Summary
Background
It is well established that severe hypertriglyceridemia can lead to pancreatitis. At present, medical treatment for patients with severe hypertriglyceridemia and repeat pancreatitis attacks is not adequate. The aim of this study was to assess the effectiveness of laparoscopic bariatric surgery in these patients.
Methods
A review of 20 morbidly obese patients with severe hypertriglyceridemia (a triglyceride level of >1000 mg/dL) who received laparoscopic bariatric surgery was performed. The study population comprised 14 males and six females, with an average age of 35.0 years (range 24–52 years), and the mean body mass index was 38.2 kg/m2 (range 25–53 kg/m2). The preoperative mean plasma triglyceride level was 1782.7 mg/dL (range 1043–3884 mg/dL). Four patients had a history of hypertriglyceridemic pancreatitis and 13 patients had associated diabetes.
Results
Of the 20 patients, 17 (85%) received gastric bypass, whereas three (15%) received restrictive-type surgery. Laparoscopic access was used in all of the patients. Hypertriglyceridemia in morbidly obese patients was more commonly associated with male sex and a poorly controlled diabetic state. The mean weight reduction was 25.5% 1 year after surgery, with a marked improvement in diabetes management. As early as 1 month following surgery, the plasma mean triglyceride levels had decreased to 254 mg/dL (range 153–519 mg/dL), and this was further reduced to mean levels of 192 mg/dL (range 73–385 mg/dL) 1 year after surgery. One patient developed acute pancreatitis during the perioperative period, but none of the patients suffered an episode of pancreatitis in the follow-up period (from 6 months to 13 years).
Conclusion
Bariatric surgery can be successfully used as a metabolic surgery in severe hypertriglyceridemia patients at risk of acute pancreatitis. However, control of triglyceride levels prior to bariatric surgery is indicated.
Keywords
hypertriglyceridemia;laparoscopic bariatric surgery;metabolic surgery;pancreatitis
1. Introduction
Hypertriglyceridemia (HTG) is an independent risk factor for atherosclerotic heart disease.1 ; 2 Moreover, patients with serum triglyceride (TG) levels >1000 mg/dL are at high risk of acute pancreatitis.3 ; 4 Diet control, statins, nicotinic acid, and fibric acid derivatives may be used in the treatment of HTG. It has been reported that in patients with severe HTG-associated pancreatitis, which had been previously unresponsive to treatment, plasmapheresis had proved useful, particularly in pregnant individuals.5; 6; 7 ; 8 However, none of these treatments can provide a cure for patients with severe HTG accompanied by repeated attacks of pancreatitis.
Metabolic surgery, defined as surgery aimed at anatomical and functional change to treat a metabolic disorder, has been proposed to overcome this problem.9 Partial ileal bypass has been reported as an effective treatment for resolving HTG, and providing a cure for patients with severe HTG and repeated pancreatitis attacks.10 ; 11 In this study, the effectiveness of bariatric surgery was evaluated in morbidly obese patients with severe HTG.
2. Materials and methods
2.1. Patient selection and data sources
The Min-Sheng General Hospital institutional review board approved the present study (MSIRB2011003). We identified 20 patients with severe HTG (serum TG levels >1000 mg/dL), who had undergone laparoscopic bariatric surgery during the period 1997 to 2011, by retrospective review of our prospectively collected bariatric database. The distribution of the plasma TG levels is shown in Table 1, and patients with severe HTG comprised only 0.6% of all the bariatric patients. The study group consisted of 14 males and six females, with a mean age of 35.0 years (range 24–52 years) and a mean body mass index of 38.2 kg/m2 (range 25–53 kg/m2). The mean body weight was 109.8 kg (range 67–144 kg). Four patients had a history of recurrent hypertriglyceridemic pancreatitis, and 13 (65%) patients had associated type 2 diabetes. The clinical characteristics and follow-up data were collected and compared with those of other bariatric patients. Insulin resistance was measured using the Homeostatic Model Assessment (HOMA) index, which can be calculated as follows: plasma glucose (mmol/L) × insulin (μU/mL)/22.5.12
| Triglycerides (mg/dL) | No. of patients | % |
|---|---|---|
| <500 | 3042 | 96.9 |
| 500–1000 | 80 | 2.5 |
| ≥1000 (severely high) | 20 | 0.6 |
| Total | 3142 | 100.0 |
2.2. Operative techniques
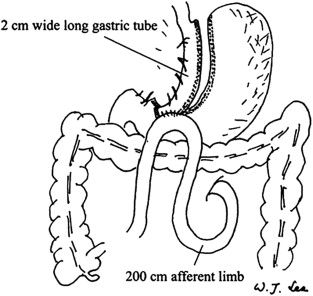
Three patients received restrictive-type surgery (two had vertical banded gastroplasty and one had adjustable gastric banding), and 17 patients received gastric bypass surgery (13 underwent a minigastric bypass and 4 had Roux-en-Y gastric bypass). All procedures were performed using laparoscopic techniques and have been described elsewhere.13; 14 ; 15 The minigastric bypass is a modification of Masons loop gastric bypass with a long lesser-curvature tube from the antrum to the Angle of His (outer diameter 1–2 cm, volume 60–80 mL). The jejunum is measured 200 cm from the ligament of Treitz and is brought up to the distal end of the gastric tube in an antecolic position (Fig. 1).16 The bypass limb in the minigastric bypass measured 200 cm (biliopancreatic limb), and in the Roux-en-Y bypass it measured 100 cm in the biliopancreatic limb and 150 cm in the alimentary limb.
|
|
|
Figure 1. Laparoscopic minigastric bypass (LMGB). |
2.3. Statistical analysis
All statistical analyses were performed using SPSS version 12.01 (SPSS Inc., Chicago, IL, USA), with baseline comparison made using Chi-square tests and a two-sample t test. Continuous variables were expressed as the mean (standard deviation), with differences expressed as the mean (95% confidence interval). A two-sided p value = 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
In comparison with the other bariatric patients, patients with severe HTG were predominantly male and were more associated with having problems managing their diabetes (Table 2). The severe HTG group had significantly higher fasting plasma glucose levels than the other patients (222.2 mg/dL vs. 115.7 mg/dL, p < 0.05), and exhibited considerably higher mean glycosylated hemoglobin (HbA1C) levels than the other patients (9.5% vs. 6.2%, p < 0.05). The severe HTG group was also more linked with hypercholesterolemia, increase in liver enzymes, insulin resistance, and uric acid level increases.
| Factor | Severe HTG (n = 20) | Others (n = 3172) | p |
|---|---|---|---|
| Age (y) | 35.0 ± 8.1 | 32.5 ± 9.5 | 0.146 |
| Sex (M/F) | 13/7 | 864/2318 | < 0.001∗ |
| Weight (kg) | 109.8 ± 22.3 | 107.4 ± 25.4 | 0.430 |
| BMI (kg/m2) | 38.2 ± 6.8 | 39.4 ± 7.7 | 0.531 |
| Waist (cm) | 117.7 ± 14.8 | 115.7 ± 17.7 | 0.502 |
| TG (mg/dL) | 1782.7 ± 769.7 | 171.7 ± 117.4 | < 0.001∗ |
| FPG (mg/dL) | 222.2 ± 109.2 | 108.5 ± 44.3 | < 0.001∗ |
| AST (IU/L) | 70.9 ± 95.4 | 32.1 ± 30.0 | 0.007∗ |
| ALT (IU/L) | 98.4 ± 118.1 | 46.1 ± 46.1 | 0.001∗ |
| Insulin (IU/L) | 30.0 ± 27.2 | 23.1 ± 29.4 | 0.156 |
| HbA1C (%) | 9.5 ± 3.1 | 6.1 ± 1.3 | < 0.001∗ |
| C-peptide (ng/mL) | 6.1 ± 3.6 | 4.4 ± 7.1 | 0.003∗ |
| HOMA-IR | 17.0 ± 15.3 | 6.6 ± 11.5 | < 0.001∗ |
| Cholesterol, total (mg/dL) | 298.0 ± 85.9 | 194.2 ± 38.2 | < 0.001∗ |
| LDL-C (mg/dL) | 73.0 ± 28.0 | 127.1 ± 33.6 | < 0.001∗ |
| Uric acid (mg/dL) | 8.1 ± 2.3 | 7.1 ± 2.6 | 0.045∗ |
| WBC (k/μL) | 8.4 ± 2.0 | 13.7 ± 4.8 | 0.842 |
∗p < 0.05.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BMI = body mass index; FPG = fasting plasma glucose; HbA1C = glycosylated hemoglobin; HOMA-IR = Homeostatic Model Assessment-Insulin Resistance; HTG = hypertriglyceridemia; LDL-C = low-density lipoprotein C; TG = triglycerides; WBC = white blood cell count.
3.2. Weight loss
An average weight reduction of 28.9 kg (25.5% reduction) was observed in HTG patients 1 year after surgery, with an accompanying reduction in mean postoperative body mass index of 23% (reduced to 29.4 kg/m2) being demonstrated (Table 3). The weight reduction curve was similar between the HTG group and the other patients (data not shown).
| Factor | After surgery | Δa | p |
|---|---|---|---|
| Weight (kg) | 82.8 ± 12.0 | 32.7 ± 11.4 | 0.005∗ |
| BMI (kg/m2) | 29.4 ± 3.5 | 11.8 ± 4.1 | 0.005∗ |
| Waist (cm) | 92.6 ± 7.3 | 25.1 ± 9.1 | 0.028∗ |
| TG (mg/dL) | 143.6 ± 65.5 | 1142.0 ± 177.5 | < 0.001∗ |
| FPG (mg/dL) | 86.5 ± 11.9 | 135.7 ± 118.4 | 0.012∗ |
| AST (IU/L) | 32.3 ± 27.2 | 38.6 ± 41.0 | 0.263 |
| ALT (IU/L) | 29.1 ± 21.6 | 69.3 ± 59.7 | 0.036∗ |
| Insulin (IU/L) | 4.9 ± 3.5 | 25.1 ± 24.1 | 0.043∗ |
| HbA1C (%) | 5.1 ± 0.4 | 4.4 ± 3.4 | 0.028∗ |
| C-peptide (ng/mL) | 2.3 ± 1.1 | 3.8 ± 2.9 | 0.068 |
| HOMA-IR | 1.1 ± 0.8 | 15.9 ± 19.4 | 0.043∗ |
| Cholesterol, total (mg/dL) | 187.0 ± 37.7 | 111.0 ± 57.5 | 0.011∗ |
| Uric acid (mg/dL) | 7.6 ± 2.2 | 0.5 ± 1.9 | 0.237 |
| WBC (k/μL) | 7.0 ± 1.3 | 1.4 ± 2.1 | 0.042∗ |
∗ p < 0.05.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BMI = body mass index; FPG = fasting plasma glucose; HbA1C = glycosylated hemoglobin; HOMA-IR = Homeostatic Model Assessment-Insulin Resistance; TG = triglycerides; WBC = white blood cell count.
a. Δ = difference between the preoperative and postoperative value.
As in different types of surgery, including restrictive and gastric bypass types, the mean body weight loss 1 year after surgery was 28.2% and 24.7%, respectively (Table 4).
| Type | Surgical method (laparoscopy) | No. of patients | Mean body weight (kg) | Mean weight loss (%) | TG reduction (%) |
|---|---|---|---|---|---|
| Restrictive | Vertical banded gastroplasty | 2 | 126.6 | 28.2 | 86.0 |
| Adjustable gastric banding | 1 | ||||
| Gastric bypass | Minigastric bypass | 13 | 106.8 | 24.7 | 90.0 |
| Roux-en-Y gastric bypass | 4 |
TG = triglycerides.
3.3. Sequential metabolic parameters
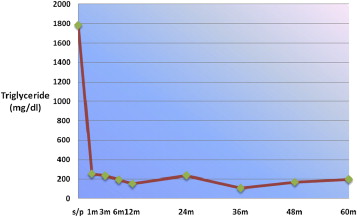
Immediately after surgery, the high plasma TG levels returned to nearly normal values (Fig. 2). The mean total plasma TG level 1 year after surgery was 192.1 mg/dL (89.2% reduction). There was no difference in the mean postoperative TG level between patients who received a restrictive-type procedure and those who received gastric bypass surgery. The reduction in the restrictive-type procedure was 86.0% (with no significant differences) and 90.0% in the gastric bypass group (Table 4). All the metabolic disorders of the study group still showed a significant improvement 1 year after surgery (Table 3). The mean HbA1C decreased from 9.5% to 5.1%. Among the 13 patients with diabetes, 12 patients had their diabetes in remission (HbA1C < 6.0%) 1 year after surgery.
|
|
|
Figure 2. Reduction in serum triglyceride levels after surgery. |
3.4. Clinical findings
No patient experienced an episode of pancreatitis during the follow-up period (from 6 months to 13 years). However, one patient developed acute pancreatitis during the operation. This was a 43-year-old male patient receiving a laparoscopic Roux-en-Y gastric bypass for revision surgery of a previous vertical banded gastroplasty operation. The operation was uneventful, but the patient developed acute pancreatitis and renal shutdown during the operation. He developed multiorgan failure and required a prolonged stay in the intensive care unit. The preoperative plasma TG level in this patient was 2061 mg/dL. Thereafter, we delayed the bariatric procedure until the TG level was reduced to <1000 mg/dL in other patients.
4. Discussion
This study confirmed that bariatric surgery can be an excellent management tool for patients with severe HTG (>1000 mg/dL), particularly in those cases where this is accompanied by repeated pancreatitis.11 Using current laparoscopic bariatric surgery, we achieved an 89.2% reduction in the preoperative HTG level 1 year after surgery, and this reduction was still observed up to 5 years after surgery. More importantly, none of the patients developed pancreatitis after the severe HTG was controlled. This result strongly supports the use of bariatric surgery, as a metabolic surgery, in patients with severe HTG-induced repeated pancreatitis.
HTG is a common clinical problem that can be exacerbated by numerous medications and medical conditions, including obesity and diabetes. However, severe HTG is rarely seen in morbidly obese patients seeking bariatric surgery. Buchwald and Schone11 reported a series of six cases and proposed that different genetic defects may be responsible for morbid obesity and severe HTG. Here, we report on a series of 20 cases, which only comprised 0.6% of the bariatric surgery cohort in this study. Most of the cases in this series were associated with type 2 diabetes and there was male predominance. Poorly controlled diabetes appears to be the most important trigger factor for severe HTG in morbidly obese patients. The morbid obesity-related diabetes is usually associated with very high insulin resistance and nonalcoholic steatohepatitis, which in turn results in elevated liver enzymes in this specific group of patients.17
Because insulin resistance is the underlying mechanism of HTG in morbidly obese patients, the dramatic diabetes treatment effect of bariatric surgery probably plays an important role in significantly reducing the severe HTG.18 ; 19 It also explains why three patients, who had received restrictive-type procedures in this study, also showed a very good reduction in severe HTG. In our cases, both restrictive and gastric bypass procedures achieved >24% mean weight reduction 1 year after surgery (Table 4)—which means that the weight loss is more important than the procedure.
Although rarely seen in morbidly obese patients seeking bariatric surgery, HTG is the next most common cause of pancreatitis after gallstones and excessive alcohol consumption.3 In up to 7% of all cases of pancreatitis, HTG or chylomicronemia is the underlying cause.4 The exact mechanisms underlying HTG-related pancreatitis are unclear. Chylomicrons are TG-rich lipoprotein particles, believed to be responsible for pancreatic inflammation.4 HTG-related pancreatitis rarely occurs if the TG level is <500 mg/dL, and people with HTG-related pancreatitis usually have TG levels of >1000 mg/dL. Therefore, long-term control of plasma TG levels of <500 mg/dL in patients with severe HTG is essential to reduce their risk of developing pancreatitis.
Initially, when treating HTG, removal of offending medications, lifestyle modifications (e.g., diet, exercise, abstention from alcohol), and use of drugs such as fibrates and nicotinic acid should be considered.3 The serum TG response to diet and weight loss is approximately 25%, with marked variation among patients. The fibrates can reduce TG levels by up to 50% and daily consumption of up to 3 g of nicotinic acid (niacin) can lower TG levels by up to 45%.20 However, none of the medical treatments can achieve the long-term 90% reduction observed with bariatric surgery.
Apheresis is also proposed for serum TG removal, and to treat or prevent HTG-induced pancreatitis.5; 6; 7 ; 8 One series of seven patients, with an average TG level of 1406 mg/dL, reported a 41% decrease in TG levels after one plasma exchange session.21 In another case study, TG was lowered from 2410 mg/dL to 138 mg/dL following 3 days of apheresis.22 Nevertheless, the effect of apheresis is transient, and repeat procedures may be required. In addition, each course of apheresis will cost approximately US$ 3000, which is not cost effective for long-term treatment.5 Therefore, the use of bariatric surgery as a metabolic surgery is probably the best option for long-term management of patients with severe HTG and repeat pancreatitis attacks.
The first metabolic surgery for hyperlipidemia was a partial ileal bypass. Performed in 1963, this procedure was carried out to specifically reduce plasma lipids.10 For morbidly obese patients with hypercholesterolemia, gastric obesity surgery with partial ileal bypass was also recommended.11 Initially, these procedures were designed for the treatment of hypercholesterolemia, but following the success in medical treatment, they went out of favor. However, these procedures may still play an important role in the treatment of severe HTG, particularly in patients where this is accompanied by repeated pancreatitis. The majority of our patients received laparoscopic minigastric bypass as a metabolic surgery for the treatment of their intractable HTG. This procedure is very similar to the former partial ileal bypass procedure.11 In our previous study, this simplified gastric bypass had proven to be low risk and was also an effective procedure in comparison with the laparoscopic Roux-en-Y gastric bypass.15
Although bariatric surgery is very effective in the treatment of severe HTG, surgery itself may be potentially dangerous in the presence of HTG. HTG-related pancreatitis is usually induced in patients who have TG levels >1000 mg/dL. One of our patients, whose TG level was 2061 mg/dL, had a life-threatening pancreatitis attack during the operation. Therefore, we recommend that bariatric surgery be performed only after the TG level has been reduced to <1000 mg/dL. These patients should be admitted prior to surgery and closely monitored under fasting conditions, with regular lipid-lowering medications being administered. Other treatments, such as using insulin and heparin to enhance lipoprotein lipase activity,23 and aphresis5; 6; 7 ; 8 may also be considered. Once the TG levels have been lowered to <1000 mg/dL, metabolic or bariatric surgery can then be performed. In addition to the major complication due to pancreatitis, we also had another patient who had a minor complication of gastric stasis after the surgery.
In conclusion, we report on a series of severe HTG patients who were effectively treated using bariatric surgery. Bariatric surgery, as a metabolic surgery, can be successfully applied in patients with severe HTG, particularly if it is accompanied by repeated pancreatitis attacks.
References
- 1 J. Veppesen, H.O. Hein, P. Suadican, F. Gyntelberg; Triglyceride concentration and ischemic heart disease: an eight year follow up in the Copenhagen Male Study; Circulation, 97 (1998), pp. 1029–1036
- 2 W.P. Castelli; Epidemiology of triglyceride: a view from Framingham; Am J Cardiol, 70 (1992), pp. 3H–9H
- 3 D. Yudav, C.S. Pitchumoni; Issues in hyperlipidemic pancreatitis; J Clin Gastroenterol, 36 (2003), pp. 54–62
- 4 S.I. Gan, A.L. Edwards, C.J. Symonds, P.L. Beck; Hypertriglyceridemia-induced pancreatitis: a case-based review; World J Gastroenterol, 12 (2006), pp. 7197–7202
- 5 H. Yamauchi, M. Sunamura, K. Takeda, T. Suzuki, K. Itoh, K. Miyagawa; Hyperlipidemia and pregnancy associated pancreatitis with reference to plasma exchange as a therapeutic intervention; Tohoku J Exp Med, 148 (1986), pp. 197–205
- 6 K.M. Ho, J. Yeo; Plasmapheresis in the management of pancreatitis related to hypertriglyceridaemia; Anaesth Intensive Care, 27 (1999), pp. 117–119
- 7 K. Swoboda, K. Derfler, R. Koppensteiner, et al.; Extracorporeal lipid elimination for treatment of gestational hyperlipidemic pancreatitis; Gastroenterology, 104 (1993), pp. 1527–1531
- 8 A. Piolot, F. Nadler, E. Cavallero, J.L. Coquard, B. Jacotot; Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis; Pancreas, 13 (1996), pp. 96–99
- 9 H. Buchwald, R.D. Rucker; The history of metabolic surgery for morbid obesity; World J Surg, 5 (1981), pp. 781–787
- 10 H. Buchwald, R.B. Moore, R.L. Varco; Ten years clinical experience with partial ileal bypass in management of the hyperlipidemias; Ann Surg, 180 (1974), pp. 384–392
- 11 H. Buchwald, J.L. Schone; Gastric obesity surgery combined with partial ileal bypass for hypercholesterolemia; Obes Surg, 7 (1997), pp. 313–316
- 12 D.R. Matthews, J.P. Hosker, A.S. Rudensky, B.A. Naylor, D.F. Treacher, R.C. Turner; Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentration in man; Diabetologia, 28 (1985), pp. 412–419
- 13 W.J. Lee, I.R. Lai, M.T. Huang, C.C. Wu, P.L. Wei; Laparoscopic versus open vertical banded gastroplasty for the treatment of morbid obesity; Surg Laparosc Endosc, 11 (2001), pp. 9–13
- 14 W.J. Lee, P.Y. Yu, W. Wang, T.C. Chen, P.L. Wei, M.T. Huang; Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial; Ann Surg, 242 (2005), pp. 20–28
- 15 W.J. Lee, W. Wang, P.J. Yu, P.L. Wei, M.T. Huang; Gastrointestinal quality of life following laparoscopic adjustable gastric banding in Asia; Obes Surg, 16 (2006), pp. 586–591
- 16 W. Wang, P.L. Wei, Y.C. Lee, M.T. Huang, C.C. Chiu, W.J. Lee; Short-term results of laparoscopic mini-gastric bypass; Obes Surg., 15 (2005), pp. 648–654
- 17 W.J. Lee, Y.C. Lee, K.H. Ser, J.C. Chen, S.C. Chen; Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures; Obes Surg, 18 (2008), pp. 1119–1125
- 18 H. Buchwald, Y. Avidor, E. Braunwald, et al.; Bariatric surgery: a systematic review and meta-analysis; JAMA, 292 (2004), pp. 1724–1737
- 19 W.J. Lee, W. Wang, Y.C. Lee, M.T. Huang, K.H. Ser, J.C. Chen; Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI >35 and <35 kg/m2; J Gastrointest Surg, 12 (2008), pp. 945–952
- 20 G.T. Gerhard, A. Ahmann, K. Meeuws, M.P. McMurry, P.B. Duell, W.E. Connor; Effects of a low-fat diet compared with those of a high-monounsaturated fat diet on body weight, plasma lipids and lipoproteins, and glycemic control in type 2 diabetes; Am J Clin Nutr, 80 (2004), pp. 668–673
- 21 G. Kadikoylu, I. Yavasoglu, Z. Bolaman; Plasma exchange in severe hypertriglyceridemia a clinical study; Transfus Apher Sci, 34 (2006), pp. 253–255
- 22 R.S. Kohli, W. Bleibel, A. Shetty, U. Dhanjal; Plasmapheresis in the treatment of hypertriglyceridemic pancreatitis with ARDS; Dig Dis Sci, 51 (2006), pp. 2287–2289
- 23 C. Henzen, M. Rock, C. Schnieper, K. Heer; Heparin and insulin in the treatment of acute hypertriglyceridemia-induced pancreatitis; Schweiz Med Wochenschr, 129 (1999), pp. 1242–1248
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?