Summary
Background and aim
Patients with stage T3 or T4 rectal cancer are candidates for neoadjuvant chemoradiation therapy. The aim of this study is to clarify the usefulness of circumferential tumor extent determined by computed tomography (CT) colonography in differentiating T3 or T4 from T1 or T2 rectal cancer.
Methods
Seventy consecutive rectal cancer patients who underwent curative-intent surgery were enrolled in this study. All patients underwent colonoscopy and CT colonography on the same day. The circumferential tumor extent was estimated in 10% increments. The pathological T stage was used as the reference.
Results
The median circumferential tumor extent evaluated by colonoscopy for T1 (n = 6), T2 (n = 21), and T3/T4 (n = 43) were 10%, 30%, and 80%, respectively (T1/T2 vs. T3/T4, p < 0.0001). The median circumferential tumor extent evaluated by CT colonography for T1, T2, and T3/T4 is 10%, 30%, and 70%, respectively (T1/T2 vs. T3/T4, p < 0.0001). The correlation coefficient between colonoscopy and CT colonography was very high (0.94). By defining a circumferential tumor extent ≥50% by CT colonography as the criterion for stage T3 or T4, the sensitivity, specificity, positive predictive value and accuracy were 72%, 88%, 91%, and 79%, respectively.
Conclusion
Circumferential tumor extent ≥50% determined by CT colonography is a simple and potentially useful marker to identify candidates for neoadjuvant chemoradiation therapy.
Keywords
circumferential tumor extent;CT colonography;rectal cancer;T stage
1. Introduction
Patients with stage T3 or T4 rectal cancers are potential candidates for neoadjuvant chemoradiation therapy. Therefore, the preoperative T stage is important for rectal cancer patients. Endorectal ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) scan are useful tools to assess the T stage, and some or all of these studies have been used to evaluate patients for neoadjuvant therapy.
Preoperatively determining the T stage of colorectal cancer by CT was initially disappointing.1 ; 2 However, along with the development of CT scanners and improved techniques for bowel preparation, the accuracy improved.3; 4 ; 5 Multidetector CT (MDCT) scanners now allow high-quality three-dimensional images to be obtained within several seconds, and various kinds of postprocessing techniques have become available for CT colonography (CTC). In our previous study, CTC was useful for preoperative assessment of T stage in colorectal cancer.6
The circumferential extent of a tumor is one factor that represents the amount and invasion area of a rectal cancer. A circumferential extent of a tumor of 75% or more has been shown to be a risk factor for the intramural distal spread of rectal cancer.7 The circular growth of the tumor was associated with more advanced T stage and positive lymph node status, and patients with circular tumors were more likely to receive preoperative adjuvant radiation.8 ; 9 Circumferential tumor extent was reported to be a simple predictor of tumor response and downstaging by preoperative chemoradiation therapy for rectal cancer.10 Circumferential tumor extent was also reported to be a good prognostic indicator in patients with rectal cancer.11; 12 ; 13 However, no report has analyzed the relationship between circumferential tumor extent and T stage in patients with rectal cancer.
The aim of this study is to demonstrate the accuracy of differentiating T3/T4 from T1/T2 lesions by determining the T stage of rectal cancer using circumferential tumor extent evaluated by CTC.
2. Materials and methods
From April 2003 to June 2007, consecutive rectal cancer patients who underwent resection with curative-intent at Jichi Medical University Hospital were enrolled in this study. Patients who received preoperative radiation or who had familial adenomatous polyposis or colitic cancer were excluded. The study was approved by the Jichi Medical University Institutional Review Board, and informed consent was waived.
All patients underwent colonoscopy and subsequently CTC on the same day. The details of the CTC procedure are described in a previous study.6 Colonoscopy was performed using standard techniques, and the circumferential tumor extent was determined by a single experienced endoscopist (KT). CTC was performed using four-row or 16-row MDCT with the patient in the prone position after insufflation with room air. A single radiologist (K.U.) reconstructed the CTC images with the use of a workstation (ZAIO M900; ZAIO Software, Tokyo, Japan; slice thickness, 2 mm) and determined the circumferential tumor extent without knowing the results of the colonoscopy. Circumferential tumor extent was estimated in 10% increments for both colonoscopy and CTC by independent examiners. Circumferential tumor extent was expressed as the median and interquartile range, and compared using the Mann–Whitney U test. Sensitivity, specificity, and predictive values were calculated based on pathological T stage.
3. Results
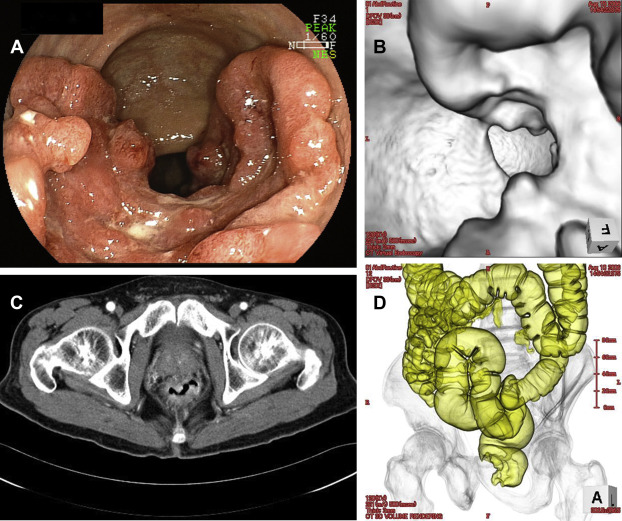
Images from a single patient with rectal cancer estimated by both colonoscopy and CTC (endoluminal image) to be 60% in circumferential extent are shown in Fig. 1. The median (interquartile range) circumferential tumor extent evaluated by colonoscopy for T1, T2, and T3/T4 were 10% (10–20), 30% (20–30), and 80% (20–100), respectively (Table 1). Circumferential tumor extent evaluated by colonoscopy for T1/T2 was significantly smaller than that for T3/T4 (p < 0.0001). The median (interquartile range) of circumferential extent evaluated by CTC (endoluminal image) for T1, T2, and T3/T4 were 10% (10–10), 30% (20–30), and 70% (20–100), respectively. Circumferential tumor extent evaluated by CTC for T1/T2 was significantly smaller than that for T3/T4 (p < 0.0001). The circumferential tumor extent could not be estimated by CTC for one patient in this study. In that particular patient, the tumor was T2, near the anal verge, and the insufflation was incomplete, but the circumferential tumor extent was estimated at 20% by colonoscopy. The correlation coefficient was very high (0.94) between the circumferential tumor extent evaluated by colonoscopy and CTC ( Table 2).
|
|
|
Figure 1. (A) Colonoscopy image of a tumor with 60% circumferential extent. (B) Computed tomography (CT) colonography, endoluminal image of a tumor with 60% circumferential extent. (C) CT axial image of a tumor with 60% circumferential extent. (D) CT colonography, air enema image of a tumor with 60% circumferential extent. |
| Colonoscopy | CTC (endoluminal image) | |
|---|---|---|
| pT1 (n = 6) | 10% (10–10%) | 10% (10–10%) |
| pT2 (n = 21) | 30% (20–30%) | 30% (20–30%) |
| pT3/T4 (n = 43) | 80% (20–100%) | 70% (20–100%) |
Circumferential tumor extent is expressed as the median (interquartile range).
CTC = CT colonography.
| CTC | Colonoscopy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% | Total | |
| 10% | 6 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| 20% | 1 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| 30% | 0 | 1 | 10 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 14 |
| 40% | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| 50% | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 6 |
| 60% | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 5 |
| 70% | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 4 |
| 80% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 4 |
| 90% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 100% | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 11 | 14 |
| Total | 7 | 11 | 15 | 4 | 3 | 4 | 2 | 7 | 3 | 13 | 69 |
Bold figures represent concordance of colonoscopy with CTC.
Correlation coefficient = 0.94.
CTC = CT colonography.
Based on these results, we propose a criterion for differentiating T1/T2 lesions from T3/T4 lesions as a circumferential extent of 10–40% and 50–100%, respectively. The sensitivity, specificity, positive predict value, and accuracy of differentiating T3/T4 from T1/T2 by this criterion are 72% (31/43), 88% (23/26), 91% (31/34), and 78% (54/69), respectively (Table 3).
| Circumferential extent | Pathological T stage | Total | |
|---|---|---|---|
| T1/T2 | T3/T4 | ||
| 10–40% | 23 | 12 | 35 |
| 50–100% | 3 | 31 | 34 |
| Total | 26 | 43 | 69 |
Sensitivity, 72%; specificity, 88%. Positive predictive value, 91%; negative predictive value, 78%.
4. Discussion
A high level of agreement between physicians was reported for determining the circumferential extent of a tumor by physical examination in the assessment of patients with rectal cancer.14 In this study, the correlation coefficient was very high between the circumferential extent evaluated by colonoscopy and CTC (Table 2). Therefore, the circumferential tumor extent can be an objective and reliable feature of a rectal cancer with small interobserver variability. The circumferential tumor extent increased along with the pathological T stage (Table 1). The result indicated that the circumferential tumor extent had correlation with pathological T stage, and a circumferential tumor extent ≥50% is a good criterion for classifying stage T3/T4 lesions with a high positive predictive value (91%).
However, three pT2 tumors were determined to have a circumferential extent ≥50% by CTC (Table 3). Two of these three pT2 tumors were near the anus and insufflation was incomplete, but had a circumferential extent <50% by colonoscopy. The third of these three pT2 tumor was a laterally spreading tumor (LST).15 ; 16 However, 12 of 43 T3/T4 tumors were found to have a circumferential tumor extent <50% by CTC. Therefore, T staging using circumferential extent by CTC is thought to be incomplete for the tumors near the anus, LSTs, and tumors with a circumferential extent <50%. Other modalities should be added to evaluate the T stage for these tumors.
In general, EUS, MRI, and CT scan are useful for preoperatively determining the T stage, and some or all of them have been used for assessment. However, their optimal role is not fully defined. EUS is reported to be most accurate for the staging of superficial rectal tumors, but not as useful for the staging of advanced rectal cancers.17; 18 ; 19 EUS has high operator dependence and is reported to have substantial interobserver variability.19 MRI has high spatial and organ resolution, and is suitable for the staging of both superficial and advanced rectal tumors. Endorectal MRI could be as accurate as EUS for staging of superficial tumors,20 and MRI was superior to conventional CT (not MDCT) for the prediction of tumor invasion in surrounding pelvic structures.21 ; 22 However, it is very difficult to differentiate stage T2 from stage T3 lesions even with MRI. MRI could not distinguish between spiculation in the perirectal fat caused by fibrosis alone (pT2 stage) and spiculation caused by fibrosis that contains tumor cells (pT3 stage).23 High cost, lengthy acquisition time, and a limited field of view are other problems associated with MRI.
MDCT has superior contrast and spatial resolution and capability for reconstruction in multiple planes, provides better performance than conventional CT,24 ; 25 and is equal to MRI in the preoperative local staging of rectal carcinoma.26 Maras-Simunic et al27 reported that T staging by CTC in patients with obstructive colon cancer showed very high overall accuracy (97.6%). The accuracy was higher than the results in the present study (78%). The low proportion of T1/T2 tumor and high proportion of T3/T4 in their study (T1/T2:T3/T4 = 2:39) may have contributed to the high accuracy observed. The results showed that T staging by CTC was useful in patients with advanced tumors. In addition, MDCT has the advantage in that a single investigation can be used to combine local, regional, and distant staging, with fast acquisition time and relatively low cost. Therefore, to evaluate a patient for neoadjuvant chemoradiation therapy, we recommend that CTC be performed in addition to colonoscopy for patients with advanced rectal cancers. Other testing modalities should be added for patients with tumors near the anus, LSTs, and tumors with a circumferential extent <50%.
In conclusion, a circumferential tumor extent >50% determined by CTC is a simple and potentially useful marker to identify candidates for neoadjuvant chemoradiation therapy with a high positive predictive value.
References
- 1 P.C. Freeny, W.M. Marks, J.A. Ryan, J.W. Bolen; Colorectal carcinoma evaluation with CT: preoperative staging and detection of postoperative recurrence; Radiology, 158 (1986), pp. 347–353
- 2 W.M. Thompson, R.A. Halvorsen, W.L. Foster Jr., L. Roberts, R. Gibbons; Preoperative and postoperative CT staging of rectosigmoid carcinoma; Am J Roentgenol, 146 (1986), pp. 703–710
- 3 C.J. Harvey, Z. Amin, C.M. Hare, et al.; Helical CT pneumocolon to assess colonic tumors: radiologic–pathologic correlation; Am J Roentgenol, 170 (1998), pp. 1439–1443
- 4 A. Filippone, R. Ambrosini, M. Fuschi, T. Marinelli, D. Genovesi, L. Bonomo; Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography—initial experience; Radiology, 231 (2004), pp. 83–90
- 5 K. Nagata, S. Endo, S.E. Kudo, T. Kitanosono, T. Kushihashi; CT air-contrast enema as a preoperative examination for colorectal cancer; Dig Surg, 21 (2004), pp. 352–358
- 6 K. Utano, K. Endo, K. Togashi, et al.; Preoprerative T staging of colorectal cancer by CT colonography; Dis Colon Rectum, 51 (2008), pp. 875–881
- 7 H. Ueno, H. Mochizuki, Y. Hashiguchi, et al.; Preoperative parameters expanding the indication of sphincter preserving surgery in patients with advanced low rectal cancer; Ann Surg, 239 (2004), pp. 34–42
- 8 S.H. Lee, E. Hernandez de Anda, C.O. Finne, R.D. Madoff, J. Garcia-Aguilar; The effect of circumferential tumor location in clinical outcomes of rectal cancer patients treated with total mesorectal excision; Dis Colon Rectum, 48 (2005), pp. 2249–2257
- 9 A. Ulrich, K. Himmer, M. Koch, P. Kienle, M.W. Büchler, J. Weitz; Location of rectal cancer within the circumference of the rectum does not influence lymph node status; Ann Surg Oncol, 14 (2007), pp. 2257–2262
- 10 P. Das, J.M. Skibber, M.A. Rodriguez-Bigas, et al.; Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer; Cancer, 109 (2007), pp. 1750–1755
- 11 J. Emslie, R. Beart, M. Mohiuddin, G. Marks; Use of rectal cancer position as a prognostic indicator; Am Surg, 64 (1998), pp. 958–961
- 12 P. Das, J.M. Skibber, M.A. Rodriguez-Bigas, et al.; Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer; Am J Clin Oncol, 29 (2006), pp. 219–224
- 13 T. Akasu, M. Takawa, S. Yamamoto, et al.; Intersphincteric resection for very low rectal adenocarcinoma: univariate and multivariate analyses of risk factors for recurrence; Ann Surg Oncol, 15 (2008), pp. 2668–2676
- 14 N.F. Boyd, B.J. Cummings, A.R. Harwood, W.D. Rider, G.M. Thomas; Observer variation in the assessment of patients with rectal cancer; Dis Colon Rectum, 25 (1982), pp. 664–668
- 15 S. Kudo, H. Kashida, T. Tamura, et al.; Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer; World J Surg, 24 (2000), pp. 1081–1090
- 16 S. Hiraoka, J. Kato, M. Tatsukawa, et al.; Laterally spreading type of colorectal adenoma exhibits a unique methylation phenotype and K-ras mutations; Gastroenterology, 131 (2006), pp. 379–389
- 17 S. Bipat, A.S. Glas, F.J. Slors, A.H. Zwinderman, P.M. Bossuyt, J. Stoker; Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis; Radiology, 232 (2004), pp. 773–783
- 18 M.J. Solomon, R.S. McLeod; Endoluminal transrectal ultrasonography: accuracy, reliability, and validity; Dis Colon Rectum, 36 (1993), pp. 200–205
- 19 J. Garcia-Aguilar, J. Pollack, S.H. Lee, et al.; Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors; Dis Colon Rectum, 45 (2002), pp. 10–15
- 20 G.F. Gualdi, E. Casciani, A. Guadalaxara, et al.; Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging: comparison with histologic findings; Dis Colon Rectum, 43 (2000), pp. 338–345
- 21 R.G. Beets-Tan, G.L. Beets, A.C. Borstlap, et al.; Preoperative assessment of local tumor extent in advanced rectal cancer: CT or high-resolution MRI?; Abdom Imaging, 25 (2000), pp. 533–541
- 22 L. Blomqvist, T. Holm, S. Nyrén, R. Svanström, Y. Ulvskog, L. Iselius; MR imaging and computed tomography in patients with rectal tumours clinically judged as locally advanced; Clin Radiol, 57 (2002), pp. 211–218
- 23 R.G. Beets-Tan, G.L. Beets, R.F. Vliegen, et al.; Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery; Lancet, 357 (2001), pp. 497–504
- 24 M. Chiesura-Corona, P.C. Muzzio, G. Giust, M. Zuliani, S. Pucciarelli, P. Toppan; Rectal cancer: CT local staging with histopathologic correlation; Abdom Imaging, 26 (2001), pp. 134–138
- 25 H. Matsuoka, A. Nakamura, T. Masaki, et al.; Preoperative staging by multidetector-row computed tomography in patients with rectal carcinoma; Am J Surg, 184 (2002), pp. 131–135
- 26 H. Matsuoka, A. Nakamura, T. Masaki, et al.; A prospective comparison between multidetector-row computed tomography and magnetic resonance imaging in the preoperative evaluation of rectal carcinoma; Am J Surg, 185 (2003), pp. 556–559
- 27 M. Maras-Simunic, N. Druzijanic, M. Simunic, J. Roglic, S. Tomic, Z. Perko; Use of modified multidetector CT colonography for the evaluation of acute and subacute colon obstruction caused by colorectal cancer: a feasibility study; Dis Colon Rectum, 52 (2009), pp. 489–495
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?