Abstract
In this study, three different kinds of simultaneous saccharification and fermentation (SSF) of washed pretreated corn stover with water-insoluble solids (WIS) content of 20% were investigated to find which one resulted in highest ethanol yield at high-solids loadings. The different methods were batch SSF, prehydrolysis followed by batch SSF and fed-batch SSF. Batch-SSF resulted in an ethanol yield of 75–76% and an ethanol concentration of 53 g/L. Prehydrolysis prior to batch SSF did not improve the ethanol yield compared with batch SSF. Fed-batch SSF, on the other hand, increased the yield, independent of the feeding conditions used (79–81%, 57–60 g/L). If the initial amount of solids during fed-batch SSF was lowered, the yield could be improved to some extent. When decreasing the enzyme dosage, the greatest decrease in yield was seen in the fed-batch mode (75%), while lower or the same yield was seen in batch mode with and without prehydrolysis (73%). This resulted in similar ethanol yields in all methods. However, the residence time to achieve the final ethanol yield was shorter using fed-batch. This shows that fed-batch can be a better alternative also at a lower enzyme loading.
Introduction
The demand for fuel is increasing alongside population growth due to requirements from an expanding transport and energy sector. However, it is well known that the combustion of fossil fuels has a negative impact on the environment [1]. One way of reducing environmental effects is to minimize the emission of greenhouse gases. This can be done by using biomass such as agricultural or forest residues to produce energy for heating or fuel. These materials bind atmospheric carbon dioxide during growth and can, therefore, reduce greenhouse gas emissions. However, progress in the introduction of biomass-based fuels is slow since their production is often more expensive than fossil fuels [2]. Therefore, efforts should be devoted to reducing the process cost, for example, increasing the overall yield from the substrate and increasing the ethanol concentration [2].
We have previously investigated the conversion of corn stover into ethanol, methane, and a lignin-rich solid residue [3-5]. The goal of those studies was to evaluate the combined production of ethanol and methane to determine which process or processes will result in the highest energy recovery from the raw material, while at the same time producing high amounts of ethanol. Two different process configurations were investigated, one based on whole slurry and the other on separate liquid and solid streams. The configuration that resulted in the highest energy recovery was the one in which the solid and the liquid phases were separated after pretreatment. The solid phase was used for ethanol production using simultaneous saccharification and fermentation (SSF), while the liquid phase was used for methane production by anaerobic digestion. However, although the energy recovery in ethanol, methane, and lignin was high (76–88%), the ethanol concentrations were only moderate (20–26 g/L).
The cost of producing ethanol from lignocellulosic materials is considerably higher than producing ethanol from starch or sugar, or fuels derived from oil. Less energy would be required in the distillation step if the ethanol concentration in the fermentation broth could be increased, leading to a reduction in the production cost [6]. One way of increasing the ethanol concentration is to use higher solids loading in SSF. However, it has been shown in many studies that higher solids loading decrease the ethanol yield [7-11]. The production cost is very sensitive to both the ethanol yield and the ethanol concentration. The latter is mainly due to the energy requirement for distillation, but also the capital cost, mainly for reactors (i.e. pretreatment, hydrolysis and fermentation tanks) as lower flow rates are processed.
One of the main problems associated with high solids loading is the increased resistance to mass transfer enhanced by the difficulty of proper mixing in the beginning [7, 12, 13]. However, other studies, for example, Kristensen et al. [14], conclude that insufficient mixing may not be the main cause for decreased yield, but suggest that inhibition may be the reason for decreased yield. Prehydrolysis of the substrate to reduce the viscosity before adding the yeast to the reactor improves mixing. This allows a higher hydrolysis temperature to be used, which is favourable for the enzymes. This commonly results in a shorter hydrolysis time and also a shorter running time at high viscosity, reducing stirring problems. Another option is to use the fed-batch mode in the SSF, where the amount of solids initially added to the reactor is smaller than in the batch mode, resulting in less power being required for stirring [15].
The aim of this study was to increase the ethanol concentration in SSF by increasing the water insoluble solids (WIS) content from 10% to 20%, while minimizing the effect on the ethanol yield. The same basic process configuration was used as in previous studies [3-5], that is, after pretreatment, the liquid and solid phase were separated, and the solid phase was used in SSF. In this study, different SSF conditions on the solid phase were investigated and compared with batch-wise SSF. The time and temperature during prehydrolysis prior to batch SSF were varied, and different fed-batch conditions were tested. The effect of decreasing the enzyme dosage on the different SSF conditions was also investigated.
Materials and Methods
Raw material and steam pretreatment
Moist corn stover was provided by the State Grid Corporation of China (Handan City, Hebei Province in China). The corn stover was air dried at room temperature for some weeks, and turned over many times during drying to reduce the risk of molding during storage. The dry matter (DM) content after drying was 90%. The dried corn stover was soaked in an aqueous solution containing 0.4% H3PO4 by mass, at room temperature for 1 h. The liquid/solid ratio was 20 kg/kg dry corn stover. The material was then dewatered in a small laboratory high-pressure press (Tinkturenpressen HP5M; Fischer Maschinenfabrik GmbH, Burgkunstadt, Germany) to a DM content of 48–50%, and then steam pretreated. Steam pretreatment was carried out in a preheated 10 L reactor, as described previously [4], at 190°C for 10 min. Several runs were performed and then the material was thoroughly mixed to form a single large batch that was stored at 4°C.
Simultaneous saccharification and fermentation
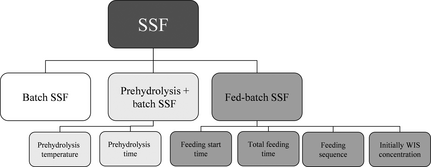
Three kinds of SSF set-ups were performed: batch SSF, prehydrolysis followed by batch SSF and fed-batch SSF, as illustrated in Figure 1. For these set-ups different conditions were investigated. During prehydrolysis different times (4, 8, 24 and 48 h) and temperatures (45°C, 50°C, 55°C, and 60°C) of the prehydrolysis step were studied, and during fed-batch SSF, different substrate feeding strategies were investigated. The amount of material being added from the beginning (50% and 33% of total), the starting time of feeding (4, 8 and 12 h), the feeding interval (every second, fourth and 12th hour) and the total feeding time (12, 16 and 24 h) were investigated in six different feeding strategies (Table 1). The results from the different conditions in these cases were then compared with each other and with batch SSF. In addition, the effect of a decreasing enzyme dosage (7.5 FPU/g WIS instead of 10) was investigated in one case in the three kinds of SSF. All the experiments were performed on washed material except one that was run on unwashed material. In this case, the configuration was performed with fed-batch and feeding strategy F.
| Feeding strategy | WIS added at start as % of total amount used | Total feeding time (h)a | Time of first addition (h) | Feeding interval (h) |
|---|---|---|---|---|
| ||||
| A | 50 | 12 | 4 | 2 |
| B | 50 | 12 | 4 | 4 |
| C | 50 | 24 | 12 | 12 |
| D | 50 | 16 | 4 | 4 |
| E | 50 | 16 | 8 | 4 |
| F | 33 | 16 | 4 | 4 |
|
|
|
Figure 1. Overview of the modes of SSF and parameters studied. SSF, simultaneous saccharification and fermentation. |
All SSF experiments were carried out in 2 L fermenters (Infors AG, Bottmingen, Switzerland) equipped with a pitched-blade impeller turbine and an anchor impeller. The final working weight in the fermenters was 1.0 kg. The material was washed before SSF, using the same procedure as in previous studies [3-5]. The pretreated material was dewatered in a filter press to a DM content between 40% and 50%. The same amount of water as had been pressed out was added, and the material was then dewatered to the same DM content as before water addition. In the case where the material was unwashed, the material was pressed to a DM content between 40% and 50% and the liquid was added back during SSF instead of sterile water.
The total duration of each of the SSF experiments was 144 h. The batch and batch with prehydrolysis experiments were carried out with WIS contents of 19.2% or 20%. The lower WIS content was due to a slightly incorrect measurement of the WIS content in the starting material. However, the difference in WIS is small, which will have a negligible effect on the final results. In the batch experiments all the solids, the enzymes, the nutrients and the yeast were added together at start. The SSF temperature was set to 35°C. In the batch experiments with prehydrolysis, only the enzymes, the nutrients and substrate were added initially. Four prehydrolysis temperatures were investigated: 45°C, 50°C, 55°C and 60°C, and four residence times: 4, 8, 24 and 48 h. After prehydrolysis the reactor was cooled to 35°C and the yeast was added. The time required for the reactor to cool down was between 15 and 30 min.
The initial WIS content in fed-batch SSF was 13% or 9.5%. Yeast, enzymes, nutrients, and required water was initially added to the reactor together with 50% or 33% of the total amount of WIS, respectively. The remaining material was added in equal amounts after 4, 8 or 12 h at regular intervals (Table 1). The total amount of solid material corresponded to an overall WIS content of 20%. The washed pretreated material was diluted with sterile water to obtain the desired WIS concentration in all experiments. In the case of unwashed material pretreatment liquid, which was pressed out, was added instead. Nutrients were added to the fermenter in all experiments to final concentrations of 0.5 g/L (NH4)2HPO4 and 0.025 g/L MgSO4·7H2O. The enzyme mixture, Cellic CTec2 (Novozymes, Bagsvaerd, Denmark), was added at an amount corresponding to 7.5 or 10 FPU/g WIS (based on the overall amounts of pretreated material added). Dry bakers yeast, Saccharomyces cerevisiae (Jästbolaget AB, Rotebro, Sweden), was added to the fermenters to give a concentration of 3 g/L based on the volume when all material was added. The experiments started with the addition of the enzyme mixture and the yeast (except when performing prehydrolysis). The substrates with high WIS contents were difficult to mix, and stirring was thus started after some enzymatic hydrolysis had occurred (after ~1 h). The pH was set to 5 using a 10% NaOH solution (by mass). Samples were collected regularly throughout SSF for analysis using high performance liquid chromatography (HPLC).
Analysis
All DM contents were determined by drying the material in an oven at 105°C until constant weight. The WIS contents of the different slurries were determined from the DM content of the slurry and the DM content of the hydrolysate, according to the method of Weiss et al. [16].
The compositions of raw corn stover and WIS after pretreatment were determined using the standard procedure “Determination of structural carbohydrates and lignin in biomass” from the National Renewable Energy Laboratory (NREL) [17]. All measurements were performed in triplicate. The total sugar content of the liquid fractions after pretreatment (hydrolysate) was analyzed according to the corresponding NREL procedure [18].
HPLC was used for the analysis of sugars, ethanol and by-products using a chromatographic system equipped with a differential refractive index detector (RID-10A) (both from Shimadzu, Kyoto, Japan). All samples were passed through a filter with a pore diameter of 0.20 μm prior to analysis to remove particles. The filtered samples were stored at −20°C before analysis. The liquid from the slurry after steam pretreatment and samples from SSF were diluted if necessary, and analyzed using an Aminex HPX-87H column (Bio-Rad, Hercules, CA) at 50°C with 5 mmol/L H2SO4 as eluent, at a flow rate of 0.5 mL/min, to separate ethanol, lactic acid, acetic acid, formic acid, levulinic acid, HMF and furfural. The liquid from the NREL analysis, the liquid after steam pretreatment and that obtained after SSF were analyzed with an Aminex HPX-87P column (Bio-Rad) at 85°C with deionized water as eluent, at a flow rate of 0.5 mL/min, to separate monomeric sugars (glucose, xylose, galactose, arabinose and mannose). All acidic samples analyzed on this column had been previously neutralized using solid CaCO3.
Results and Discussion
Raw material and pretreatment
The raw material consisted mainly of glucan (40.4%), xylan (24.5%) and lignin (22.5%, including both acid-soluble and acid-insoluble lignin), together with small amounts of galactan (3.0%) and arabinan (5.3%). The ash content was 2.3%. The corn stover was steam pretreated with 0.4% phosphoric acid in two different batches at 190°C for 10 min. The results of pretreatment of the two batches differed slightly, as can be seen in Table 2, where the glucan content and glucose concentrations were lower in the second batch, and the lignin content was somewhat higher. However, a mass balance on glucose results in the same glucan recovery (results not shown). Also the results from the batch experiments that were run with the two different batches gave similar yields (75% and 76%, respectively). Therefore, the results from SSF runs from the different pretreatment batches are comparable even though the lignin content differs.
| Batch 1 | Batch 2 | |
|---|---|---|
| ||
| Total solids (%) | 13.5 | 13.9 |
| WIS content (%) | 10.2 | 10.6 |
| Solid fraction (% of WIS) | ||
| Glucan | 59.6 | 55.7 |
| Xylan | 9.1 | 8.8 |
| Galactan | nd | nd |
| Arabinan | 0.5 | 0.7 |
| Lignin | 26.2 | 30.5 |
| Ash | 5.6 | 5.9 |
| Liquid fraction (g/L)a | ||
| Glucose | 6.6 | 3.8 |
| Xylose | 21.4 | 19.1 |
| Galactose | 2.2 | 2.2 |
| Arabinose | 1.6 | 1.6 |
Effect on ethanol yield of increasing WIS
The ethanol yields obtained with batch SSF were similar to, or slightly higher than, those obtained in our previous study [5], where the same pretreatment conditions were used but only 10% WIS. In that study, the ethanol yield was found to be 74%, which can be compared with yields ranging from 75% to 76% in this study using 20% WIS in batch SSF. A similar pattern has also been observed in a previous study, where the ethanol yield did not decrease with increasing WIS concentration when washed material was used [19]. Others have reported a decrease in the ethanol yield from SSF with increasing solids loading [7, 9-11]. However, unwashed material was used in these studies, which contains all the inhibitors formed during the pretreatment. Therefore, one experiment was made in this study to verify that lower yields were obtained with unwashed material. The experiment was run in the same way as the SSF resulting in highest yield to have conditions that were suitable for the yeast (fed-batch, feeding strategy F). In this experiment, pretreatment liquid was added instead of water to get the correct WIS content. This resulted in only a small ethanol formation. However, when using the same pretreatment conditions and unwashed material in our previous study, ethanol was formed to the same extent as with washed material. It can thus be concluded that the ethanol yield does not decrease to the same extent with washed material as unwashed material. This was also confirmed in a study by Hodge et al. [20], showing that enzyme inhibition is due to soluble components rather than insoluble components. Mohagheghi et al. [21]. reported that washed material resulted in a decrease in ethanol yield with increasing solids loading, and concluded that the yeast cells were partially inhibited at ethanol concentrations above 55 g/L. This was not seen in the present study, where the ethanol yield did not decrease compared with that using 10% WIS, despite ethanol concentrations between 44 and 60 g/L.
Influence of prehydrolysis temperature on batch SSF
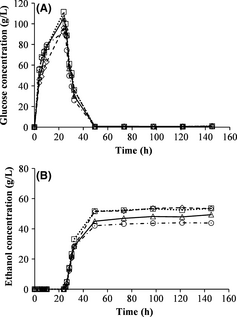
The yeast was added to batch SSF after 24 h of prehydrolysis at the temperatures 45°C, 50°C, 55°C and 60°C. The highest glucose concentration prior to yeast addition (111.5 g/L) was obtained with a prehydrolysis temperature of 50°C, while 55°C resulted in a slightly lower concentration of glucose (Fig. 2A). Prehydrolysis temperatures of 45°C and 60°C yielded lower glucose concentrations of 96.1 and 92.4 g/L, respectively. Fermentation was rapid, and started as soon as the yeast was added to the reactor in all cases. The highest ethanol concentration and yield were obtained with prehydrolysis temperatures of 45°C and 50°C (Fig. 2B and Table 3). The ethanol concentration decreased as the prehydrolysis temperature was increased above 50°C. The optimal temperature for the activity of the enzymes is 50°C, and they are partly deactivated at higher temperatures, resulting in lower yields. However, when investigating the ethanol yield, a lower temperature, for example, 45°C can also be used to obtain the same yield. A lower temperature can be beneficial to decrease the cost of heating the reactor if the residence time is the same for both the processes. It has also been shown that the optimal hydrolysis temperature is depending on the residence time [22].
| Expt no. | SSF mode | Pretreatment batch | Experimental conditionsa | WIS (%)b | Enzyme dosage (FPU/g WIS) | Ethanol yield (%)c | Ethanol (g/L) | Glucose (g/L) | Glycerol (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| 1 | Batch | 1 | 0 h | 19.2 | 10 | 75 (69) | 53.8 | 0.5 | 4.0 |
| 2 | Batch | 2 | 0 h | 20.0 | 10 | 76 (70) | 53.4 | 0.3 | 3.8 |
| 3 | Prehydrol. | 1 | 24 h (45°C) | 19.2 | 10 | 74 (68) | 53.4 | 1.2 | 4.3 |
| 4 | Prehydrol. | 1 | 24 h (50°C) | 19.2 | 10 | 75 (69) | 53.5 | 1.0 | 5.1 |
| 5 | Prehydrol. | 1 | 24 h (55°C) | 19.2 | 10 | 69 (63) | 49.4 | 0.6 | 5.2 |
| 6 | Prehydrol. | 1 | 24 h (60°C) | 19.2 | 10 | 60 (56) | 44.8 | 0.4 | 5.3 |
| 7 | Prehydrol. | 1 | 4 h (50°C) | 19.2 | 10 | 73 (67) | 52.3 | 1.1 | 4.1 |
| 8 | Prehydrol. | 1 | 8 h (50°C) | 19.2 | 10 | 72 (66) | 51.5 | 0.5 | 4.6 |
| 9 | Prehydrol. | 1 | 48 h (50°C) | 19.2 | 10 | 70 (65) | 49.2 | 0.5 | 5.9 |
| 10 | Fed-batch | 1 | A | 20.0 | 10 | 79 (73) | 60.2 | 0.5 | 3.9 |
| 11 | Fed-batch | 1 | B | 20.0 | 10 | 79 (73) | 60.0 | 0.2 | 3.7 |
| 12 | Fed-batch | 1 | C | 20.0 | 10 | 79 (73) | 59.5 | 0.2 | 4.1 |
| 13 | Fed-batch | 2 | D | 20.0 | 10 | 80 (73) | 56.9 | 0.4 | 3.6 |
| 14 | Fed-batch | 2 | E | 20.0 | 10 | 80 (73) | 56.8 | 0.2 | 3.6 |
| 15 | Fed-batch | 2 | F | 20.0 | 10 | 81 (75) | 58.0 | 0.3 | 3.4 |
| 16 | Fed-batch | 2 | Fd | 20.0 | 10 | – | 0.7 | 84.2 | 0.8 |
| 17 | Batch | 2 | 0 h | 20.0 | 7.5 | 73 (67) | 51.8 | 0.4 | 3.7 |
| 18 | Prehydrol. | 2 | 8 h (50°C) | 20.0 | 7.5 | 73 (67) | 52.6 | 0.3 | 3.4 |
| 19 | Fed-batch | 2 | F | 20.0 | 7.5 | 75 (69) | 53.4 | 0.2 | 4.3 |
|
|
|
Figure 2. Measured concentrations of glucose (A) and ethanol (B) during batch simultaneous saccharification and fermentation with 24 h prehydrolysis at prehydrolysis temperatures of: 45°C (–◊–), 50°C (∙∙∙□∙∙∙), 55°C (—∆—) and 60°C (– ∙○– ∙). |
Influence of prehydrolysis time on batch SSF
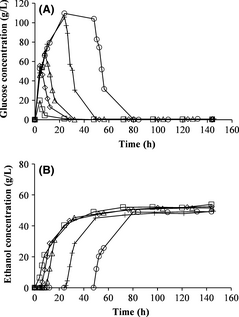
The effect of different prehydrolysis times at a prehydrolysis temperature of 50°C on batch SSF was also evaluated. The glucose concentration, before yeast addition, was lower with shorter prehydrolysis time but the same after 24 and 48 h of prehydrolysis (Fig. 3A). The ethanol concentration and yield were slightly lower or approximately the same with prehydrolysis as without prehydrolysis (Fig. 3B and Table 3). The largest deviation was seen with prehydrolysis for 48 h. The glycerol concentration increased with longer prehydrolysis time (Table 3).
|
|
|
Figure 3. Measured concentration of glucose (A) and ethanol (B) during batch simultaneous saccharification and fermentation with prehydrolysis at 50°C for various times: 0 h (no prehydrolysis) (□), 4 h (◊), 8 h (∆), 24 h (+), 48 h (○). |
The ethanol yield was only slightly affected by varying the duration of prehydrolysis, but the glycerol concentration increased with increasing prehydrolysis time. This indicates that a longer prehydrolysis time favours cell growth. No effect, or a small negative effect, has been reported in some previous studies after prehydrolysis prior to batch SSF [7, 23], while a clear improvement in ethanol yield has been found in other studies when using prehydrolysis [7, 24]. However, in those studies was unwashed material used. The conclusion from Hoyer et al. [7, 24]. was that when batch SSF results in high yields, prehydrolysis does not improve the outcome. This was also confirmed in the present study. Although the effect on ethanol yield was small, prehydrolysis resulted in faster liquefaction. It has been shown that power consumption is highly dependent on the viscosity of the slurry [25]. Therefore, less stirring power will be required when using prehydrolysis than in batch SSF without prehydrolysis.
Fed-batch SSF
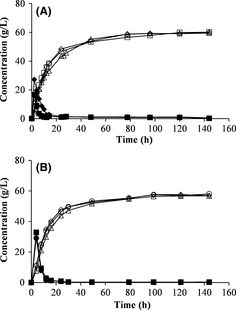
The fed-batch mode was employed to reduce stirring problems during SSF. No major differences were seen when varying the feeding interval or the total feeding time during fed-batch SSF (Fig. 4A). The ethanol yield was the same with 2-h feeding intervals and 4-h feeding intervals, during a total duration of 12 h. The same results were also obtained using a feeding interval of 12 h for a total feeding time of 24 h.
|
|
|
Figure 4. Concentrations of glucose (filled symbols) and ethanol (open symbols) during fed-batch SSF. (A) Fed-batch with feeding intervals of 2 h (□), 4 h (◊) and 12 h (∆). (B) Fed-batch with a total feeding time of 16 h and feeding interval of 4 h, with different initial WIS loadings at different starting times: 50% of total WIS after 4 h (◊), 50% of total WIS after 8 h (∆) and 33% of total WIS after 4 h (○). SSF, simultaneous saccharification and fermentation; WIS, water-insoluble solids. |
A total feeding time of 16 h was also investigated, where feeding was started after 4 or 8 h with a 4-h feeding interval. The second pretreatment batch was used in these experiments, which resulted in lower ethanol concentrations. The total ethanol yields were very similar in all experiments. These results is in agreement with results presented in previous studies where the feeding interval was varied [26, 27]. In those studies the total ethanol yield was also similar, independent on feeding interval. The initial solid loading was also varied in this series of experiments. One-third of the total WIS used in SSF was added at the beginning of one experiment (no. 15, Table 3) instead of 50% as in the other experiments. This might have resulted in a slightly higher ethanol concentration and yield than with an initial loading of 50% of the total WIS (Fig. 4B). More experiments are needed to verify if there is a significant difference when using a lower initial loading.
The ethanol yield was higher in fed-batch SSF than in batch SSF with and without prehydrolysis (Table 3). The glycerol concentration was similar to, or lower than, that obtained with batch SSF. This indicates that there was less cell growth during fed-batch SSF, resulting in more glucose being available for ethanol production. The findings of other studies are rather diverse. It has been reported that the ethanol yield was the same using fed-batch and batch SSF [21, 26], while others have reported fed-batch to be superior to batch SSF [28, 29]. In a study by Hoyer et al. [30], it was concluded that the enzyme-feeding strategy affected the ethanol yield. The strategy depends on a number of factors, for example, WIS content and inhibitor concentration. Different enzyme feeding strategies were not investigated in the present study.
Influence of enzyme loading
The enzyme loading can be reduced to minimize the overall production cost. However, this often results in a lower ethanol yield or a longer residence time. The enzyme loading was decreased from 10 to 7.5 FPU/g WIS to study the effects on SSF. A small or no decrease in ethanol yield was seen in batch SSF with and without prehydrolysis (comparing experiments 1 and 2 with 17 and experiment 8 with 18 in Table 3). But there is a difference in residence time as lower enzyme dosage results in longer residence time to reach the final ethanol yield. This is probably due to that more enzymes being partially or completely deactivated, but also because the amount of active enzyme is lower. The greatest decrease in ethanol yield was seen in fed-batch SSF (comparing experiment 15 with 19 in Table 3). The difference between the different configurations disappears with the lower enzyme dosage (Fig. 5). This could indicate that a lower enzyme dosage is more favourable using the batch mode than fed-batch. One reason is that all enzymes are added in the beginning, which can result in unproductive binding of the enzyme on the initially added substrate. Using fed-batch, there is a risk that the enzymes will not efficiently hydrolyze the fed material.
|
|
|
Figure 5. Concentrations of glucose (filled symbols) and ethanol (open symbols) during different SSF experiments. Batch SSF with 10 FPU/g WIS (◊); batch SSF with 7.5 FPU/g WIS (○); batch SSF with 8 h prehydrolysis at 50°C with 7.5 FPU/g WIS (□), fed-batch SSF with an initial WIS load of 33% of the total WIS and a total feeding time of 16 h with 7.5 FPU/g WIS (∆). SSF, simultaneous saccharification and fermentation; WIS, water-insoluble solids. |
Since the ethanol yield was similar in all configurations at the lower enzyme dosage, the residence time in the various configurations is an important factor to establish the most suitable SSF mode. The time to reach high ethanol concentration is lower with fed-batch than with batch with lower enzyme dosage. The residence time is also similar to the batch with original enzyme loading. As the residence time is shorter using the fed-batch mode, this indicates that fed-batch is still a better alternative, even when the enzyme loading is lowered by 25%.
Conclusions
Prehydrolysis did not improve the ethanol yield from batch SSF. A higher ethanol yield was seen when using fed-batch than when using batch, indicating that fed-batch is a better alternative at high solids loading. When decreasing the enzyme dosage, a small or no decrease in ethanol yield was seen in batch SSF. The decrease in ethanol yield was greater during fed-batch SSF, resulting in similar ethanol yield in both batch and fed-batch. However, the residence time to achieve the final ethanol yield is shorter with fed-batch. This shows that fed-batch is the better alternative also at a lower enzyme loading.
Acknowledgments
The State Grid Corporation of China is most gratefully acknowledged for financial support of this project.
Conflict of Interest
None declared.
References
- IPCC. 2013. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change, Cambrige University Press, New York, NY.
- Carriquiry, M. A., X. Du, and G. R. Timilsina. 2011. Second generation biofuels: economics and policies. Energy Policy39:4222–4234.
- Bondesson, P.-M., M. Galbe, and G. Zacchi. 2013. Ethanol and biogas production after steam pretreatment of corn stover with or without the addition of sulphuric acid. Biotechnol. Biofuels6:11.
- Bondesson, P.-M., M. Galbe, and G. Zacchi. 2014. Comparison of energy potentials from combined ethanol and methane production using steam-pretreated corn stover impregnated with acetic acid. Biomass Bioenergy67:413–424.
- Bondesson, P.-M., A. Dupuy, M. Galbe, and G. Zacchi. 2015. Optimizing ethanol and methane production from steam-pretreated, phosphoric acid-impregnated corn stover. Appl. Biochem. Biotechnol.175:1371–1388. doi: 10.1007/s12010-014-1358-4
- Wingren, A., M. Galbe, and G. Zacchi. 2003. Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol. Prog.19:1109–1117.
- Hoyer, K., M. Galbe, and G. Zacchi. 2009. Production of fuel ethanol from softwood by simultaneous saccharification and fermentation at high dry matter content. J. Chem. Technol. Biotechnol.84:570–577.
- Humbird, D., A. Mohagheghi, N. Dowe, and D. J. Schell. 2010. Economic impact of total solids loading on enzymatic hydrolysis of dilute acid pretreated corn stover. Biotechnol. Prog.26:1245–1251.
- Jorgensen, H., J. Vibe-Pedersen, J. Larsen, and C. Felby. 2007. Liquefaction of lignocellulose at high-solids concentrations. Biotechnol. Bioeng.96:862–870.
- Öhgren, K., A. Rudolf, M. Galbe, and G. Zacchi. 2006. Fuel ethanol production from steam-pretreated corn stover using SSF at higher dry matter content. Biomass Bioenergy30:863–869.
- Varga, E., H. B. Klinke, K. Reczey, and A. B. Thomsen. 2004. High solid simultaneous saccharification and fermentation of wet oxidized corn stover to ethanol. Biotechnol. Bioeng.88:567–574.
- Kadić, A., B. Palmqvist, and G. Lidén. 2014. Effects of agitation on particle-size distribution and enzymatic hydrolysis of pretreated spruce and giant reed. Biotechnol. Biofuels7:77.
- Rosgaard, L., P. Andric, K. Dam-Johansen, S. Pedersen, and A. S. Meyer. 2007. Effects of substrate loading on enzymatic hydrolysis and viscosity of pretreated barley straw. Appl. Biochem. Biotechnol.143:27–40.
- Kristensen, J. B., C. Felby, and H. Jorgensen. 2009. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels2:11.
- Palmqvist, B., and G. Liden. 2012. Torque measurements reveal large process differences between materials during high solid enzymatic hydrolysis of pretreated lignocellulose. Biotechnol. Biofuels5:57.
- Weiss, N. D., J. J. Stickel, J. L. Wolfe, and Q. A. Nguyen. 2010. A simplified method for the measurement of insoluble solids in pretreated biomass slurries. Appl. Biochem. Biotechnol.162:975–987.
- Sluiter, A., B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton, et al. 2008. Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure, NREL, Golden, CO.
- Sluiter, A., B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, and D. Templeton. 2006. Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Laboratory analytical procedure, NREL, Golden, CO.
- Lu, Y., Y. Wang, G. Xu, J. Chu, Y. Zhuang, and S. Zhang. 2010. Influence of high solid concentration on enzymatic hydrolysis and fermentation of steam-exploded corn stover biomass. Appl. Biochem. Biotechnol.160:360–369.
- Hodge, D. B., M. N. Karim, D. J. Schell, and J. D. McMillan. 2008. Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Bioresour. Technol.99:8940–8948.
- Mohagheghi, A., M. Tucker, K. Grohmann, and C. Wyman. 1992. High solids simultaneous saccharification and fermentation of pretreated wheat straw to ethanol. Appl. Biochem. Biotechnol.33:67–81.
- Tengborg, C., M. Galbe, and G. Zacchi. 2001. Influence of enzyme loading and physical parameters on the enzymatic hydrolysis of steam-pretreated softwood. Biotechnol. Prog.17:110–117.
- Öhgren, K., J. Vehmaanperä, M. Siika-Aho, M. Galbe, L. Viikari, and G. Zacchi. 2007. High temperature enzymatic prehydrolysis prior to simultaneous saccharification and fermentation of steam pretreated corn stover for ethanol production. Enzyme Microb. Technol.40:607–613.
- Hoyer, K., M. Galbe, and G. Zacchi. 2013. The effect of prehydrolysis and improved mixing on high-solids batch simultaneous saccharification and fermentation of spruce to ethanol. Process Biochem.48:289–293.
- Palmqvist, B., M. Wiman, and G. Lidén. 2011. Effect of mixing on enzymatic hydrolysis of steam-pretreated spruce: a quantitative analysis of conversion and power consumption. Biotechnol. Biofuels4:10.
- Rudolf, A., M. Alkasrawi, G. Zacchi, and G. Lidén. 2005. A comparison between batch and fed-batch simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme Microb. Technol.37:195–204.
- Zhang, M., F. Wang, R. Su, W. Qi, and Z. He. 2010. Ethanol production from high dry matter corncob using fed-batch simultaneous saccharification and fermentation after combined pretreatment. Bioresour. Technol.101:4959–4964.
- Nilsson, A., M. J. Taherzadeh, and G. Lidén. 2001. Use of dynamic step response for control of fed-batch conversion of lignocellulosic hydrolyzates to ethanol. J. Biotechnol.89:41–53.
- Rudolf, A., M. Galbe, and G. Liden. 2004. Controlled fed-batch fermentations of dilute-acid hydrolysate in pilot development unit scale. Appl. Biochem. Biotechnol.113:601–617.
- Hoyer, K., M. Galbe, and G. Zacchi. 2010. Effects of enzyme feeding strategy on ethanol yield in fed-batch simultaneous saccharification and fermentation of spruce at high dry matter. Biotechnol. Biofuels3:14.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?