early surgery (within 1 week) might be beneficial for patients with the most severe forms of IE, including S. aureus infection, congestive heart failure, and large vegetations, to avoid the risk of in-hospital mortality associated with an increased frequency of relapse and prosthetic valve dysfunction. However, there is still some built-in diversity and heterogeneity in the groups, and that contributes to uncertainty in interpreting the data.
Regarding S. aureus endocarditis, the results suggest that early surgery is indicated for patients with staphylococcus infection because the adverse event rate was approximately 2% per day for the staphylococcal infection group and 1% per day for the non-staphylococcal infection group ( Figure 2). S. aureus endocarditis is characterized by a rapid onset, high fever, and the frequent involvement of normal cardiac valves at initial presentation 18 and is associated with a high complication rate. 8; 19 ; 20 Therefore, several studies have also recommended early surgery for IE with an associated staphylococcal infection.21
4.1. Study limitations
The present study suggests a delay in operation of 30 days for IE with hemorrhagic stroke despite an adverse event risk of 45%. Due to the limited case number, however, it is difficult to compare two heterogeneous groups to strongly demonstrate that early surgery is beneficial for IE patients with neurologic complication. Furthermore, the surgical treatment offered is somehow delayed by several variables, such as treating physicians discretion and families' hesitation, and makes the results somewhat confounding and uncertain. We also noticed three hemorrhagic transformations in the initial ischemic group, which all happened within 36 hours of admission. By either intervention we could not change the result. Prospective studies are needed to clarify the impact of early surgery on patients presenting with IE and a hemorrhagic CVA. It is hoped that this study will stimulate future research in this field.
The rate of adverse events after IE with stroke is high, so early surgical intervention for IE with ischemic stroke may prevent adverse events, particularly in patients with impaired renal function, diabetes, or staphylococcal infection. A delay in operation of > 30 days after hemorrhagic stroke is recommended, although the exact timing for IE with hemorrhagic stroke remains uncertain. Furthermore, we suggest cardiac echography and brain CT with angiography be regularly performed during the waiting period in these unique populations.
References
- 1 J.G. Gaca, S. Sheng, M.A. Daneshmand, et al.; Outcomes for endocarditis surgery in North America: a simplified risk scoring system; J Thorac Cardiovasc Surg, 141 (2011), pp. 98–106
- 2 N.J. Howell, I.C. Wilson; Timing for surgery in patients with infective endocarditis and cerebrovascular complications–waiting may be best but results of early surgery are acceptable and improvements in neurology are common; Eur J Cardiothorac Surg, 41 (2012), pp. 476–477
- 3 J.G. Byrne, K. Rezai, J.A. Sanchez, et al.; Surgical management of endocarditis: the society of thoracic surgeons clinical practice guideline; Ann Thorac Surg, 91 (2011), pp. 2012–2019
- 4 F. Thuny, J.F. Avierinos, C. Tribouilloy, et al.; Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: a prospective multicentre study; Eur Heart J, 28 (2007), pp. 1155–1161
- 5 D.H. Kim, D.H. Kang, M.Z. Lee, et al.; Impact of early surgery on embolic events in patients with infective endocarditis; Circulation, 122 (11 suppl) (2010), pp. S17–S22
- 6 E. Ruttmann, J. Willeit, H. Ulmer, et al.; Neurological outcome of septic cardioembolic stroke after infective endocarditis; Stroke, 37 (2006), pp. 2094–2099
- 7 F. Thuny, S. Beurtheret, J. Mancini, et al.; The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis; Eur Heart J, 32 (2011), pp. 2027–2033
- 8 R.O. Bonow, B.A. Carabello, C. Kanu, et al.; ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons; Circulation, 114 (2006), pp. e84–e231
- 9 L. Derex, E. Bonnefoy, F. Delahaye; Impact of stroke on therapeutic decision making in infective endocarditis; J Neurol, 257 (2010), pp. 315–321
- 10 H.A. Cooper, E.C. Thompson, R. Laureno, et al.; Subclinical brain embolization in left-sided infective endocarditis: results from the evaluation by MRI of the brains of patients with left-sided intracardiac solid masses (EMBOLISM) pilot study; Circulation, 120 (2009), pp. 585–591
- 11 B.D. Prendergast, P. Tornos; Surgery for infective endocarditis: who and when?; Circulation, 121 (2010), pp. 1141–1152
- 12 G. Habib, B. Hoen, P. Tornos, et al.; Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer; Eur Heart J, 30 (2009), pp. 2369–2413
- 13 K. Eishi, K. Kawazoe, Y. Kuriyama, Y. Kitoh, Y. Kawashima, T. Omae; Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan; J Thorac Cardiovasc Surg, 110 (1995), pp. 1745–1755
- 14 A.M. Gillinov, R.V. Shah, W.E. Curtis, et al.; Valve replacement in patients with endocarditis and acute neurologic deficit; Ann Thorac Surg, 61 (1996), pp. 1125–1130
- 15 J.S. Gammie, S.M. O'Brien, B.P. Griffith, E.D. Peterson; Surgical treatment of mitral valve endocarditis in North America; Ann Thorac Surg, 80 (2005), pp. 2199–2204
- 16 I.M. Tleyjeh, J.M. Steckelberg, G. Georgescu, et al.; The association between the timing of valve surgery and 6-month mortality in left-sided infective endocarditis; Heart, 94 (2008), pp. 892–896
- 17 D.H. Kang, Y.J. Kim, S.H. Kim, et al.; Early surgery versus conventional treatment for infective endocarditis; N Engl J Med, 366 (2012), pp. 2466–2473
- 18 F.D. Lowy; Staphylococcus aureus infections; N Engl J Med, 339 (1998), pp. 520–532
- 19 E. Mylonakis, S.B. Calderwood; Infective endocarditis in adults; N Engl J Med, 345 (2001), pp. 1318–1330
- 20 T. Lalani, C.H. Cabell, D.K. Benjamin, et al.; Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias; Circulation, 121 (2010), pp. 1005–1013
- 21 J.P. Remadi, G. Najdi, A. Brahim, F. Coviaux, Y. Majhoub, C. Tribouilloy; Superiority of surgical versus medical treatment in patients with Staphylococcus aureus infective endocarditis; Int J Cardiol, 99 (2005 Mar 18), pp. 195–199
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?