Summary
Schmallenberg virus (SBV) is a member of the family Bunyaviridae and mainly affects ruminants. It is transmitted by biting midges, first and foremost Culicoides spp., and causes congenital malformations reflected in arthrogryposis–hydranencephaly (AH) syndrome. The aim of this study was to collect data on the emergence of SBV as a new arthropod-borne disease introduced into Europe in 2011. Germany was located in the core region of the 2011/2012 epidemic. Following two seroprevalence studies in the north-west of Germany in 2012, this study focused on the epidemiology and distribution of SBV throughout 130 small ruminant flocks in the whole country. Blood samples were obtained of 30 animals per flock and a SBV-specific questionnaire was used to collect operating data of the farms. The median within-herd seroprevalence for all 130 flocks tested was 53.3% with a total range from 0% to 100%. The median within-herd seroprevalence for goats was 30% [interquartile range (IQR): 40.3%] and 57% for sheep (IQR: 43.3%). Small ruminant flocks kept permanently indoors or housed overnight had a significantly lower seroprevalence than flocks kept permanently outdoors. In addition, this study revealed a significantly lower seroprevalence in the north-east of Germany. These results show that small ruminants in Germany are still at risk of contracting new SBV infections following incomplete seroconversion of flocks especially in the north-east of Germany. This might contribute to SBV becoming enzootic in central and northern Europe. Furthermore, the survey revealed that housing animals at least during mating and early pregnancy may reduce the risk of new SBV infections and may thus be an option to reduce losses as long as there is no licensed vaccine available on the German market.
Introduction
Schmallenberg virus (SBV) was first detected in Europe in autumn 2011. It was named after the place where the original isolate derived from, a town called Schmallenberg, located in the north-west of Germany. In autumn 2011, dairy cattle farmers from the Netherlands and Germany almost simultaneously reported disease outbreaks in their herds including mild fever, therapy-resistant diarrhoea and a reduction in milk yield (Hoffmann et al. 2012; Muskens et al. 2012). After ruling out several classical bovine endemic and emerging viruses as cause of these outbreaks, the Friedrich-Loeffler-Institute (FLI, Federal Research Institute for Animal Health, Germany) identified a new virus as causative agent utilising a meta-genomic approach with next-generation sequencing (Hoffmann et al. 2012). SBV belongs to the Simbu serogroup of the family Bunyaviridae, genus Orthobunyaviruses and is closely related to other arthropod-borne viruses, which are known to primarily infect ruminants such as Akabane, Sathuperi, Aino and Shamonda virus. All these viruses are transmitted by vectors such as mosquitoes and biting midges and can induce congenital malformations in neonates if a susceptible dam is infected during a vulnerable period in early pregnancy (Hoffmann et al. 2012).
In two real-time PCR (RT-PCR) studies on the occurrence of SBV-RNA in Culicoides subspecies (C. ssp.) performed in autumn 2011 in Belgium and Denmark, several C. ssp. (C. obsoletus complex, C. dewulfi, C. chiopterus) were tested positive for SBV, strongly suggesting that these species are relevant natural vectors for the virus (De Regge et al. 2012; Rasmussen et al. 2012).
Based on the pathogenesis of Akabane virus, it is believed that in small ruminants an infection between day 1 and 28 of gestation may lead to early embryonic death and abortion, followed by an increased rate of animals returning to oestrus. If infection takes place between day 28 and 56 of gestation, it might result in birth of congenitally malformed or stillborn lambs. Thus, this period represents the most critical period in pregnancy. After day 56 of gestation, the foetus becomes immunocompetent and has the ability to fight the virus with its matured immune system (The Center for Food and Security and Public Health, Iowa State University 2009). So far there is no evidence to refute the assumption that SBV infection induces a long-term immunity in affected animals, so that clinical signs can only be observed in ruminants infected for the first time [The European Food Safety Authority (EFSA), 2013]. Elbers et al. (2014) found that 80% of adult dairy cows still had measurable antibodies against SBV at least 24 months after the estimated introduction of the virus into the herd. This field study supports the assumption that natural SBV infection in adult cows induces a long-term immunity of at least 2 years. Whether these assumptions can be completely transferred to small ruminants remains to be clarified.
Typical congenital malformations can be summarised by the arthrogryposis–hydranencephaly (AH) syndrome, reflected in stiffening of the joints and cranial distension, spinal malformations (scoliosis, lordosis, kyphosis and torticollis) and brachygnathia or agnathia. Frequent pathomorphological findings are malformations of the central nervous system such as hydranencephaly, anencephaly, porencephaly, cerebellar hypoplasia and brain stem hypoplasia besides several musculoskeletal and vertebral malformations which are most likely a result of failures in the development of the central nervous system. True skeletal defects could not be observed in malformed neonates (Herder et al. 2012).
Two studies on the zoonotic potential of SBV conducted in Germany and in the Netherlands in 2012 revealed a lack of evidence for transmission of SBV to humans (Ducomble et al. 2012; Reusken et al. 2012).
From September 2011 until April 2013 more than 8000 holdings with laboratory confirmed SBV cases were recorded in 22 European countries [The European Food Safety Authority (EFSA), 2013]. The FLI provided case numbers and maps of the distribution and spread of approved SBV cases in Germany which were monthly updated from January 2012 until today. These maps revealed that the core region of the 2011/2012 epidemic was located in the north-west of Germany. First cases of SBV infection were not reported until winter 2012/2013 of federal states located more southerly or easterly. To date, 2504 SBV cases have been proven in Germany by RT-PCR with 1478 cattle herds, 973 sheep flocks and 53 goat flocks being SBV positive with a high estimate of unreported cases (FLI, Federal Research Institute for Animal Health, 24 March 2014). So far SBV has been detected by RT-PCR in cattle, sheep, goats, bison, deer, moose, buffalos, alpacas [The European Food Safety Authority (EFSA), 2013] and dogs (Sailleau et al. 2013).
Germany was located in the core region of the 2011/2012 epidemic. After two seroprevalence studies performed in the north-western parts of Germany (Helmer et al. 2013a,b) and another survey based on 462 sheep and 125 goat sera gained from 14 different German federal states (Wernike et al. 2014), this study focused on the epidemiology and distribution of SBV in small ruminant flocks throughout the entire German country. The main objectives were to possibly detect regional differences in inter-herd and within-herd prevalences and to check for the potential influence of certain variables on the within-herd prevalence [e.g. species, flock size, production type, location, type of wool (coarse wool, crossbred wool, fine wool, hair sheep/goats), treatment with repellents, housing management, exposure to wet- and/or woodland, other species kept on the farm with special focus on cattle].
Materials and methods
Germany is divided into 16 federal states whereof three states are free cities (Free Hanseatic City of Bremen, Free and Hanseatic City of Hamburg and Berlin). The total population size of small ruminants (sheep and goats) in Germany is 2,023,500 animals kept on 33,492 farms in March 2013 (Federal Statistical Office of Germany, 2013). In detail, 130,200 goats were housed on 10,800 farms and 1,893,300 sheep were housed on 20,000 farms with an average flock size of 12.06 in goats and 94.7 in sheep (Federal Statistical Office of Germany, 2013). The three free cities were not taken into consideration for this serosurvey as the sheep and goat population in these areas are extremely low (in total 200 goats and 3600 sheep). With an assumed true prevalence of 10%, an assumed test sensitivity of 90% and specificity of 99.75% (according to the manufacturers instructions), a confidence level of 95% and a desired precision of 20%, the sample size to estimate the true prevalence was 10 flocks per federal state (AusVet. Animal Health Services: EpiTools – epidemiological Calculators, 2013). One hundred and thirty small ruminant holdings were therefore tested from the 13 remaining federal states. These comprised 27 goat and 103 sheep flocks. Herd size ranged from 10 to 2200 in goats and 15 to 2100 in sheep. Venous blood samples were taken from 30 animals per flock (population size: 2,238,477, confidence level: 95%, assumed true prevalence: 10%). If there were less than 30 animals on the farm all adult females were tested. A SBV-specific questionnaire was used to collect information on potential risk factors (RFs) at the time of blood sampling. The questionnaire had been pre-tested and adapted after previous studies (Helmer et al. 2013a,b).
Ethic statement
The samples were taken in accordance with the principles outlined by the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes. Most of the blood samples were taken by veterinarians of the University of Veterinary Medicine Hannover, Foundation with help of veterinarians of the Clinic for Ruminants, LMU Munich (Bavaria) and of the Small Ruminant Health Services of the federal states of Baden-Wuerttemberg, Saxony and Thuringia, as well as by veterinarians of a private practice (Schafpraxis Stoffenried, Bavaria, Germany).
Samples
Between January and the beginning of October 2013, a total of 3779 serum samples were obtained from female sheep and goats (>1 year) in 13 German federal states. One hundred and fifteen of the 130 tested sheep and goat holdings were visited between January and May 2013 representing the vector season 2012. The remaining 15 farms were visited during the summer period representing the vector period 2013. The 15 flocks sampled during the summer months were evenly distributed over the study area. Blood samples were taken from the vena jugularis externa or the vena cava cranialis (Ganter et al. 2001). The blood was centrifuged at 10,000 g for 10 min and the serum was stored at −18°C until analysis.
ELISA
Serum samples were analysed for the presence of antibodies against SBV using a commercial ELISA (ID Screen®, SBV Indirect; IDvet Laboratories, Montpellier, France) according to the manufacturers instructions. The test can be used with individual bovine, ovine and caprine serum or plasma.
SBV-specific questionnaire (Supplementary Material)
Participating farmers were interviewed to complete a SBV-specific questionnaire to collect information on potential RFs at the time of blood sampling. The questionnaire was filled in by the veterinarian who visited the flock and obtained the blood samples which was in >75% of the cases the first author herself. The evaluation of the questionnaire data was performed by the first author only. The questionnaire had been pre-tested and adapted following previous studies (Helmer et al. 2013a,b). Information was gathered on species, flock size, location, breed, type of wool, production type, clinical signs of SBV infection, RT-PCR results (if existing), treatment with repellents, deworming management, vaccinations, additional treatments, other known health problems, housing conditions, exposure to wetland/woodland, other animals kept on the farm (e.g. cattle), mating period, number of lambs per ewe, number of lambs born alive, number of abortions, number of stillbirths, number of malformed lambs and clinical signs in adult animals during the possible time of first SBV infection (fever, languor, reduced milk yield, diarrhoea).
Statistical analysis
Explorative statistical analyses were performed using SAS 9.1 (SAS Institute Inc, Cary, NC, USA). Putative RF for infection were investigated using the herd-level serological response (k out of n animals tested positive) as outcome by means of a fixed effects logistic regression for grouped (i.e. flocks) binomially scaled observations (R command glm, R) (R Core Team, 2014). The final model was obtained after stepwise backwards elimination of variables based on the Akaikes information criterion (AIC) (compared to a chi-squared statistic). This final model was also fitted with a random effects term on the level of the flock (R command glmmML) (Broström 2014). Multilevel RF where introduced into the model by choosing a suitable reference category. Statistical significance was assumed if the P-values were 5% or below.
Results
Clinical signs
Forty-nine per cent of the farmers (n = 66) reported higher than usual proportions of abortions and stillbirths as well as lambs born with congenital malformations consistent with intrauterine SBV infection. Forty-eight per cent of these (n = 31) only reported individual cases of malformed neonates (1–5 cases per flock). Twenty-eight per cent (n = 18) reported 5–20 cases of congenital malformation, 11.5% (n = 7) reported 20–50 cases per flock and 12.5% (n = 8) of the farmers reported massive losses due to intrauterine SBV infections with more than 50 lambs showing typical signs of AH syndrome. The congenital malformations observed in the flocks can mainly be summarised as AH syndrome with lambs showing stiffening and deformation of the joints and of the vertebral column as well as cranial distension. Brachygnathia and agnathia were also frequently observed. In many cases the malformed foetuses led to dystocia resulting in higher numbers of caesarean sections and fetotomies. Some ewes died or had to be euthanised due to dystocia. These problems during parturition often resulted in endometritis followed by reduced fertility in the next mating season. None of the farmers reported clinical signs in adult small ruminants during viremia as described for cattle (mild fever, languor, reduced milk yield and diarrhoea) (Muskens et al. 2012). Only a small number of flocks (n = 9) suffered from losses due to SBV infections in two consecutive years. None of these farmers repeatedly observed malformed offspring from one and the same animal. These statements were derived from the answers to the questionnaire and could not be followed up by the veterinarians.
Serological response
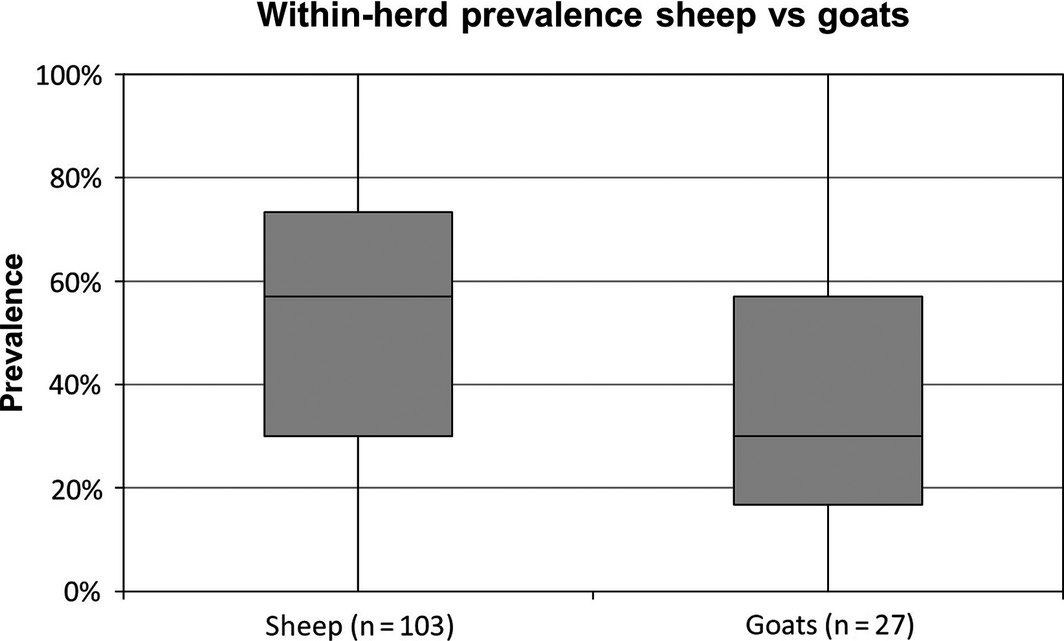
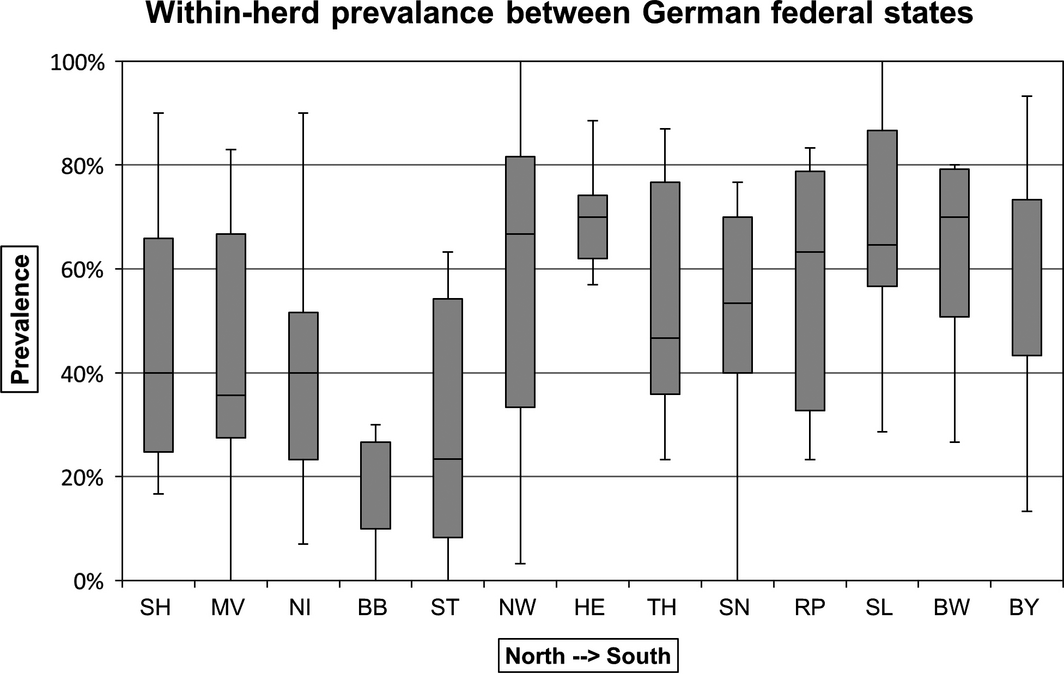
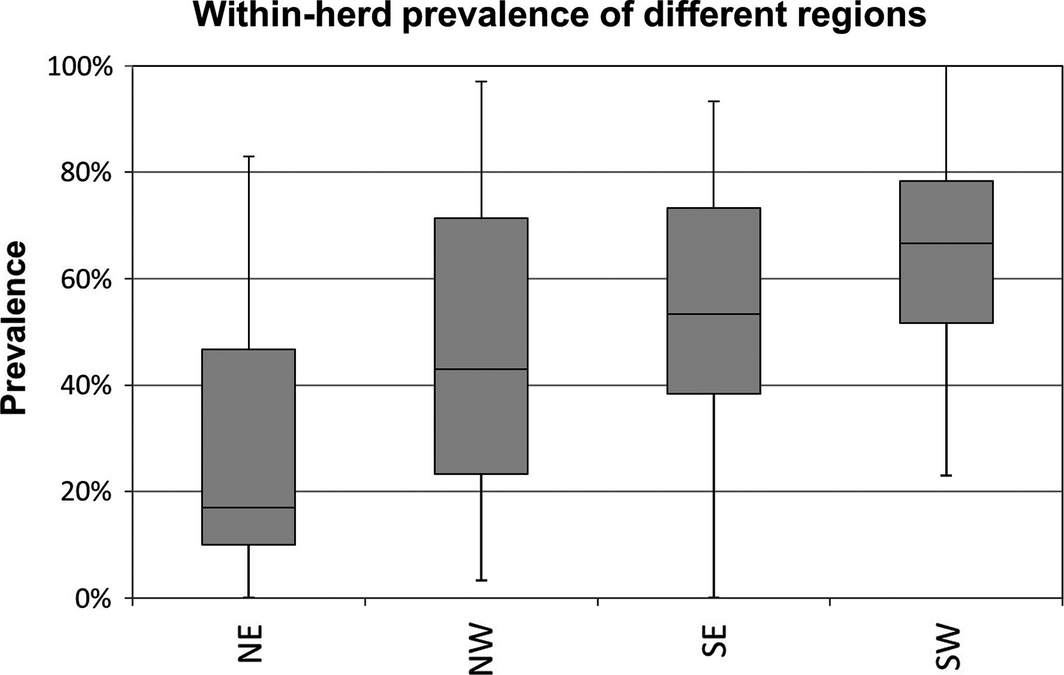
The median within-herd prevalence for all 130 flocks tested was 53.3% with a range from 0% to 100%. A significantly lower median within-herd prevalence of 30% (IQR: 40.3%) was observed in goat flocks (n = 27) compared to sheep flocks (n = 103), where a median of 57% (IQR: 43.3%) of the animals were seropositive (Fig. 1). Two sheep and four goat flocks were seronegative for SBV antibodies. Both sheep flocks were located in the north-eastern state of Brandenburg, administrative district Maerkisch-Oderland. The four goat flocks were located in the eastern German federal states Brandenburg, Mecklenburg Western-Pomerania, Saxony and Saxony-Anhalt. All seronegative goat flocks were dairy goats and were either kept indoors all year round or stabled during the night. On one goat and two sheep farms all animals sampled for this survey tested positive for SBV antibodies (seroprevalence of 100%). All three of these farms were located in the Western part of the country, in the Saarland (n = 2) and North Rhine-Westphalia (n = 1). All these flocks were kept outdoors all year round with only a shed for shelter. The calculated median within-herd prevalences for the different federal states are as follows (listed from north to south): Schleswig-Holstein (SH) 40%, Mecklenburg Western-Pomerania (MV) 36%, Lower Saxony (NI) 42%, Brandenburg (BB) 10%, Saxony-Anhalt (ST) 23%, North Rhine-Westphalia (NRW) 67%, Hesse (HE) 73%, Thuringia (TH) 48%, Saxony (SN) 59%, Rhineland-Palatinate (RP) 52%, Saarland (SL) 65%, Baden-Wuerttemberg (BW) 70% and Bavaria (BY) 68% (Fig. 2). We divided the Federal Republic of Germany into the four regions northeast (NE: BB, MV, ST), northwest (NW: NI, NRW, SH), southeast (SE: BY, SN, TH) and southwest (SW: BW, HE, RP, SL) for a more illustrative presentation. According to Eurostat, 12,685,990 heads of livestock were kept on German farms in December 2013 (European Commission: Eurostat Data Explorer, 2013), 5,234,050 of these animals are kept on farms in the NW of Germany (41.4%), 4,088,290 are kept on farms in the SE of Germany (32.3%), 1,880,890 are kept on farms located in the SW of Germany (14.8%) and 1,465,410 heads of livestock are kept on farms located in the NE of Germany (11.5%). Regarding sheep and goats 657,900 (34%) are kept in the SE, 565.300 (28%) are reared in the NW, 521,000 (25%) sheep and goats are kept in the SW and 269,100 (13%) in the NE of Germany (Federal Statistical Office of Germany, 2013). The calculated median within-herd prevalences for these regions are given in Figure 3.
|
|
|
Figure 1. Comparison of the median within-herd prevalence of sheep and goats; the difference concerning the median within-herd prevalence was statistically significant in a regression model without random effect on flock basis (P = 0.00308). |
|
|
|
Figure 2. Comparison of the median within-herd prevalences of the different federal states of Germany listed from north to south. SH, Schleswig-Holstein; MV, Mecklenburg Western-Pomerania; NI, Lower Saxony; BB, Brandenburg; ST, Saxony-Anhalt; NW, North Rhine-Westphalia; HE, Hesse; TH, Thuringia; SN, Saxony; RP, Rhineland-Palatinate; SL, Saarland; BW, Baden-Wuerttemberg; BY, Bavaria. |
|
|
|
Figure 3. Comparison of regional differences in the distribution of Schmallenberg virus (SBV) infection throughout Germany. NE, north-east; NW, north-west; SE, south-east; SW, south-west; the NE of Germany is significantly less affected by SBV infection than the other regions of Germany in both logistic regression models (P-value without random effect on flock basis = 9.3 × 10−8; P-value with random effect on flock basis = 0.005). |
Questionnaire results
Sixty per cent of the flocks (n = 77) included in this survey are kept for environmental grazing projects and meat production. Twenty per cent are kept for milk production (n = 27) or as hobby breeding animals (n = 26), respectively. Roughly 60% of the farmers treated their flocks against external parasites (n = 77). More than 80% of these used products containing deltamethrin as active agent (n = 64). Ten farmers used products containing phoxim as active agent and four farmers used alternative treatments such as neem oil or garlic compounds. Only 4% (n = 5) of the farmers treated their animals during the mating period or early gestation. On most farms the animals were treated after shearing which is generally performed in spring. RT-PCR results were available for 19 flocks. Lambs of 15 of these were tested positive for SBV by RT-PCR, while lambs submitted from the other four were tested negative despite all submitted foetuses showing evident clinical signs of congenital malformations consistent with SBV infection. Eighty per cent of the flocks (n = 102) included in the study were kept permanently outdoors and were only housed for lambing, whereas 5% were kept permanently indoors throughout the entire year (n = 8). The remaining 15% were regularly housed during the night (n = 20). Thirty per cent of the farmers interviewed stated that their farms and pastures were located in dry areas not adjacent to stagnant water or woodland (n = 40). Another 30% reported that their farm and pastures were located close to wetland (n = 36), while 20% responded that their farm area and pastures were located close to woodland (n = 27). The remaining 20% stated that their farms and pastures were surrounded by both wet- and woodland (n = 27). The presence of other ruminant species was also of interest. Forty per cent of the farmers interviewed stated that their sheep and goats did not have any contact with other animals, except wildlife such as deer and wild boar or companion animals such as cats and dogs (n = 51). Of the remaining farms, 40% also kept cattle (n = 33) and 22% of the sheep farmers also kept goats (n = 17). Four of the 103 sheep flocks came into contact with transhumance flocks. One sheep farmer kept a herd of bison on his farm. In addition, several small ruminant flocks, especially the hobby flocks, were kept together with horses, donkeys, swine and poultry.
Risk analysis of RFs of SBV infection
The final fixed effect and random effect logistic regression models, denoted as FEM (residual deviance 731.49 on 114 degrees of freedom; AIC: 1185) and REM (residual deviance of 369 on 111 degrees of freedom; AIC: 407), respectively, contained seven explanatory variables (Table 1). Sheep flocks showed a higher level of SBV infection than goat flocks (significant in FEM but not in REM). Flock sizes of 1–56, 57–255, 251–500 and >500 animals were categorised into the quartiles Q1, Q2, Q3 and Q4, respectively. Large flocks (third and fourth quartiles) displayed a lower level of SBV infections than small (first quartile) flocks (significant in FEM, but not in REM). Flocks located in the north-east of Germany showed a lower level of SBV infections than flocks located in the north-west of Germany (significant in both FEM and REM). Flocks located in the south-east (SE) and the south-west (SW) of Germany displayed a higher risk of SBV infections than flocks located in the north-west of Germany (SE: significant in FEM, but not in REM; SW: significant in both FEM and REM). The production type was not included in the final model due to lack of significance. The type of wool showed no statistically significant differences in the final models. Farms using deltamethrin pour on treatments revealed a lower level of SBV infection compared to flocks where deltamethrin was not used against external parasites (significant in both FEM and REM). Flocks kept permanently indoors or housed overnight showed a lower level of SBV infection than those kept permanently outdoors (significant in both FEM and REM). Flocks exposed to both wetland and forest/woodland and those exposed to forest/woodland only displayed a higher level for SBV infection than those without any exposure (wetland and woodland: significant in both FEM and REM; forest/woodland: significant in FEM, but not in REM). No significant effects were found for exposure to wetlands only. The presence of cattle on the premises was not included in the final models due to lack of significance.
| Risk factor (reference) | FEM | REM | ||
|---|---|---|---|---|
| Estimate | P-value | Estimate | P-value | |
| Intercept | −0.16 | 0.350 | −0.29 | 0.530 |
| Species (goat) | ||||
| Sheep | 0.58 | 0.002** | 0.77 | 0.110 |
| Flock size (Q1)† | ||||
| Q2 | 0.21 | 0.067 | 0.31 | 0.290 |
| Q3 | −0.47 | 0.000*** | −0.42 | 0.180 |
| Q4 | −0.35 | 0.007** | −0.28 | 0.420 |
| Location (NW) | ||||
| NE | −0.66 | 0.000*** | −0.91 | 0.003** |
| SE | 0.46 | 0.000*** | 0.52 | 0.091 |
| SW | 0.56 | 0.000*** | 0.66 | 0.025* |
| Type of wool (hair) | ||||
| Coarse | −0.24 | 0.253 | −0.37 | 0.520 |
| Crossbred | 0.14 | 0.398 | 0.02 | 0.960 |
| Fine | −0.19 | 0.296 | −0.44 | 0.370 |
| Treatment (none) | ||||
| Butox | −0.56 | 0.000*** | −0.62 | 0.014* |
| Other | −0.11 | 0.283 | −0.10 | 0.710 |
| Housing (no) | ||||
| Night | −0.86 | 0.000*** | −0.95 | 0.005** |
| Yes | −1.66 | 0.000*** | −2.09 | 0.000*** |
| Exposure (none) | ||||
| Forest and wetland | 0.73 | 0.000*** | 0.85 | 0.005** |
| Forest | 0.36 | 0.001*** | 0.51 | 0.069 |
| Wetland | −0.08 | 0.446 | −0.01 | 0.960 |
*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. †Flock size has been categorised into four quartiles (Q1–Q4). | ||||
Discussion
While most conducted seroprevalence studies on SBV focused on cattle, the focus of this serosurvey was on the distribution and epidemiology of the disease in small ruminants which were more affected by SBV infection than cattle. Dominguez et al. (2014) found that congenital SBV morbidity was on average moderate, although higher in sheep than in other ruminant species. They estimated typical congenital SBV deformities of 8% in lambs, 3% in calves and 2% in goat kids, respectively. A study focusing on the impact of SBV on British sheep farms found a mortality of 10.4% for lambs born on farms where SBV infection was confirmed. Moreover, they found that 25% of farmers where SBV was confirmed or suspected perceived a high impact of SBV on emotional well-being and that 13% of these farmers believed that the disease would have a large impact on flock welfare and financial performance (Harris et al. 2014). Two previous seroprevalence studies focusing on small ruminants were performed in north-western parts of Germany in 2012 (Helmer et al. 2013a,b). Moreover, the FLI conducted a seroprevalence study in cattle and small ruminants for which 462 sheep and 125 goat sera were obtained from 14 different federal states in 2012 (Wernike et al. 2014). The study area for this seroprevalence study was extended to the entire German country with the aim of gaining additional insight into the developments of SBV infection in small ruminants in the north-west as well as to ascertain whether and how much the infection had spread to southern and eastern parts of Germany in 2013. In addition, the present study focused on potential RFs for SBV infection which were assessed by questionnaire.
The estimated seroprevalence of antibodies against SBV in dairy cows in the Netherlands was 72.5% (Elbers et al. 2012) and 90.8% for cattle in Belgium (Garigliany et al. 2012) in 2012. A serosurvey conducted in France in winter 2011–2012 revealed seroprevalences of 90% for cattle herds and 30% for sheep flocks in highly affected areas (Gache et al. 2014). A seroprevalence study focusing on ruminants performed in Germany in 2012 revealed an average seroprevalence among German cattle, sheep and goats of 61%, 24.7% and 26.4%, respectively. In the core region of the epidemic in north-western Germany up to 98% of the animals tested positive for SBV antibodies (Wernike et al. 2014). The median within-herd prevalence calculated for German small ruminant holdings in this seroprevalence study was 30% (IQR: 40.3%) for goats and 57% for sheep (IQR: 43.3%) (Fig. 1). Small ruminant flocks seem therefore to be less exposed to SBV than cattle during the recent SBV outbreak in Europe.
This seroprevalence study confirmed earlier findings (Helmer et al. 2013a,b; Veldhuis et al. 2013a) revealing that goat flocks have a lower risk of contracting SBV infection than sheep flocks. This species-specific statistically significant difference in within-herd prevalence of sheep and goat flocks is, however, no longer sustainable in a logistic regression model with random effect on flock basis. The within-herd seroprevalence for SBV in Belgium was estimated at 84.3% for sheep and 40.7% for goats (Méroc et al. 2013), showing similar results for goat flocks to those observed in the current survey, whereas the within-herd prevalence for sheep was much higher in Belgium than in Germany. Veldhuis et al. (2013a) found a within-herd prevalence of 89% in sheep and 50.8% showing that a sheep flock had a 13.7 times higher odds to be seropositive than a goat herd. The aforementioned study of Wernike et al. (2014) revealed an average seroprevalence of 24.7% in sheep and 26.4% in goats, not showing any difference concerning the within-herd prevalence of these two small ruminant species. The difference in seroprevalence between sheep and goat flocks found in this survey is probably due to the fact that 74% of the goat flocks tested were dairy herds (20 out of 27) and were therefore kept permanently indoors or at least housed overnight (18 out of 27). Both models used for statistical analyses of the obtained data revealed a significantly lower risk of SBV infection for small ruminants permanently stabled or at least housed overnight. This finding is statistically confirmed using a fixed effects model (FEM), but not using a random effects model (REM). The application of a REM has been recommended for studying RFs in farmed animals to account for the clustering of animals in herds or flocks (McDermott & Shukken 1994). The results of the FEM can be regarded as indicative while conclusions should be based on the REM. Thus, the present study does not provide conclusive evidence for a differential risk of seropositivity between sheep and goats. The lower seroprevalence in goat flocks compared to sheep flocks is therefore probably due to the housing system rather than a species-specific factor. This is supported by the observation that several goat flocks which were permanently kept outdoors throughout the entire year showed seroprevalences for SBV antibodies of 70% and more (n = 3). The four goat flocks that were serologically negative for SBV were all dairy goat flocks and therefore kept permanently indoors or housed overnight. In the Belgian seroprevalence study the housing management is not reported so the possible influence of housing on seroprevalence could not be addressed (Méroc et al. 2013). According to a personal communication of Stephen Valas and Anses Niort cited by Wernike et al. (2014), the between-herd seroprevalence of goats located in the central–western parts of France was 62%. Differences between extensive and intensive goat farming was noticed in this study as well.
As reported by other groups, cattle seem to be more exposed to biting midges than small ruminants even when they are kept permanently indoors. The reasons for these differences concerning the within-herd prevalences of cattle and small ruminants might be explained as follows:
- Different host preferences of the vectors: Most Culicoides (C.) spp. prefer to feed on cattle if present (Bartsch et al. 2009; Lassen et al. 2011, 2012; Ninio et al. 2011). The thick fleece of sheep makes the reachable surface for midge bites fairly small as only non-woollen areas of the skin or those with a short hair coat such as the udder, the inner shank and thigh, the interdigital skin, the head and the bottom side of the tail are approachable for bites by C. spp. Possibly the species-specific odour of goats might be a reason for the lower attractiveness for biting midges compared to cattle and sheep.
- Different housing conditions and uses: Most small ruminant flocks in Germany are kept extensively on pasture day and night. The main reasons for sheep farming are landscape protection, meat production and breeding, while the main reasons for goat farming are milk production and breeding. The only time of year when animals are stabled is during the lambing period. Exceptions are dairy sheep and goats, which are mainly kept indoors on deep litter year round or are at least housed during the night. It is expected that animals kept outdoors are more exposed to vectors than animals kept indoors, which could also be shown by Baylis et al. (2010). In addition, grazing has been identified as a potential RF for bluetongue disease, which is another vector-borne disease transmitted by C. spp. (Santman-Berends et al. 2010). This study confirmed findings of a previous survey (Helmer et al. 2013a) revealing that permanently housed sheep and goat flocks were considerably less affected by SBV infection than flocks kept permanently outdoors. Thus, housing of animals, at least during the night, is an adequate method to reduce SBV infection in small ruminants. In contrast to most small ruminant flocks, German cattle herds are mainly kept indoors year round on slatted floors with cesspools below for the storage of dung. Although most cattle are permanently housed throughout the entire year, the reported seroprevalences are much higher for cattle than for sheep and goats (Elbers et al. 2012; Garigliany et al. 2012; Gache et al. 2014; Wernike et al. 2014). This is most probably due to the storage of the liquid and nutrient-rich manure which provides perfect conditions for the reproduction and development of C. spp. Several studies outlined that C. spp. breed in cattle manure and may even use it for hibernation (Meiswinkel et al. 2008; Zimmer et al. 2008). In contrast, no larval stages of biting midges could be found in sheep manure or soil inside sheep barns (Gonzáles et al. 2013). Veldhuis et al. (2013b) found that cattle herds that were grazed in 2011 had increased odds of a high seroprevalence for SBV compared to cattle herds that were kept indoors. Moreover, when animals were grazed in 2011, the odds of malformations in newborn calves tended to be 2.6 times higher compared to herds in which cattle was kept indoors. However, the study also revealed that keeping cattle indoors year round did not prevent SBV infection as an average within-herd prevalence of 63.9% was found in herds that stated not to have been grazed in 2011.
The presence of other animals on the same farm area, namely cattle, could not be identified as a RF for SBV infection. Several authors indicated that C. spp. prefer to feed on cattle rather than on small ruminants (Bartsch et al. 2009; Lassen et al. 2011, 2012; Ninio et al. 2011). Nevill (1978) stated that keeping cattle near sheep appear to have appreciably reduced the incidence of bluetongue disease in sheep. This could not be confirmed for SBV in our study.
This study found that large sheep and goat flocks (third and fourth quartiles) displayed a lower level of SBV infections than small (first quartile) flocks. These findings could be confirmed by Veldhuis et al. (2013a) who also found a significantly lower mean seroprevalence in large sheep and goat flocks compared to small ones.
The logistic regression models indicated a lower level of SBV infections in the NE of Germany compared to the NW, SW and SE, putting them at greater risk of infection in the future season. First outbreaks of the new viral disease were reported almost simultaneously in the NW of Germany and in the Netherlands in autumn 2011 (Hoffmann et al. 2012; Muskens et al. 2012). The core region of the epidemic during the lambing season 2011/2012 was also located in the north-western parts of Germany. Due to an incomplete infection of small ruminant flocks in this first season, the viral disease spread to southern and eastern parts of Germany in 2012/2013 which can be monitored on the homepage of the FLI, which provides case numbers and maps of the spread of SBV throughout Germany from January 2011 until today (FLI, Federal Research Institute for Animal Health, 24 March 2014). As this serosurvey shows, small ruminant flocks in north-eastern parts of Germany [Brandenburg (BB), Mecklenburg Western-Pomerania (MV) and Saxony-Anhalt (ST)] had significantly lower median within-herd prevalences (17%) than the other regions (NW: 46.5%, SE: 58%, SW: 67%) and are therefore still at risk of SBV outbreaks during the next lambing seasons. BB had the lowest median within-herd prevalence of all federal states with 10%. Two sheep flocks and one goat flock tested in BB were seronegative for SBV antibodies. Both sheep flocks were located in the administrative district Maerkisch-Oderland close to the Polish border. Both flocks were kept permanently outdoors close to dykes of the river Oder and were only stabled during the lambing period. Several seroprevalence studies performed in small ruminants, cattle and elk in Poland showed that SBV had already entered Poland (Kaba et al. 2013; Larska et al. 2013a,b). Even more Eastern countries such as Turkey had reported cases of SBV infections (Azkur et al. 2013; Yilmaz et al. 2014). The seronegative goat flock was a dairy flock which was kept indoors overnight. This might explain why the flock did not come into contact with the virus-transmitting vectors. It seems that SBV did not occur in the NE of Germany at such a high level as in the other regions although there are many large woods, rivers and lakes located in these federal states. Similar observations were made during the bluetongue epidemic, which hit Germany in 2006/2007, when the north-eastern parts of Germany were less affected by bluetongue virus (BTV) than the other parts of the country [Friedrich-Loeffler-Institute (FLI), Federal Research Institute for Animal Health, Germany 2009]. Hesse (HE), which is located in the middle of Germany, had the highest median within-herd prevalence of all German federal states with 73%, followed by Baden-Wuerttemberg with 70% and Bavaria with 68%. It seems that especially the SE (58%) and the SW (67%) of Germany were affected by this new emerging disease. A possible explanation for these findings might be the livestock density of the individual federal states. On the basis of the livestock density, we would expect the lowest within-herd prevalence in the NE of Germany as this region has the lowest livestock density and should therefore not be as attractive for biting midges as other regions of the country. Moreover, we would expect the other regions to be affected in the following order (from low to high): SW, SE and NW. On the basis of the numbers of sheep and goats reared in these regions, we would expect the SE to be most affected, followed by the NW and SW. However, the serology results revealed that the SW of Germany has the highest median within-herd prevalence throughout the country. Hence, livestock density might have an influence on the within-flock prevalence as seen in the NE of Germany, but other factors must also be involved. Wernike et al. (2014) obtained 462 sheep and 125 goat sera from 14 different federal states for their serosurvey conducted in 2012. They found the lowest seroprevalence for goats in MV which is also located in the north-east of Germany. The highest within-herd prevalence for goats was found in Schleswig-Holstein which is located in the North of the country. No data are available for goat holdings located in the states Bavaria, Hamburg, Hesse and Thuringia. For sheep, the lowest within-herd prevalence was also found in MV and the highest seroprevalence was found in Rhineland-Palatinate followed by North Rhine-Westphalia. No data are available for sheep holdings located in Hamburg or Thuringia. Thus, the survey of Wernicke et al. confirms findings revealed in the current study showing that the lowest seroprevalences for sheep and goat holdings could be found in north-eastern parts of Germany.
None of the models revealed a significant difference regarding the production type of the flocks. We would have expected a lower risk for dairy sheep and goats compared to the other production types, as most dairy flocks are kept permanently indoors or are at least housed overnight. They should therefore be better protected against C. spp. due to the housing management. However, this effect might be masked by the housing variable in the multivariable model.
Both models showed a statistically significant difference regarding the treatment with repellents. Farms with flocks treated with products containing deltamethrin as an active agent against external parasites were at a lower risk for SBV infection than flocks that were treated with other pharmaceutical products or that were not treated at all. Deltamethrin is a pyrethroid ester insecticide and a neurotoxin, which induces paralysis and convulsions resulting in death of the insects after absorption. It needs to be mentioned that none of the farmers treated their flocks during the mating period or during early pregnancy so that protection against SBV due to treatment with repellents is not absolutely proven.
As most small ruminants are kept out on pasture day and night with only a shed for shelter against adverse weather conditions, they are not protected against biting midges during the main flight time. Exceptions are dairy sheep and goats which are mainly kept permanently indoors or at least housed indoors overnight. Both logistic regression models revealed a higher risk of SBV infection for flocks that were exposed to both wet- and woodland. Due to the fact that C. spp. develop aquatically and are dependent on humid substrate, it is hardly surprising that flocks with an exposure to wet- and woodland have a higher risk of contracting SBV infection than flocks without any exposure to wet- and/or woodland. In summary, this study shows that German sheep and goat flocks are still at risk of contracting new SBV infections due to incomplete seroconversion of flocks especially in the north-eastern parts of Germany. This might contribute to establishing an enzootic situation in central and northern Europe for SBV. Furthermore, this survey showed that small ruminant flocks that are housed permanently or at least kept indoors overnight have a lower risk of contracting SBV infection than flocks kept permanently outdoors. Housing animals at least during mating and early pregnancy might therefore reduce the risk of new SBV infection during the critical time of early gestation. Housing might therefore be a valuable tool to protect small ruminants against SBV, while there is no vaccine available in the German market.
Since SBV very recently entered Europe in autumn 2011, further studies are needed to understand the pathogenesis of the disease and to find possible routes of entry into central and northern Europe. Further studies are also needed to ascertain the so far assumed hypothesis of long-term immunity in adults. The field study of Elbers et al. (2014) revealed a persistence of SBV-specific antibodies in adult cows for at least 24 months. Whether these findings can be extrapolated to small ruminants remains to be clarified. Furthermore, it would be useful to investigate after which time specific antibodies against SBV start to decline. Since two vector-borne diseases have hit Europe in recent years (BTV and SBV), more studies on the biology and distribution of biting midges are needed to gain more detailed knowledge about these species which might be potential carriers of not only animal pathogens, but also their potential role in transmission of human pathogens.
Acknowledgements
The authors are very grateful for the excellent laboratory technical assistance given by the laboratory staff of the Clinic for Swine and Small Ruminants of the University of Veterinary Medicine Hannover, Foundation. Special thanks go to Christina Boss. Furthermore, we would like to use this opportunity to thank the Small Ruminant Health Services, the Chambers of Agriculture and the Tierseuchenkassen (animal diseases fund) of the following federal states: BW, MV, SN, ST, TH and RP for helping us collect blood samples and establish contact to sheep and goat farms in their states. Moreover, we wish to thank Frances Sherwood-Brock for reading the English manuscript. We also use this opportunity to thank all farmers of the 130 flocks investigated for the study for their support.
Source of funding
This work was supported by the German Federal Ministry of Food, Agriculture and Consumer Protection (BMELV) through the Federal Office for Agriculture and Food (BLE), grant number 2812HS008.
Conflict of interest
The authors declare that they have no competing interests.
References
- AusVet. Animal Health Services: EpiTools – epidemiological Calculators (2013) Available at: http://epitools.ausvet.com.au/content.php?page=PrevalenceSS (accessed 15 January 2013).
- Azkur A.K., Albayarek H., Risvanli A., Pestil Z., Ozan E., Yilmaz O.et al. (2013) Antibodies to Schmallenberg virus in domestic livestock in Turkey. Tropical Animal Health and Production45, 1825–1828.
- Bartsch S., Bauer B., Wiemann A., Clausen P.H. & Steuber S. (2009) Feeding patterns of biting midges of the Culicoides obsoletus and Culicoides pulicaris groups on selected farms in Brandenburg, Germany. Parasitology Research105, 373–380.
- Baylis M., Parkin H., Kreppel K., Carpenter S., Mellor P.S. & McIntyre K.M. (2010) Evaluation of housing as a means to protect cattle from Culicoides biting midges, the vectors of bluetongue virus. Medical and Veterinary Entomology24, 38–45.
- Broström G. (2014) Package ‘glmmML’: Generalized linear models with clustering. Available at: http://cran.r-project.org/web/packages/glmmML/glmmML.pdf (accessed 5 July 2014).
- De Regge N., Deblauwe I., De Deken R., Vantieghem P., Madder M., Geysen D.et al. (2012) Detection of Schmallenberg virus in different Culicoides ssp. by real-time RT-PCR. Transboundary and Emerging Diseases59, 471–475.
- Dominguez M., Gache K., Touratier A., Perrin J.B., Fediaevsky A., Collin E.et al. (2014) Spread and impact of the Schmallenberg virus epidemic in France 2012–2013. BMC Veterinary Research10, 248.
- Ducomble T., Wilking H., Stark K., Takla A., Askar M., Schaade L.et al. (2012) Lack of evidence for Schmallenberg virus infection in highly exposed persons, Germany, 2012. Emerging Infectious Diseases18, 1333–1335.
- Elbers A.R.W., Loeffen W.L.A., Quak S., De Boer-Luijtze E., Van Der Spek A.N., Bouwstra R.et al. (2012) Seroprevalence of Schmallenberg virus antibodies among dairy cattle, the Netherlands, Winter 2011–2012. Emerging Infectious Diseases18, 1065–1071.
- Elbers A.R.W., Stockhofe-Zurwieden N. & van der Poel W.H.M. (2014) Schmallenberg virus antibody persistence in adult cattle after natural infection and decay of maternal antibodies in calves. BMC Veterinary Research10, 103.
- European Commission: Eurostat Data Explorer (2013) Lifestock Holding in December after NUTS-2-regions: Available at http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=agr_r_animal&lang=de (accessed 30 May 2014).
- Federal Statistical Office of Germany (2013) Livestock Holding of farms – survey of agricultural structure fachserie 3 reihe 2.1.3 – 2013. Available at: https://www.destatis.de/DE/Publikationen/Thematisch/LandForstwirtschaft/ViehbestandTierischeErzeugung/Viehhaltung2030213109004.pdf?_blob=publicationFile (accessed 15 January 2014).
- Friedrich-Loeffler-Institute (FLI), Federal Research Institute for Animal Health, Germany (2015) Schmallenberg virus. Available at: http://www.fli.bund.de/de/startseite/aktuelles/tierseuchengeschehen/schmallenberg-virus.html (accessed 24 March 2015).
- Friedrich-Loeffler-Institute (FLI), Federal Research Institute for Animal Health, Germany (2009) Qualitative risk assessment to the abolition of the mandatory vaccination against bluetongue disease, serotype 8, in 2010. Available at: http://www.fli.bund.de/fileadmin/dam_uploads/tierseuchen/Risikobewertung_Impfung_BTV-8_091007.pdf (accessed 25 June 2014).
- Gache K., Dominguez M., Pelletier C., Petit E., Calavas D., Hendrikx P. & Touratier A. (2014) Schmallenberg virus: a seroprevalence survey in cattle and sheep, France, winter 2011–2012. The Veterinary Record173, 141.
- Ganter M., Hermann J. & Waibl H. (2001) Blood sampling from the vena cava cranialis in sheep and goat using sampling systems armed by canula. Tierarztl Prax Ausg G Grosstiere Nutztiere29, 37–40.
- Garigliany M.M., Bayrou C, Kleijnen D, Cassart D, Desmecht D, 2012. Schmallenberg virus in domestic cattle, Belgium, 2012. Emerging Infectious Diseases18, 1512–1514.
- Gonzáles M., López S., Mullens B.A., Baldet T. & Goldarazena A. (2013) A survey of Culicoides developmental sites on a farm in northern Spain, with a brief review of immature habitats of European species. Veterinary Parasitology191, 81–93.
- Harris K.A., Eglin R.D., Hayward S., Milnes A., Davies I., Cook A.J.C. & Downs S.H. (2014) Impact of Schmallenberg virus on British sheep farms during the 2011/2012 lambing season. The Veterinary Record175, 16–23.
- Helmer C., Eibach R., Tegtmeyer P.C., Humann-Ziehank E. & Ganter M. (2013a) Survey of Schmallenberg virus (SBV) infection in German goat flocks. Epidemiology and Infection141, 2335–2345.
- Helmer C., Eibach R., Tegtmeyer P.C., Humann-Ziehank E., Runge M., Ganter M., 2013b: Serosurvey of Schmallenberg virus infections in sheep and goat flocks in Lower Saxony, Germany. Transboundary and Emerging Disease62, 425–436.
- Herder V., Wohlsein P., Peters M., Hansmann F. & Baumgärtner W. (2012) Salient lesions in domestic ruminants infected with the emerging so-called Schmallenberg virus in Germany. Veterinary Pathology49, 588–591.
- Hoffmann B., Scheuch M., Höper D., Jungblut R., Holsteg M., Schirrmeier H.et al. (2012) Orthobunyavirus in cattle. Emerging Infectious Diseases18, 469–472.
- Kaba J., Czopowicz M. & Witkowski L. (2013) Schmallenberg virus antibodies detected in Poland. Transboundary and Emerging Disease60, 1–3.
- Larska M., Polak M.P., Grochowska M., Lechowski L., Zwiazek J.S. & Zmudzinski J.F. (2013a) First report of Schmallenberg virus in cattle and midges in Poland. Transboundary and Emerging Disease60, 97–101.
- Larska M., Krzysiak M., Smreczak M., Polak M.P. & Zmudzinski J.F. (2013b) First detection of Schmallenberg virus in elk (Alces alces) indicating infection of wildlife in Bialwoieza National Park in Poland. Veterinary Journal198, 279–281.
- Lassen S.B., Nielsen S.A., Skovgard H. & Kristensen M. (2011) Molecular identification of blood meals from midges (Diptera: Ceratopogonidae: Culicoides Latreille) in Denmark. Parasitology Research108, 823–829.
- Lassen S.B., Nielsen S.A. & Kristensen M. (2012) Identity and diversity of blood meal hosts of biting midges (Diptera: Ceratopogonidae: Culicoides latreille) in Denmark. Parasites and Vectors5, 143.
- McDermott J.J. & Shukken Y.H. (1994) A review of methods used to adjust for cluster effects in explanatory epidemiological studies of animal populations. Preventive Veterinary Medicine18, 155–173.
- Meiswinkel R., Baldet T., De Deken R., Takken W., Delécolle J.C. & Mellor P.S. (2008) The 2006 outbreak of bluetongue in northern Europe – the entomological perspective. Preventive Veterinary Medicine87, 55–63.
- Méroc E., De Regge N., Riocreux F., Caij A.B., Van den Berg T., Van der Stede Y., 2013: Distribution of Schmallenberg virus and seroprevalence in belgian sheep and goats. Transboundary and Emerging Diseases61, 425–431.
- Muskens J., Smolenaars A.J., Van Der Poel W.H., Mars M.H., Van Wuijckhuise L., Holzhauer M.et al. (2012) Diarrhoea and loss of production on Dutch dairy farms caused by the Schmallenberg virus. Tijdschrift voor Diergeneeskunde137, 112–115.
- Nevill E.M. (1978) The use of cattle to protect sheep from bluetongue infection. Journal of the South African Veterinary Association49, 129–130.
- Ninio C., Augot D., Delecolle J.C., Dufour B. & Depaquit J. (2011) Contribution to the knowledge of Culicoides (Diptera: Cerapogonidae) host preferences in France. Parasitology Research108, 657–663.
- R Core Team (2014) The R project for statistical computing. Available at: http://www.R-project.org/ (accesses 15 April 2014).
- Rasmussen L.D., Kristensen B., Kirkeby C., Rasmussen T.B., Belsham G.J., Bodker R. & Botner A. (2012) Culicoides as vectors of Schmallenberg virus. Emerging Infectious Diseases18, 1204–1206.
- Reusken C., Van Den Wijngaard C., Van Beek P., Beer M., Bouwstra R., Godeke G.J.et al. (2012) Lack of evidence for zoonotic transmission of Schmallenberg virus. Emerging Infectious Diseases18, 1746–1753.
- Sailleau C., Boogaerts C., Meyrueix A., Laloy E., Bréard E., Viarouge C.et al. (2013) Schmallenberg virus infection in dogs, France, 2012. Emerging Infectious Diseases19, 1896–1898.
- Santman-Berends I.M.G.A., Bartels C.J.M., van Schaik G., Stegeman J.A. & Vellema P. (2010) The increase in seroprevalence of bluetongue virus (BTV) serotype 8 infections and associated risk factors in Dutch dairy herds, in 2007. Veterinary Microbiology142, 268–275.
- The Center for Food and Security and Public Health, Iowa State University (2009) Akabane disease. Available at: http://www.cfsph.iastate.edu/Factsheets/pdfs/akabane.pdf (accessed 5 May 2012).
- The European Food Safety Authority (EFSA) (2013) “Schmallenberg” virus: analysis of the epidemiological data (May 2013). Available at: http://www.efsa.europa.eu/en/supporting/pub/429e.htm (accessed 15 June 2013).
- Veldhuis A.M.B., Van Schaik G., Vellema P., Elbers A.R.W., Bouwstra R., Van der heijden H.M.J.F., Mars M.H., 2013a: Schmallenberg virus epidemic in the Netherlands: spatiotemporal introduction in 2011 and seroprevalence in ruminants. Preventive Veterinary Medicine112, 35–47.
- Veldhuis A.M.B., Carp-van Dijken S., Wuijckhuise L., Witteveen G. & van Schaik G. (2013b) Schmallenberg virus in Dutch dairy herds: potential risk factors for high within-herd seroprevalence and malformations in calves, and its impact on productivity. Veterinary Microbiology168, 281–293.
- Wernike K., Conraths F., Zanella G., Granzow H., Gache K., Schirrmeier H.et al. (2014) Schmallenberg virus – Two years of experiences. Preventive Veterinary Medicine116, 423–434.
- Yilmaz H., HoffmannB, TuranN, CizmecigilUY, RichtJA, Van der PoelWHM, 2014: Detection and partial sequencing of Schmallenberg virus in cattle and sheep in turkey. Vector Borne Zoonotic Diseases14, 223–225.
- Zimmer J.Y., Haubruge E., Francis F., Bortels J., Simonon G., Losson B.et al. (2008) Breeding sites of bluetongue vectors in northern Europe. The Veterinary Record162, 700.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?