Abstract
Background
Late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (CMR) has been recommended to distinguish Tako-tsubo cardiomyopathy (TTC) from either acute myocardial infarction or myocarditis.
Method
44 consecutive patients with confirmed Mayo Clinic criteria for TTC underwent CMR imaging at 1.5 Tesla during the acute phase. 10 patients who had CMRI to exclude scar related ventricular tachycardia, and had negative studies, were used as negative controls. LGE was quantitated at two signal intensity thresholds (CircleCVi software) at > 2 and > 5 standard-deviations (SD) above reference myocardium, and compared to biomarkers.
Findings
Mean door-to-CMR time was 57 hours. 18 patients (41%) had LGE > 2 SD localized to the area of abnormal wall motion, representing 28.9 ± 11.2% LV mass. In 16 of these 18 patients (89%) LGE signal intensity was > 5 SD above normal myocardium, representing 12.1 ± 10% LV mass. LGE signal intensity was significantly greater in TTC than in matched controls (p < 0.05) but lower than in STEMI patients (p < 0.05). Mean troponin was significantly higher in LGE positive patients (2.5 ± 1.8 vs 4.4 ± 6.9, p = 0.001). Mean ejection fraction (EF) by CMR was 45% ± 8.7 in LGE-negative, and 40% ± 7.1 in LGE-positive patients (p = 0.37). Recovery of segmental function was confirmed at follow-up, mean EF was 59% in both groups.
Conclusion
LGE was present in 41% of cases of TTC, 89% of which had intense enhancement > 5 SD above normal myocardium. Presence of LGE was associated with worse myocardial injury in the acute setting, with no difference in recovery of function.
Keywords
Tako-tsubo;Stress cardiomyopathy;Cardiac magnetic resonance;Infarct quantitation;Late gadolinium enhancement;Speckle tracking strain echocardiography
1. Introduction
The “Tako-tsubo” cardiomyopathy (TTC), also known as apical ballooning syndrome or stress cardiomyopathy [1], is an emerging clinical syndrome presenting with acute chest pain or dyspnoea, associated with new ST-T segment abnormalities, regional transient myocardial dysfunction, typically localized at left ventricular (LV) apex and usually extended beyond a single vessel territory, serum cardiac enzyme release and absence of significant coronary lesions at coronary angiography [2]. Among patients presenting with clinically suspected acute coronary syndrome (ACS), its prevalence is reported to range between 1.2%–2.0% and it occurs almost exclusively in postmenopausal women, being often, but not exclusively, associated with emotional or physical stress [3] ; [4]. Tako-tsubo cardiomyopathy now accounts for up to 1% of admissions for suspected myocardial infarction in Japan, and is increasingly recognised in the West owing to primary coronary intervention, accounting for up to 1 in 30 cases of primary angioplasty [5] ; [6]. The pattern of left ventricular dysfunction has been labelled in Japanese as “Tako-tsubo” cardiomyopathy due to its resemblance to the traditional Japanese octopus-pot [7], and elsewhere in the world has also been termed “transient left ventricular apical ballooning” and “stress cardiomyopathy” [1] ; [8]. Cardiac MRI has been used to evaluate myocardial viability and all patients had coronary angiography to rule out atherosclerotic CAD as a substrate for the ventricular dysfunction [8]. Given that positive serum troponin and oedema occur in patients with Tako-tsubo cardiomyopathy, we hypothesised that Late Gadolinium Enhancement (LGE) may be detected by CMR when performed in the acute phase, and LGE has been reported in TTC cases by other centres but this has not been evaluated in a quantitative manner [9] ; [10]
2. Method
44 consecutive patients with confirmed clinical, ECG, angiographic and left ventriculographic findings of TTC using the Mayo Clinic criteria were prospectively enrolled in the study at the time of initial hospitilisation [11] [ Fig. 1]. All patients underwent invasive coronary angiography, confirming the absence of significant epicardial coronary artery stenosis with presence of wall motion defect at ventriculography (either ‘typical’ apical ballooning, or ballooning of the mid-segments of the anterior wall, referred to as ‘mid-wall variant’). In all patients screening for pheochromocytoma was performed with both serum and urine metanephrines [12].
|
|
|
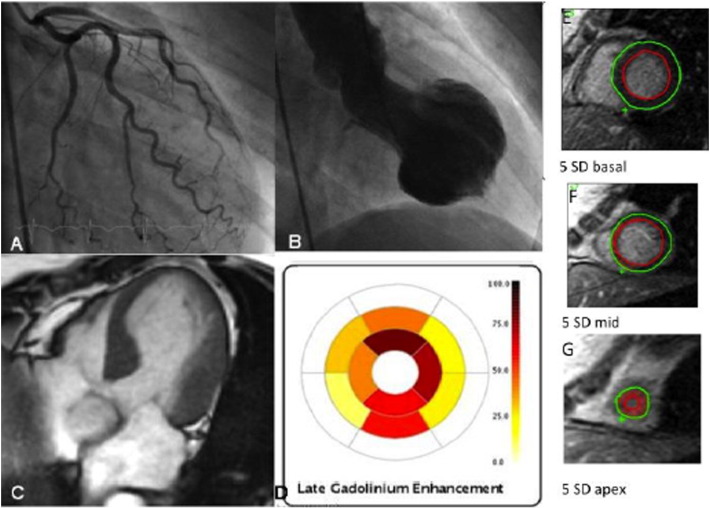
Fig. 1. Patient with normal coronary arteries (A), and the classic takotsubo “apical ballooning” wall motion abnormality at ventriculography (B). CMR end-systolic 4-chamber view showing the apical ballooning on cine imaging (C), with quantitative LGE represented by an AHA16-segment model polar plot (D). E-G show the signal analysis contours, with signal > 5 SD above remote myocardium, most obvious in the apical slice (G). |
CMR was performed in the acute phase as soon as the patient was clinically stable, using a 1.5 Tesla system (Signa Twinspeed, GE Healthcare, Milwaukee, WI, USA) with an 8-element cardiac phased array coil. Steady state free precession (SSFP) images were acquired in multiple planes using the following protocol: TE 1.5 ms, TR 3.4 ms, flip angle 45, receiver bandwidth ± 125 kHz, FOV 35 cm, slice thickness 8 mm, slice gap 2 mm, acquisition matrix 224 × 224, number of averages = 1, ECG gating, k-space segmentation 16 views per segment. Twenty cardiac phases per slice were reconstructed. T2-weighted (T2w) oedema imaging was performed in the same short axis slice locations using a fat-suppressed double inversion recovery sequence.
Late gadolinium enhancement (LGE) imaging was performed in the same slice locations as SSFP images, at 10 and 20 min following intravenous administration of 0.2 mmol/kg of gadolinium-DTPA using a segmented inversion recovery fast gradient echo sequence with the following parameters: TE 2.8 ms, TR 6 ms, TI 170–250 ms (depending on time elapsed since injection), flip angle 20, receiver bandwidth ± 31 kHz, FOV 35 cm, slice thickness 8 mm, slice gap 2 mm, acquisition matrix 256 × 160, number of averages = 2, ECG gating with k-space segmentation of 24 views per segment. All images were acquired at end expiration using respiratory bellows to confirm compliance with breathing instructions. Measures of ventricular function were derived with MASS software (MEDis Medical Imaging Systems, Leiden, NL) on an Advantage Windows workstation (GE Healthcare, Milwaukee, WI, USA). In cases where quantitative assessment of the right ventricle was required, imaging was performed using the modified RV short axis series [13].
Quantification of late gadolinium enhancement was assessed as recommended by the International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis [14] ; [15]. Using a crossplane localizer, the regions of LGE were cross-referenced to the corresponding areas of hypokinesis on SSFP images, and visually analysed for presence of increased signal intensity of LGE images. LGE was quantitated using the cvi42 software (v4.1.5, Circle Cardiovascular Imaging, Calgary, Canada). Endocardial and epicardial contours were manually drawn in the short axis and two long axis frames with careful reference to SSFP images. A region-of-interest (ROI) was drawn in an area of reference normal myocardium having normal wall motion and no edema on T2 images. Signal thresholds of both > 2 SD and > 5 SD above the reference myocardium were applied, with an automated signal detection algorithm to identify areas of abnormal myocardium (Fig. 1 ; Fig. 2). Extent of LGE was normalized to LV mass for each case, and expressed as a percentage (LGE%) (see Fig. 3).
|
|
|
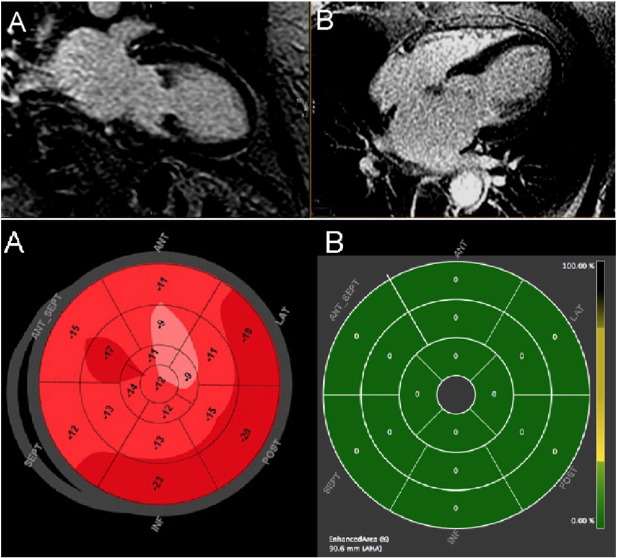
Fig. 2. TTC without LGE. Upper: CMR LGE images in 2-chamber and 4-chamber views, showing absence of late gadolinium enhancement. Lower: Quantitative analysis of 2D speckle strain (panel A) and late gadolinium enhancement (panel B) demonstrated in matching segmental polar maps (AHA segments). |
|
|
|
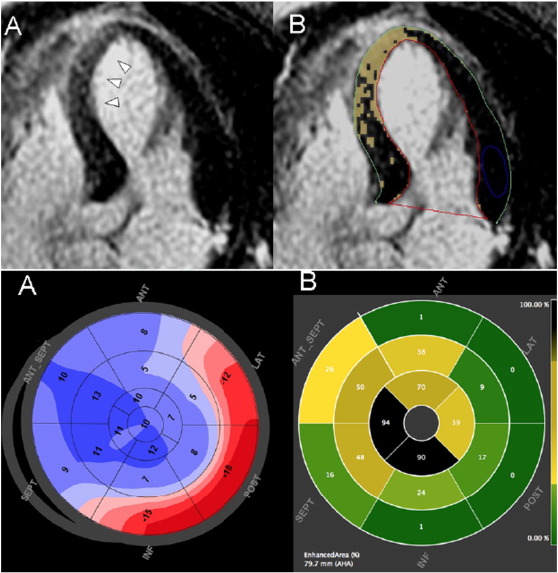
Fig. 3. TTC with LGE: Upper: 4chamber view showing increased signal intensity in the septum and apical segments (panel A, arrows), and quantitative signal intensity analysis with automated colour overlay (panel B) showing > 2 standard deviations above normal reference myocardium in the unaffected segments. Lower: Polar maps of 2D speckle strain (panel A) and LGE-CMR (panel B) demonstrating concordance between the segments with markedly abnormal strain and the degree of enhancement by CMR. |
When available, echocardiography with 2D speckle tracking for global longitudinal strain was performed during the acute admission, using a GE E9 system and quantitation with Echo-PAC software (Fig. 2).
Controls: 10 patients who had CMR to investigate supra-ventricular tachycardia, with normal CMR scans and no LGE were used as negative controls. A cohort of 10 patients post-ST Elevation myocardial infarction (STEMI) were used as positive controls, and signal ratios of infarcted myocardium to normalized (non-infarct related) myocardium were measured using cvi42 software. All controls were scanned with an identical protocol and LGE was quantitatively assessed using the same method.
2.1. Statistics
Categorical variables are expressed as number and percentage of patients. Continuous data are estimated as mean (SD) or as median. Differences between groups were assessed using the 2-tailed Fisher exact test. Statistical tests were performed using Medcalc and GraphPad Prism (V6.0, LaJolla, CA). The presence of LGE, LGE% and change in left ventricular ejection fraction (LVEF) at follow up were compared using a Students t-test. Statistically significant changes were designated to occur with a 2-sided p value of < 0.05.
3. Results
The mean age of patients with TTC was 66 years (range 41–87 years), 91% percent female, of which 90% were post-menopausal (Table 1).
| Peak TnI μg/L | Sex | Age | Door-to-MRI (hours) | RWMA variant | LVEF acute | LVEF f/up | Quantitative LGE as %LV mass | ||
|---|---|---|---|---|---|---|---|---|---|
| > 2 SD | > 5 SD | ||||||||
| LGE − ve | 1.1 | F | 83 | n/a | Apical | 28 | 55 | 0 | 0 |
| 2.3 | F | 44 | 96 | Mid | 50 | 57 | 0 | 0 | |
| 0.1 | F | 82 | 76 | Apical | 50 | n/a | 0 | 0 | |
| 0.7 | F | 69 | 110 | Apical | 48 | 54 | 0 | 0 | |
| 2.2 | F | 41 | 47 | Mid | 40 | 50 | 0 | 0 | |
| 0.8 | F | 67 | 61 | Apical | 44 | 56 | 0 | 0 | |
| 1.9 | F | 87 | 144 | Apical | 30 | 60 | 0 | 0 | |
| 6.4 | F | 79 | n/a | Apical | 50 | n/a | 0 | 0 | |
| 3.4 | F | 76 | n/a | Mid | 57 | n/a | 0 | 0 | |
| 4.7 | F | 46 | 15 | Mid | 39 | n/a | 0 | 0 | |
| 3.1 | F | 57 | 28 | Mid | 55 | 67 | 0 | 0 | |
| 3.2 | F | 62 | 92 | Mid | 42 | 66 | 0 | 0 | |
| 1.6 | F | 60 | 65 | Apical | 48 | 66 | 0 | 0 | |
| 4.3 | F | 76 | 16 | Apical | 49 | 59 | 0 | 0 | |
| 0.2 | F | 70 | 46 | Apical | 44 | 56 | 0 | 0 | |
| 0.4 | F | 67 | n/a | Mid | 52 | 58 | 0 | 0 | |
| 2.4 | F | 58 | 45 | Apical | 35 | 67 | 0 | 0 | |
| 0.5 | F | 67 | 78 | Apical | 57 | n/a | 0 | 0 | |
| 1 | F | 69 | 85 | Mid | 54 | 69 | 0 | 0 | |
| n/a | F | 66 | 73 | Apical | 46 | 51 | 0 | 0 | |
| 5.5 | F | 66 | 76 | Apical | 28 | 55 | 0 | 0 | |
| 3 | F | 70 | 108 | Mid | 55 | 59 | 0 | 0 | |
| 1.6 | F | 63 | 6 | Apical | 36 | 59 | 0 | 0 | |
| 3.9 | F | 65 | 44 | Mid | 38 | 54 | 0 | 0 | |
| 6.4 | M | 64 | 115 | Mid | 47 | n/a | 0 | 0 | |
| 2.6 | M | 71 | 53 | Mid | 53 | n/a | 0 | 0 | |
| LGE + ve | 0.2 | F | 67 | 33 | Apical | 33 | n/a | 35.15 | 0 |
| 1.5 | F | 59 | 86 | Apical | 43 | 19.27 | 0 | ||
| 1.7 | F | 76 | 37 | Apical | 51 | 25.01 | 3.54 | ||
| 7.5 | F | 63 | 63 | Apical | 32 | 62 | 30.62 | 11.43 | |
| 3.9 | F | 60 | n/a | Apical | 25 | n/a | 32.65 | 11.62 | |
| 3.5 | F | 68 | 27 | Mid | 36 | 58 | 34.8 | 24 | |
| 0.2 | M | 63 | 115 | Apical | 45 | 52 | 36.6 | 24.3 | |
| 0.7 | F | 62 | 15 | Apical | 48 | 67 | 24.42 | 24.47 | |
| 30 | F | 68 | 43 | Apical | 36 | 43 | 46 | 24.6 | |
| 1.2 | F | 63 | 48 | Mid | 50 | 64 | 46.9 | 28.75 | |
| 1.2 | F | 70 | 8 | Apical | 38 | 69 | 48.61 | 29.98 | |
| 3 | F | 83 | 76 | Api | 45 | 67 | 24.3 | 10.1 | |
| 2.6 | F | 73 | 42 | Api | 38 | n/a | 18.4 | 4.5 | |
| 3.1 | F | 66 | 50 | Mid | 40 | 56 | 11.2 | 3.3 | |
| 0.2 | M | 77 | 22 | Api | 38 | n/a | 29.3 | 3 | |
| 4.4 | F | 76 | 10 | Api | 35 | 69 | 13.9 | 1.5 | |
| 4.3 | F | 60 | 17 | mid | 49 | n/a | 18 | 14.4 | |
| 10 | F | 50 | 71 | Api | 44 | 53 | 22.4 | 7.2 | |
F = female; M = male; TnI = troponin I; RWMA = Regional wall motion abnormality; LVEF = left ventricular ejection fraction by CMR; f/up = follow up; LGE = − late gadolinium enhancement; SD = standard deviation.
Identifiable precipitating events were recorded in 82%; such events included new diagnoses of malignancy, orthopaedic trauma, attendance at daughters funeral, medical illness (diverticulitis, pneumonia), and two cases of emotive discussions. In 18% events were not recorded although it is recognised that a proportion of patients with TTC may not have a clearly identifiable precipitating event [1]. One female patient, who presented with pericardial tamponade (due to malignant effusion) and normal wall motion on baseline echocardiography, developed TTC acutely after pericardiocentesis, which is a previously unreported precipitating event. One patient had significant left ventricular outflow tract obstruction, with systolic anterior motion of the mitral valve. Screening for pheochromocytoma revealed no abnormal results in this cohort.
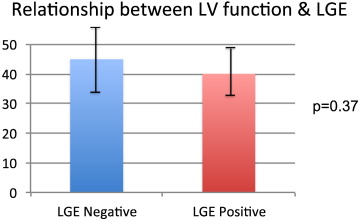
Cardiac magnetic resonance was performed in the acute phase with a mean door-to-CMR time of 57 h. Wall motion defects were present in all cases; the typical “apical ballooning” wall motion defect was present in 28 cases, and a midwall variant in 16 cases (Table 1). There was no significant difference in LVEF between these two groups (mean 40% vs 44% respectively, p = NS). 18 patients (41%) had LGE > 2 SD localized to the area of abnormal wall motion, representing 28.9 ± 11.2% LV mass. In 16 of these 18 patients (89%) LGE was > 5 SD above normal myocardium, representing 12.1 ± 10% LV mass. LGE signal intensity of > 5 SD in this condition has not been previously described. The pattern of LGE differed to that seen in both ischemia and myocarditis, in that it involved the full thickness of the affected myocardium in a diffuse manner, rather than the sub-endocardial high signal present in ischaemic injury, or the sub-epicardial layers typically seen in myocarditis. Of interest, the patient with resuscitated sudden cardiac death also exhibited right ventricular involvement manifest by dyskinesia in the mid-segments of the right ventricular free wall, which recovered on subsequent imaging. Mean ejection fraction (EF) by CMR was 45% ± 8.7 in LGE-negative, and 40% ± 7.1 in LGE-positive patients (p = 0.37). Recovery of segmental function was confirmed at follow-up, mean EF was 59% in both groups.
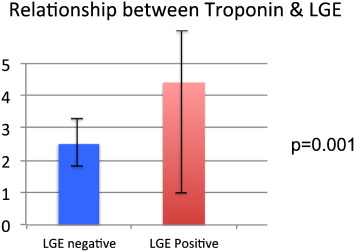
Mean troponin was significantly higher in LGE positive patients (2.5 ± 1.8 vs 4.4 ± 6.9, p = 0.001) [ Fig. 4] Mean ejection fraction (EF) by CMR was 45% ± 8.7 in LGE-negative, and 40% ± 7.1 in LGE-positive patients (p = 0.37) [ Fig. 5]. All patients experienced full recovery of function at follow-up (**p < 0.01).
|
|
|
Fig. 4. Bargraph comparing the troponin levels of TTC patients with and without LGE. |
|
|
|
Fig. 5. Bargraph comparing LVEF between TTC patients with and without LGE. |
LGE positive patients had a higher serum peak troponin-I in the acute phase (2.5 ± 1.8 vs 4.4 ± 6.9, p = 0.001) [ Fig. 4]. Of note, LGE signal intensity in TTC patients was significantly greater than in controls (p < 0.05) but significantly lower than in STEMI patients (p < 0.05).
At 6 months follow-up all patients had full resolution of left ventricular systolic function as assessed by echocardiography; acute-phase mean EF was 43% ± 8.5% (range 25–51%), and at follow-up mean EF 64.4 +/− 7%, (p = 0.001). This recovery of left ventricular function is consistent with the natural history of TTC [16]. 16 patients also received follow-up CMR at a mean duration of 8 months (range 2-27 months) after the index event. Ejection fraction had normalized in all cases (mean EF 43 +/− 10%, follow-up mean 60.2 +/− 7%, p = 0.001). In all patients with LGE, enhancement fully resolved on follow-up CMR with normalization of signal intensity and no residual LGE detectable by quantitative signal analysis.
The majority of patients were discharged on medical therapy of angiotensin converting enzyme inhibitors, beta-blockers, and aspirin, although there is little evidence to guide clinicians in the effectiveness of such therapies. One LGE-positive patient received an implantable defibrillator as secondary prevention for resuscitated sudden cardiac death, and could not therefore undergo a follow-up CMR study.
4. Discussion
Myocardial late gadolinium enhancement (LGE) does occur in the TTC syndrome, in contrast to initial reports which propose the absence of LGE as a diagnostic criterion [11]. In this single-centre series, LGE was confirmed using quantitative signal intensity analysis in 41% of TTC patients, occurring in a diffuse pattern through the areas of myocardial stunning, 89% of which were > 5 SD above normal myocardium. Late gadolinium enhancement in TTC may represent diffuse myocyte damage, which would be consistent with the higher serum troponin levels in the LGE positive group. This finding gives some insight into the nature of myocardial damage occurring in these patients.
4.1. Late gadolinium enhancement (LGE)
The LGE was diffuse in appearance compared to adjacent normal myocardium. This pattern of LGE differed to that seen in ischemia or myocarditis; ischemia produces bright subendocardial or transmural enhancement whereas myocarditis produces midwall or sub-epicardial enhancement. In TTC the LGE appears to occur in a segmental distribution associated with abnormal wall motion, and resolves with time. The pathophysiology of myocardial LGE results from the inherent relative differences in the volume of distribution of gadolinium between normal and abnormal myocardium [17] ; [18]. The distribution of gadolinium is confined to the extracellular and interstitial space, i.e. it does not penetrate intact myocardial cell membranes. In the normal myocardium, the interstitial space constitutes about 15% of the total myocardial volume. Changes to the interstitium, such as oedema or infiltration, increase the volume of distribution allowing a larger amount of gadolinium to penetrate into the tissue. Myocyte necrosis results in loss of cell membrane integrity, and allows intracellular accumulation of gadolinium which explains the LGE seen in myocardial infarction [14]; [19]; [20] ; [21].
Rolf et al. reported 15 patients with TTC using serial endomyocardial biopsy correlated with CMR imaging to explore the differences in the extent of oedema, necrosis, and fibrosis [18]. In that report LGE was assessed dichotomously as either present or absent, but the degree of LGE was not quantified. Immunohistochemical analysis of endomyocardial biopsies was reported to show disorganization of contractile and cytoskeletal proteins, elevated extracellular matrix, but not cell death. The authors concluded that the diffuse LGE pattern seen their study resulted from expansion of extracellular matrix and acute fibrosis, rather than due to cell necrosis or oedema. In the present study, however, the trend to higher mean TnI values in patients with diffuse LGE implies a greater degree of myocyte disruption or death in those patients. Troponin is specific for myocardial cell damage, and any positively detectable circulating serum troponin (> 99th percentile upper limit of normal) reflects microscopic zones of myocardial necrosis, as defined in the ESC/ACCF/AHA/WHF task force definition. The positive troponin in these patients suggests myocyte damage is occurring, possibly in a diffuse manner within the affected area of myocardial stunning. A larger sample cohort would be required to test the validity of this observation.
It should be noted, also, that endomyocardial biopsy as performed by Rolf et al. targets the sub-endocardium with limited penetration into the myocardium, and the diffuse pattern of LGE seen in the present study was not subendocardial in nature. It is possible, therefore, that endomyocardial biopsy may not have detected regions of mid-wall myocyte necrosis, or that other factors may be involved in the mechanism of diffuse LGE. To further clarify this issue, an autopsy case with a prior in-vivo CMR would be required to further correlate the true nature of LGE in TTC; much as demonstrated by Moon et al. for hypertrophic cardiomyopathy in which the histological basis for LGE for that condition was defined [22]. Partial or complete resolution of LGE over time has been well documented in myocarditis [15]; [23] ; [24], the mechanism of which is not entirely understood. The same appears to be true for LGE in the Tako-tsubo cardiomyopathy, in which LGE may be a manifestation of multiple factors including cellulary injury, oedema, expanded extracellular matrix and acute fibrosis.
The present results suggest that absence of LGE should not be a defining criterion for TTC, in contrast to previous reports [8]; [11]; [16] ; [24]. LGE signal intensity of up to 3 SD has been reported previously [25], including in midwall-variant TTC [9] ; [10].
In our study, however, LGE was observed at increased signal intensities > 5 SD using identical methods and software as previous studies. We postulate two reasons for the increase in LGE in our study. Firstly our cohort had scans early in the acute phase, and secondly the dose of gadolinium in our protocol is higher as compared to the above study (0.2 mmol/kg vs 0.1 mmol/kg). It is unclear from this small observational study whether presence of LGE confers a worse prognosis with altered myocardial recovery or greater a propensity for recurrence. There was however no difference in follow-up LV function as assessed by echocardiography or CMR in this group.
4.2. Wall motion
We noted that the LGE was pronounced in the area of wall motion abnormality. The mid-wall variant formed 45% of this patient cohort, which is similar to previous reports [8] ; [26]. There was no significant difference in ejection fraction, or propensity for diffuse LGE, between the mid-wall and apical variants; but the study was not statistically powered to detect such a difference. The patient with resuscitated sudden cardiac death also exhibited right ventricular involvement, as manifested by dyskinesia of the right ventricular free wall, resolving on follow-up imaging. Right ventricular involvement has been reported to be associated with a more severe clinical manifestation including acutely decompensated heart failure or shock [11]. There is debate as to the relevance of various wall motion defects in TTC, which simply may represent partial recovery of some segments. Recently, opinion-leaders stated that “subclassifying and renaming this cardiomyopathy according to specific LV contraction patterns could lead to more confusion” [1]. These authors recommended researchers categorize the diverse forms of wall motion abnormalities under the broad term of “Tako-tsubo cardiomyopathy”.
[17].
We believe this is an accurate, quantitative and carefully-performed study which has highlighted subtle but definite changes in myocardial tissue characterization, detected as a result of MRI very early in the clinical course of Tako-tsubo patients (short door-to-MRI time, allowing detection of diffuse LGE signal in these patients). This novel observation adds to our understanding of the pathophysiology in Tako-tsubo cardiomyopathy.
5. Conclusion
Late gadolinium enhancement does occur in the Tako-tsubo cardiomyopathy syndrome, in a diffuse manner, with signal intensity > 5 SD in some patients. The pattern of LGE is different to that seen in ischemia, myocarditis, or infiltrative disorders of the myocardium. LGE occurs in the affected area of myocardial stunning. “Absence of LGE” per se cannot therefore be used as a criterion for the diagnosis of TTC. Further collaborative multi centre studies, with agreement on imaging protocols, assessment of perfusion, follow-up timing and core-lab interpretation of CMR images and LGE quantification, coupled with biomarker and immunohistochemical analyses, are required to further define the natural history and aetiology of this disorder and of the pathophysiology underling CMR enhancement patterns. [18].
Conflicts of interest
None to declare.
Funding
A/Prof. Hamilton-Craig was funded by the DSITIA Smart Futures Fellowship Early Career GrantISF783 (QLD Government), the National Heart Foundation of Australia, Scholarship Grant #PC08B4054, and the Washington-Queensland Trans-Pacific Fund (inagural grant recipient 2010).
References
- [1] S.W. Sharkey, J.R. Lesser, M.S. Maron, B.J. Maron; Why not just call it tako-tsubo cardiomyopathy a discussion of nomenclature; J. Am. Coll. Cardiol., 57 (13) (2011 Mar 29), pp. 1496–1497 (PubMed PMID: WOS:000288695300011. English)
- [2] K.A. Bybee, T. Kara, A. Prasad, A. Lerman, G.W. Barsness, R.S. Wright, et al.; Systematic review: Transient left ventricular apical ballooning: A syndrome that mimics ST-segment elevation myocardial infarction; Ann. Intern. Med., 141 (11) (2004 Dec 7), pp. 858–865 (PubMed PMID: WOS:000225530700006. English)
- [3] M. Gianni, F. Dentali, A.M. Grandi, G. Sumner, R. Hiralal, E. Lonn; Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review; Eur. Heart J., 27 (13) (2006 Jul), pp. 1523–1529 (PubMed PMID: 16720686)
- [4] V. Kurowski, A. Kaiser, K. von Hof, D.P. Killermann, B. Mayer, F. Hartmann, et al.; Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis; Chest, 132 (3) (2007 Sep), pp. 809–816 (PubMed PMID: 17573507)
- [5] W.J. Desmet, B.F. Adriaenssens, J.A. Dens; Apical ballooning of the left ventricle: first series in white patients; Heart, 89 (9) (2003 Sep), pp. 1027–1031 (PubMed PMID: 12923018. Pubmed Central PMCID: 1767823)
- [6] P.S. Seth, G.P. Aurigemma, J.M. Krasnow, D.A. Tighe, W.J. Untereker, T.E. Meyer; A syndrome of transient left ventricular apical wall motion abnormality in the absence of coronary disease: a perspective from the United States; Cardiology, 100 (2) (2003), pp. 61–66 (PubMed PMID: 14557691)
- [7] S. Kurisu, H. Sato, T. Kawagoe, M. Ishihara, Y. Shimatani, K. Nishioka, et al.; Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction; Am. Heart J., 143 (3) (2002), pp. 448–455
- [8] I.S. Wittstein, D.R. Thiemann, J.A.C. Lima, K.L. Baughman, S.P. Schulman, G. Gerstenblith, et al.; Neurohumoral features of myocardial stunning due to sudden emotional stress; New Engl. J. Med., 352 (6) (2005 Feb 10), pp. 539–548 (PubMed PMID: WOS:000226862100004. English)
- [9] P. Alter; Mystery of myocardial midwall late enhancement?; Int. J. Cardiovasc. Imaging, 30 (8) (2014 Dec), pp. 1569–1570 (PubMed PMID: 25117644. Epub 2014/08/15)
- [10] K. Kato, Y. Sakai, I. Ishibashi, Y. Kobayashi; Recurrent mid-ventricular takotsubo cardiomyopathy; Int. J. Cardiovasc. Imaging, 30 (8) (2014 Dec), pp. 1417–1418 (PubMed PMID: 24929873. Epub 2014/06/16)
- [11] A. Prasad, A. Lerman, C.S. Rihal; Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction; Am. Heart J., 155 (3) (2008 Mar), pp. 408–417 (PubMed PMID: 18294473)
- [12] J. Abraham, J.O. Mudd, N.K. Kapur, K. Klein, H.C. Champion, I.S. Wittstein; Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists; J. Am. Coll. Cardiol., 53 (15) (2009 Apr 14), pp. 1320–1325 (PubMed PMID: 19358948)
- [13] W.E. Strugnell, I.R. Slaughter, R.A. Riley, A.J. Trotter, H. Bartlett; Modified RV short axis series—a new method for cardiac MRI measurement of right ventricular volumes; J. Cardiovasc. Magn. Reson., 7 (5) (2005), pp. 769–774 (PubMed PMID: 16353437)
- [14] A.M. Beek, O. Bondarenko, F. Afsharzada, A.C. van Rossum; Quantification of late gadolinium enhanced CMR in viability assessment in chronic ischemic heart disease: a comparison to functional outcome; J. Cardiovasc. Magn. Reson., 11 (2009), p. 6 (PubMed PMID: 19272147. Pubmed Central PMCID: 2657135)
- [15] M.G. Friedrich, U. Sechtem, J. Schulz-Menger, G. Holmvang, P. Alakija, L.T. Cooper, et al.; Cardiovascular magnetic resonance in myocarditis: a JACC white paper; J. Am. Coll. Cardiol., 53 (17) (2009), pp. 1475–1487
- [16] A. Crean, J.P. Greenwood, S. Plein; Contribution of noninvasive imaging to the diagnosis and follow-up of takotsubo cardiomyopathy; JACC Cardiovasc. Imaging, 2 (4) (2009 Apr), pp. 519–521 (PubMed PMID: 19580736)
- [17] H. Arheden, M. Saeed, C.B. Higgins, D.W. Gao, J. Bremerich, R. Wyttenbach, et al.; Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats; Radiology, 211 (3) (1999), p. 698
- [18] A. Rolf, H.M. Nef, H. Möllmann, C. Troidl, S. Voss, G. Conradi, et al.; Immunohistological basis of the late gadolinium enhancement phenomenon in tako-tsubo cardiomyopathy; Eur. Heart J., 30 (13) (2009), pp. 1635–1642
- [19] R.C. Kim, K. Judd, R., C.A. Higgins; Assessment of myocardial viability by contrast enhancement; Cardiovascular MRI and MRA 1ed, Lippincot Williams & Wilkins, Philadelphia (2003), pp. 209–236
- [20] K. Thygesen, J.S. Alpert, H.D. White; Joint ESCAAHAWHFTFftRoMI. Universal definition of myocardial infarction; J. Am. Coll. Cardiol., 50 (22) (2007), pp. 2173–2195
- [21] J.C. Weaver, J.A. McCrohon; Contrast-enhanced cardiac MRI in myocardial infarction; Heart Lung Circ., 17 (4) (2008 Aug), pp. 290–298 (PubMed PMID: 18294909)
- [22] J.C. Moon, E. Reed, M.N. Sheppard, A.G. Elkington, S.Y. Ho, M. Burke, et al.; The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy; J. Am. Coll. Cardiol., 43 (12) (2004 Jun 16), pp. 2260–2264 (PubMed PMID: 15193690)
- [23] P.A. Monney, N. Sekhri, T. Burchell, C. Knight, C. Davies, A. Deaner, et al.; Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis; Heart, 97 (16) (2011 Aug), pp. 1312–1318 (PubMed PMID: WOS:000292914900007. English)
- [24] K.H. Stensaeth, E. Fossum, P. Hoffmann, A. Mangschau, P.T. Skretteberg, N.E. Klow; Takotsubo cardiomyopathy in acute coronary syndrome; clinical features and contribution of cardiac magnetic resonance during the acute and convalescent phase; Scand. Cardiovasc. J., 45 (2) (2011 Apr), pp. 77–85 (PubMed PMID: 20979536)
- [25] I. Eitel, F. von Knobelsdorff-Brenkenhoff, P. Bernhardt, I. Carbone, K. Muellerleile, A. Aldrovandi, et al.; Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy; JAMA, 306 (3) (2011 Jul 20), pp. 277–286 (PubMed PMID: 21771988)
- [26] T. Yoshida, T. Hibino, T. Fujimaki, M. Oguri, K. Kato, K. Yajima, et al.; Transient mid-ventricular ballooning syndrome complicated by syncope: a variant of tako-tsubo cardiomyopathy; Int. J. Cardiol., 135 (1) (2009 Jun 12), pp. e20–e23 (PubMed PMID: 18582968)
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?