Abstract
Objectives
The aim of the present study was to evaluate results, including clinical and radiological outcomes and number of complications, following minimally invasive plate osteosynthesis (MIPO) of proximal humerus fractures, using the PHILOS® proximal humerus internal locking system (Synthes Holding AG, Solothurn, Switzerland).
Methods
Retrospectively evaluated were 31 patients treated with MIPO (12 male, 19 female; average age: 58.4 years). Four patients had 2-part fractures, 14 patients had 3-part fractures, and 13 patients had 4-part fractures, according to Neer classification. Healing, complications, and head-shaft angle (HSA) were radiographically evaluated. Clinical outcomes were assessed at 1-year follow-up with Constant score.
Results
Average Constant scores for fractured and normal shoulders were 73.2 ± 10.9 and 84.8 ± 5.1, respectively. Varus progression, fracture type, and age had no significant effect on functional outcome. Average postoperative and follow-up HSAs were 130.80 ± 7.70 and 128.80 ± 10.00, respectively. Significant varus progression was observed during follow-up (p = 0.01). Varus progression was more prominent in patients with postoperative HSA < 130° (p < 0.001). Inferomedial calcar screw usage, fracture type, and age had no significant effect on varus progression. Complications included 2 implant failures, 1 case of avascular necrosis (AVN), 1 primary screw cut-out, 1 axillary nerve injury, and 1 radial nerve injury (22.6% overall).
Conclusion
MIPO is a safe and effective option for the treatment of proximal humerus fractures, with good functional recovery and fewer complications, which are typically technique dependent. Reduction may be difficult, resulting in varus progression. Another disadvantage is risk of axillary nerve injury. Careful surgical technique and correct implant selection is important in the prevention of nerve injury.
Level of evidence
Level IV, Therapeutic study.
Keywords
Complication ; Minimally invasive plate osteosynthesis ; Proximal humerus fracture
Introduction
Proximal humerus fractures are very common injuries, with increasing incidence in elderly patients.1 Surgical treatment is usually preferred for displaced fractures. Various methods have been introduced, including the use of percutaneous k wires, plates, intramedullary nails, and arthroplasty.2 ; 3 After the development of angular stable locking plates, surgical fixation of proximal humerus fracture became more popular.4 Deltopectoral approach had traditionally been used for plate fixation, though the extensile approach causes additional soft tissue damage, deltoid muscle injury, and impairment of the anterior circumflex humeral artery, which may lead to complications including nonunion, avascular necrosis (AVN), and infection.5
In 2005, Gardner described the anterolateral deltoid-splitting approach for treatment of proximal humerus,6 and this approach was also used as a component of minimally invasive plate osteosynthesis (MIPO) in treatment of proximal humerus fractures. The approach has the advantages of less soft tissue stripping, better preservation of blood supply, and direct visualization of greater tuberosity. In recent years, MIPO has been extensively used to treat proximal humerus fractures.7 ; 8 ; 9 ; 10 ; 11 ; 12 ; 13 ; 14 ; 15
The aim of the present study was to evaluate results, including clinical and radiological outcomes and number of complications, following MIPO implemented with use of the PHILOS® proximal humerus internal locking system (Synthes Holding AG, Solothurn, Switzerland).
Patients and methods
The present study was approved by committee of Çankaya Hospital. Between December 2006 and August 2014, 44 patients with displaced proximal humerus fractures were treated using the MIPO technique. Four patients treated with conventional plates were excluded. Of the remaining 40 patients treated with the PHILOS® plate, 9 patients were lost during follow-up. Ultimately, medical reports of 31 patients who completed at least 1 year of follow-up were retrospectively evaluated. Twelve male and 19 female patients, with an average age of 58.4 years (range: 18–83), were included. Sixteen patients (51.6%) were younger than 60 years of age; 15 patients (48.4%) were older (Table 1 ).
| Variable | Value |
|---|---|
| Number of patients | 31 |
| Age | |
| Average | 58.4 (range: 18–83) |
| <60 years | 16 (51.6%) |
| ≥60 years | 15 (48.4%) |
| Gender | |
| Male | 12 (38.7%) |
| Female | 19 (61.3%) |
| Neer fracture type | |
| 2-part | 4 (12.9%) |
| 3-part | 14 (45.2%) |
| 4-part | 13 (41.9%) |
All fractures were classified according to Neer classification, using x-ray and computed tomography imaging. Four patients (12.9%) had 2-part, 14 patients (45.2%) had 3-part, and 13 patients (41.9%) had 4-part fractures.
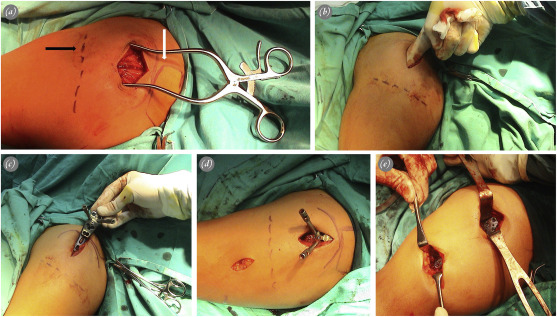
Average delay between injury and surgery was 3 days (range: 1–10). All procedures were performed under general anesthesia with the patient in beach chair position. A lateral longitudinal incision was proximally made, beginning at the anterolateral tip of the acromion, and extending at a maximum of 5 cm distally (Fig. 1 a). Deep dissection was performed through avascular deltoid raphe (Fig. 1 a). Nonabsorbable sutures were passed through the insertion sites of the subscapularis, supraspinatus, and infraspinatus tendons. These sutures were used for mobilization and reduction of the tuberosities. If necessary, k wires were used for indirect reduction of the humeral head or for temporary fixation of tuberosities. The axillary nerve was palpated blindly by the index finger through the incision (Fig. 1 b). Full exploration of the axillary nerve was not performed. Submuscular tunnel was prepared underneath the axillary nerve, using a blunt elevator.
|
|
|
Fig. 1. Surgical technique applied to a 43-year-old female patient with 2-part displaced fracture. a. Location of the proximal incision in relation to the anterolateral tip of the acromion (white arrow) and axillary nerve (black arrow). Anterior deltoid raphe is visualized inside the incision. b. Palpation of the axillary nerve through the proximal incision by index finger. c. Insertion of the locking plate through the proximal incision. d. Location of the distal incision on the lateral aspect of the humeral shaft in relation to the axillary nerve. e. Final position of the plate at the end of fixation. |
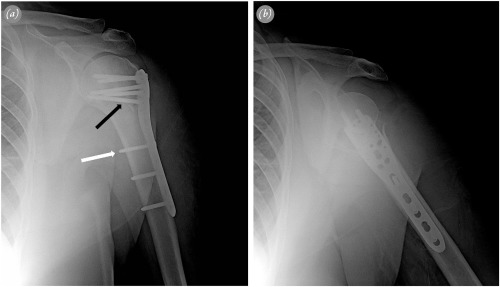
Plate was inserted percutaneously from proximal to distal (Fig. 1 c). Location of the distal incision was determined according to the length of the plate, under fluoroscopic control (Fig. 1 d). The distal plate was palpated on the midshaft of the distal humerus. Position of the proximal plate was checked under fluoroscopy. Two k wires were inserted through the first row on the plate, using locking drill sleeves to fix the plate to the humeral head. These k wires also provided information about the position of the most proximal screws. Fixation was started distally with a 3.5-mm cortical screw as a positional screw to indirectly reduce the shaft (Fig. 2 a). Proximal fixation was performed, using at least 4 3.5-mm locking screws. If metaphyseal communition was present, a long inferomedial calcar screw (IMCS) was inserted through the fourth row in the plate16 (Fig. 2 a). Additional 2 or 3 3.5-mm locking screws were inserted distally to complete fixation (Fig. 1 e). Nonabsorbable sutures were tied to anchor holes to fix the tuberosity fragments and to counterbalance the deforming forces on the fracture. No additional fixation was performed for the tuberosities.
|
|
|
Fig. 2. Radiographs of a 57-year-old female patient with 3-part fracture. a. Anteroposterior view with correct positioning of the plate and appropriate screw lengths. A conventional screw (white arrow) was used as a positional screw for indirect reduction, and an inferomedial calcar screw (black arrow) was used to support metaphyseal comminution. b. Lateral view with full internal rotation of the arm. |
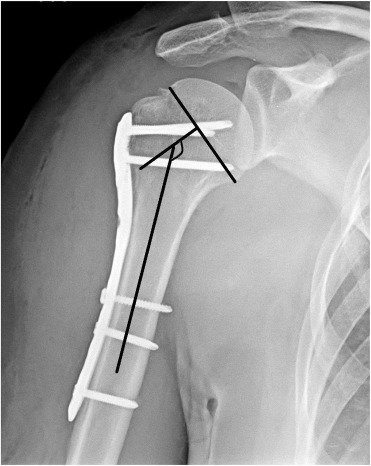
Sling immobilization was postoperatively used for 1 week, and passive- and active-assisted range of motion exercises were immediately begun. Active exercises were begun after 4 weeks. Radiographic evaluations were routinely performed at 6 weeks, 3 months, 6 months and 1 year, by using 20° external rotation projection for anteroposterior view and full internal rotation projection for lateral view (Fig. 2 ). If suspicion of fracture healing was present at 3 months, radiographic controls were performed more closely. Fracture healing, complications, and head-shaft angle (HSA) were evaluated radiographically. HSA was calculated by the same surgeon (U.G.), according to the method of Hertel et al.17 (Fig. 3 ). An angle above 130° was considered the goal of treatment. Clinical outcomes were assessed at 1 year of follow-up with Constant score. Nerve lesions were assessed clinically.
|
|
|
Fig. 3. Measurement of head-shaft angle. |
Outcomes of the present study were evaluated with SPSS statistical software (version 23.0; SPSS Inc., Chicago, IL, USA). Comparison of postoperative and follow-up HSA measurements, comparison of varus progression between patients with primary reduction less and more than 130°, and between patients with or without IMCS and varus progression, in terms of fracture type and age, were analyzed with analysis of variance for repeated measures. Comparison of functional outcomes of patients with or without varus progression, in terms of fracture type and age, were analyzed with Students t-test; p values less than 0.05 were considered statistically significant.
Results
No nonunions were observed at follow-up. Complete implant failure (cut-out of all proximal screws) after varus collapse was seen in 2 patients (6.5%) 3 and 4 months after surgery. They had 4-part fractures which were treated without IMCS. Postoperative HSA of these patients were 117° and 122°, with 22° and 5° of varus progression, respectively, at follow-up. Shoulder arthroplasty was performed for these patients. All other patients had radiographic union at 3-month follow-up. One patient (3.2%) developed AVN 6 months after surgery. He had 4-part fracture with poor greater tuberosity reduction. Short proximal screws had been used in the first surgery. Shoulder arthroplasty was performed. Other complications were primary screw perforation in 1 patient (3.2%), who was treated by changing the long screw at 1 month, deep infection in 1 patient (3.2%), who recovered completely after debridement at 3 weeks, axillary nerve injury in 1 patient (3.2%), and radial nerve injury in 1 patient (3.2%) who had relatively short arm length and was treated with a 5-hole plate. Both nerve injuries healed without clinical consequence. No subacromial impingement or secondary loss of greater tuberosity reduction were observed. Hardware removal was not performed. Overall complication rate was 22.6% (7 patients), and overall secondary operation rate was 16.1% (5 patients) (Table 2 ).
| Complication | Number n : 7 (22.6 %) | Secondary procedures n : 5 (16.1 %) |
|---|---|---|
| Varus collapse with implant failure | 2 (6.5%) | Shoulder arthroplasty |
| Avascular necrosis | 1 (3.2%) | Shoulder arthroplasty |
| Primary screw perforation | 1 (3.2%) | Renewal with a shorther screw |
| Deep infection | 1 (3.2%) | Debridement |
| Axillary nerve injury | 1 (3.2%) | |
| Radial nerve injury | 1 (3.2%) |
Upon postoperative radiographic examination, HSA measurement less than 130° was observed in 12 patients (38.7%). IMCS was used in 11 patients (35.5%), who had metaphyseal communition. Three-hole PHILOS® plate was used in 6 patients (19.4%), and 5-hole PHILOS® plate was used in 25 patients (80.6%). Average postoperative HSA measurement was 130.8°±7.7° (range: 114°–142°). At follow-up radiographic examination, average HSA measurement after fracture union was 128.8°±10.0° (range: 95°–142°). A statistically significant change of HSA measurement was found in accordance with progression of varus displacement at follow-up (p = 0.01). Varus progression was observed in 18 patients (58%). With the exception of 2 patients who underwent arthroplasty due to varus collapse, varus progression was between 1 and 4°.
Average postoperative and follow-up HSA measurements for patients with primary reduction of less than 130° were 122.8° ± 14.5° (range: 114°–128°) and 119.0° ± 8.5° (range: 95°–126°), respectively. Average postoperative and follow-up HSA measurements for patients with primary reduction of more than 130° were 135.8°±4.2° (range: 130°–142°) and 135.0° ± 4.1° (range: 128°–142°), respectively. Statistically significant difference was found between the 2 groups (p < 0.001), indicating that patients with insufficient primary reduction (HSA < 130°) had increased varus progression during follow-up.
No statistically significant difference in varus progression was found between patients treated with or without IMCS (p = 0.210). The present finding indicated the protective effect of IMCS against varus collapse. Fracture type, plate length, and age had no significant effect on varus progression (p = 0.550, p = 0.341, and p = 0.180, respectively). Three patients who had secondary arthroplasty were excluded from functional assessment. Average Constant score for the remaining 28 patients was 73.2 ± 10.9 (range: 48–91) at 1-year follow-up. Average Constant score of normal shoulder was 84.8 ± 5.1 (range: 70–95).
Average Constant scores for patients with or without varus progression at follow-up were 70.5 ± 11.5 (range: 48–86) and 76.9 ± 9.3 (range: 60–91), respectively. Although the varus progression group had lower functional scores, the difference was not statistically significant (p = 0.129). Follow-up HSA measurement, fracture type, age, and gender had no significant effect on functional outcome (p = 0.205, p = 0.715, p = 0.455, and p = 0.222, respectively).
Discussion
In the past 10 years, use of the MIPO technique with anterolateral deltoid-splitting approach has become more popular treatment for proximal humerus fracture.7 ; 8 ; 9 ; 10 ; 11 ; 12 ; 13 ; 14 ; 15 In the approach, the plate is easily placed on the lateral part of the humeral head, where an avascular bare area was described by Gardner et al.18 The area also offers better access to greater tuberosity for reduction. Less soft tissue dissection and decreased damage to the anterior humeral circumflex artery diminishes the risk of high complication rates reported for open reduction.19 Better functional outcomes with MIPO, compared to deltopectoral approach, have been reported in recent studies.20 ; 21
Significant functional improvement was presently observed, with an average Constant score of 73.2, consistent with the literature.10 ; 12 ; 13 ; 15 Patients reached 86.3% of contralateral shoulder function at 1-year follow-up. Typically, better functional outcomes have been reported in patients younger than 60 years, both in those who have undergone open surgery, and surgery in which the MIPO technique was used.15 ; 22 However, this was not a result presently observed, which may be due to the relatively young patient group in the present study.
The most significant result was varus progression at follow-up. Varus progression had previously been reported.9 ; 11 However, it was presently unexpected due to the relatively young age group. At radiographic evaluation, varus progression was seen in both age groups, possibly due to insufficient proximal fixation. With the MIPO technique, it is very difficult to use the proximal screw insertion guide block of the PHILOS® system, as it causes tenting of the axillary nerve.23 In most patients, guide sleeves were used in place of the guide block, which may have caused screw malpositioning and improper locking, leading to decreased stability and varus progression. Modifications of guide block15 and external aiming guides24 ; 25 have been described, but the present authors had no experience with these devices.
Varus progression was more prominent in patients who had postoperative HSA measurements of less than 130°, indicating the importance of primary reduction. A high rate of insufficient reduction (38.7%) was presently observed. Although various indirect reduction techniques have been described,25 ; 26 reduction may be difficult with MIPO. Use of a positional cortical screw for indirect reduction is an effective method, but peroperative fluoroscopic control is essential to avoid malreduction.
Varus malreduction has been reported to have adverse effects on clinical outcome.10 ; 27 However, HSA measurements had no significant effect on the present functional results. Although patients with varus progression had lower Constant scores, this difference was statistically insignificant, though 2 patients who needed arthroplasty after varus collapse were excluded from functional evaluation. These patients were expected to have poor Constant scores, and the omission may have skewed the present results.
No significant difference in varus progression or functional outcome was found between fracture types, in contrast to the findings of Shon et al., who reported lower Constant scores and increased varus progression in 4-part fractures.11 The present authors believe that the MIPO technique can be safely used to treat 4-part fractures, particularly in young patients.
Overall complication rate in the present study was 22.6%, comparable to that of other series, with complication rates ranging between 12 and 27%.7 ; 9 ; 10 ; 11 ; 13 ; 15 No nonunions were observed, though implant failure occurred in 2 patients with cut-out of all proximal screws. Primary reduction was poor in both patients, causing increased incidence of varus progression and implant failure.
Secondary screw cut-out is difficult to prevent due to subsidence of the humeral head, particularly in patients with metaphyseal comminution. Restoration of medial calcar is very important in order to prevent varus collapse.16 ; 28 ; 29 ; 30 . Augmentative methods designed to increase support of the medial calcar have been described, including the use of long oblique inferomedial screw16 ; 31 or strut allografts,27 ; 32 ; 33 the later of which can be used as a component of the MIPO technique.11 ; 33 In the present study, IMCSs were used in 11 patients with metaphyseal comminution. No difference in varus progression between patients treated with and without IMCS was found, supporting the protective effect of IMCS for varus collapse, though the implementation was not always easy, as the fourth row used for IMCS was usually too distal in the incision and orientation of the screw, which was oblique, causing stretching of the axillary nerve during insertion.
AVN is a major complication after open reduction, due to impairment of blood supply, and rates of 0–8.2% have been reported in series in which the MIPO technique was used.7 ; 9 ; 10 ; 11 ; 13 ; 15 In the present study, AVN developed in only 1 patient, who had 4-part fracture in which greater tuberosity was poor and proximal screws used were short. Greater tuberosity reduction and stability are important factors, affecting the revascularization of the humeral head.2
The axillary nerve is at risk when the MIPO technique is implemented. It is localized at approximately 6 cm distal to the acromion,34 ; 35 and as a result, proximal incision must not be longer than 5 cm, and screw insertion in the 5th and 6th holes must be avoided.23 A proper tunnel must be prepared underneath the nerve, and plate insertion must be gentle. Some authors prefer direct visualization of the axillary nerve by extending the anterolateral incision.36 However, as the axillary nerve has many branches in the area, there is still a risk of injury, even if the nerve is visualized. It is believed by the present authors that blind palpation of the nerve is a safe method. A 5-hole PHILOS® plate is generally preferred by the authors, in order to avoid contact with the nerve. Only 1 patient had transient axillary nerve injury in the present study, without clinical consequence.
An unexpected complication, radial nerve injury, occurred in 1 patient. The Radial nerve can be damaged when the MIPO technique is used with long plates to treat proximal humeral shaft fracture. However, to our knowledge, no radial nerve injury following MIPO used to treat proximal humerus has been reported. Irritation of the tip of the 5-hole plate in the present patient, who had a short arm, may be the cause of the discrepancy. Primary screw cut-out and infection are rare complications, each of which were present in 1 patient. Primary screw cut-out can be prevented by careful fluoroscopic control.
The primary present limitation was the absence of a control group treated with open reduction technique. In addition, discussion of long-term functional outcome is lacking, and is expected to improve after 2 years.9 ; 12
In conclusion, MIPO technique with PHILOS® plate is a safe and effective option for the treatment of proximal humerus fractures, with good functional recovery. Complications such as nonunion, AVN, and infection are rare, due to less soft tissue damage and blood supply impairment. Complications including screw cut-out, nerve injury, and malreduction are usually technique-dependent. MIPO is a technically demanding procedure, and reduction in particular may be difficult, resulting in varus progression observed on follow-up. Use of IMCS for metaphyseal comminution is also difficult, and another disadvantage is the risk of axillary nerve injury. In addition, the radial nerve may be at risk in patients with short arms. Careful surgical technique and correct implant selection is important in the prevention of nerve injuries.
References
- 1 M. Palvanen, P. Kannus, S. Niemi, et al.; Update in the epidemiology of proximal humeral fractures; Clin Orthop Relat Res, 442 (2006), pp. 87–92
- 2 D. Karataglis, S.I. Stavridis, G. Petsatodis, et al.; New trends in fixation of proximal humeral fractures: a review; Injury, 42 (4) (2011), pp. 330–338
- 3 G. Gradl, M. Knobe, H.C. Pape, et al.; Decision making in displaced fractures of the proximal humerus: fracture or surgeon based?; Int Orthop, 39 (2) (2015), pp. 329–334
- 4 S.C. Chudik, P. Weinhold, L.E. Dahners; Fixed—angle plate fixation in simulated fractures of the proximal humerus: a biomechanical study of a new device; J Shoulder Elbow Surg, 12 (6) (2003), pp. 578–588
- 5 M. Sturzenegger, E. Fornaro, R.P. Jakob; Results of surgical treatment of multifragmented fractures of the humeral head; Arch Orthop Trauma Surg, 100 (4) (1982), pp. 249–259
- 6 M.J. Gardner, M.H. Griffith, J.S. Dines, et al.; The extended anterolateral acromial approach allows minimally invasive access to the proximal humerus; Clin Orthop Relat Res, 434 (2005), pp. 123–129
- 7 G. Röderer, J. Erhardt, M. Graf, et al.; Clinical results for minimally invasive locked plating of proximal humerus fractures; J Orthop Trauma, 24 (7) (2010), pp. 400–406
- 8 S. Ruchholtz, C. Hauk, U. Lewan, et al.; Minimally invasive polyaxial locking plate fixation of proximal humeral fractures: a prospective study; J Trauma, 71 (6) (2011 Dec), pp. 1737–1744
- 9 Y.P. Acklin, K. Stoffel, C. Sommer; A prospective analysis of the functional and radiological outcomes of minimally invasive plating in proximal humerus fractures; Injury, 44 (4) (2013), pp. 456–460
- 10 S.W. Jung; Indirect reduction maneuver and minimally invasive approach for displaced proximal humerus fractures in elderly patients; Clin Orthop Surg, 5 (1) (2013), pp. 66–73
- 11 H.S. Sohn, S.J. Shin; Minimally invasive plate osteosynthesis for proximal humeral fractures: clinical and radiologic outcomes according to fracture type; J Shoulder Elbow Surg, 23 (9) (2014), pp. 1334–1340
- 12 P.A. Koljonen, C. Fang, T.W. Lau, et al.; Minimally invasive plate osteosynthesis for proximal humeral fractures; J Orthop Surg (Hong Kong), 23 (2) (2015), pp. 160–163
- 13 J. Park, S.Y. Jeong; Complications and outcomes of minimally invasive percutaneous plating for proximal humeral fractures; Clin Orthop Surg, 6 (2) (2014), pp. 146–152
- 14 H. Chen, X. Hu, H. Tang, et al.; Minimal invasive percutaneous osteosynthesis for elderly valgus impacted proximal humeral fractures with the PHILOS; Biomed Res Int, 2015 (2015), p. 971216
- 15 F. Falez, M. Papalia, A. Greco, et al.; Minimally invasive plate osteosynthesis in proximal humeral fractures: 1—year results of a prospective multicenter study; Int Orthop, 40 (3) (2016 Mar), pp. 579–585
- 16 M.J. Gardner, Y. Weil, J.U. Barker, et al.; The importance of medial support in locked plating of proximal humerus fractures; J Orthop Trauma, 21 (3) (2007), pp. 185–191
- 17 R. Hertel, U. Knothe, F.T. Ballmer; Geometry of the proximal humerus and implications for prosthetic design; J Shoulder Elbow Surg, 11 (4) (2002), pp. 331–338
- 18 M.J. Gardner, J.E. Voos, T. Wanich, et al.; Vascular implications of minimally invasive plating of proximal humerus fractures; J Orthop Trauma, 20 (9) (2006), pp. 602–607
- 19 S. Brorson, J.V. Rasmussen, L.H. Frich, et al.; Benefits and harms of locking plate osteosynthesis in intraarticular (OTA Type C) fractures of the proximal humerus: a systematic review; Injury, 43 (7) (2012), pp. 999–1005
- 20 T. Lin, B. Xiao, X. Ma, et al.; Minimally invasive plate osteosynthesis with a locking compression plate is superior to open reduction and internal fixation in the management of the proximal humerus fractures; BMC Musculoskelet Disord, 15 (2014), p. 206
- 21 K. Liu, P.C. Liu, R. Liu, et al.; Advantage of minimally invasive lateral approach relative to conventional deltopectoral approach for treatment of proximal humerus fractures; Med Sci Monit, 21 (2015), pp. 496–504
- 22 A.S. Parmaksizoğlu, S. Sökücü, U. Ozkaya, et al.; Locking plate fixation of 3— and 4—part proximal humeral fractures; Acta Orthop Traumatol Turc, 44 (2) (2010), pp. 97–104
- 23 J. Smith, G. Berry, Y. Laflamme, et al.; Percutaneous insertion of a proximal humeral locking plate: an anatomic study; Injury, 38 (2) (2007), pp. 206–211
- 24 N. Saran, S.G. Bergeron, B. Benoit, et al.; Risk of axillary nerve injury during percutaneous proximal humerus locking plate insertion using an external aiming guide; Injury, 41 (10) (2010), pp. 1037–1040
- 25 Y.P. Acklin, C. Sommer; Plate fixation of proximal humerus fractures using the minimally invasive anterolateral delta split approach; Oper Orthop Traumatol, 24 (1) (2012), pp. 61–73
- 26 D.M. Rouleau, G.Y. Laflamme, G.K. Berry, et al.; Proximal humerus fractures treated by percutaneous locking plate internal fixation; Orthop Traumatol Surg Res, 95 (1) (2009), pp. 56–62
- 27 C.M. Robinson, J.R. Wylie, A.G. Ray, et al.; Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate; J Bone Jt Surg Br, 92 (5) (2010), pp. 672–678
- 28 C.W. Lee, S.J. Shin; Prognostic factors for unstable proximal humeral fractures treated with locking—plate fixation; J Shoulder Elbow Surg, 18 (1) (2009), pp. 83–88
- 29 J. Lescheid, R. Zdero, S. Shah, et al.; The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support; J Trauma, 69 (5) (2010), pp. 1235–1242
- 30 W.B. Jung, E.S. Moon, S.K. Kim, et al.; Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate?; BMC Musculoskelet Disord, 14 (2013), p. 102
- 31 L. Zhang, J. Zheng, W. Wang, et al.; The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study; Int Orthop, 35 (11) (2011), pp. 1655–1661
- 32 M.J. Gardner, S. Boraiah, D.L. Helfet, et al.; Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant; J Orthop Trauma, 22 (3) (2008), pp. 195–200
- 33 F. Matassi, R. Angeloni, C. Carulli, et al.; Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus; Injury, 43 (11) (2012), pp. 1939–1942
- 34 C.Z. Esenyel, S. Dedeoğlu, Y. Imren, et al.; Relationship between axillary nerve and percutaneously inserted proximal humeral locking plate: a cadaver study; Acta Orthop Traumatol Turc, 48 (5) (2014), pp. 553–557
- 35 M.J. Gardner, M.H. Griffith, J.S. Dines, et al.; A minimally invasive approach for plate fixation of the proximal humerus; Bull Hosp Jt Dis, 62 (1—2) (2004), pp. 18–23
- 36 N. Aksu, S. Karaca, A.N. Kara, et al.; Minimally invasive plate osteosynthesis (MIPO) in diaphyseal humerus and proximal humerus fractures; Acta Orthop Traumatol Turc, 46 (3) (2012), pp. 154–160
Document information
Published on 31/03/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?