Abstract
The purpose of this article is to study the impact of oxyfuel combustion applied to a rotary kiln producing lime. Aspects of interest are product quality, energy efficiency, stack gas composition, carbon dioxide emissions, and possible benefits related to carbon dioxide capture. The method used is based on multicomponent chemical equilibrium calculations to predict process conditions. A generic model of a rotary kiln for lime production was validated against operational data and literature. This predicting simulation tool is used to calculate chemical compositions for different recirculation cases. The results show that an oxyfuel process could produce a high-quality lime product. The new process would operate at a lower specific energy consumption thus having also a reduced specific carbon dioxide emission per ton of product ratio. Through some processing, the stack gas from the new process could be suitable for carbon dioxide transport and storage or utilization. The main conclusion of this paper is that lime production with an oxyfuel process is feasible but still needs further study.
Introduction
It is the purpose of this article to study the impact of oxyfuel combustion applied to a rotary kiln- producing lime. Aspects of interest are product quality, energy efficiency, stack gas composition, carbon dioxide emissions, and possible benefits related to carbon dioxide capture.
Oxyfuel combustion is combustion of a fuel with pure oxygen or a mixture of oxygen, water, and carbon dioxide [1] in contrast to conventional combustion which is done with air. This oxyfuel technology study involves combustion with pure oxygen and flue gas recirculation.
Lime (calcium oxide, CaO) is produced by calcination of limestone, containing a high concentration of calcium carbonate (CaCO3). Limestone is an abundant natural raw material. Lime is used for environmental purposes, e.g., waste neutralization or flue gas desulphurization, and in industrial processes, e.g., for formation of metallurgical slags or for production of paper pigments.
The method used is based on multicomponent chemical equilibrium calculations to predict process conditions. A predicting simulation tool is used to model processes and calculate chemical compositions for different cases. A generic model of a rotary kiln for lime production was validated against operational data and literature and used to examine different oxyfuel recirculation cases.
Oxyfuel technology applied to lime production would cause several major changes to the lime plant. The implications of all these changes, e.g., air separation unit, new piping, and fans for gas recirculation, are not discussed in this work.
Conventional lime production in rotary kilns
Lime is produced by calcination of calcium carbonates in industrial kilns. The mineral calcite containing the calcium carbonates is the main component in naturally abundant limestone. The limestone is quarried or mined, mechanically pretreated and delivered to the lime plant. One of the most common kiln types is the rotary kiln.
Calcination is an endothermic reaction requiring heat to evolve gaseous carbon dioxide from the calcite to form lime [2].
|
|
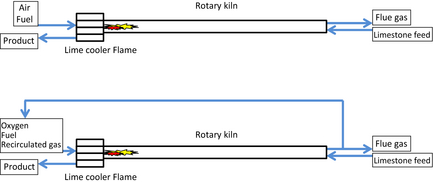
The calcination starts between 800°C and 900°C and the operational solid temperature usually reaches 1000–1200°C. The calcination temperature is dependent on the partial pressure of carbon dioxide in the kiln. A simplified illustration of a rotary kiln for lime production is presented in Figure 1. The upper setup shows the conventional air-fired process used as a reference case in this work. The lower setup shows the oxyfuel process with added air separation unit and flue gas recirculation are used for simulation cases in this work.
|
|
|
Figure 1. Simplified illustration of conventional and oxyfuel rotary kiln technology for lime production. |
The limestone is fed in the upper end. The feed is usually a 10–40 mm fraction of the limestone produced in the quarry. A small inclination of the kiln enables the material to move downward during rotation. The rotation is slow and the residence time is usually in the range of 5–10 h. It is a countercurrent process. When the limestone enters the kiln, it is dried and heated by the flue gases flowing in the opposite direction. At the lower end, the fuel is fed into the kiln through a burner. When exiting the kiln through the lower end, the limestone has released its content of carbon dioxide and the majority of the material is calcined into calcium oxide. Residual content of carbon dioxide in the product is usually in the range of 0.1–2.0 mass percent, depending on customer application. The mass balance shows that at full calcination, up to 44% of the feed is released as carbon dioxide gas during the process. After the kiln the lime product enters a cooler. The cooling is done with air to suitable product temperature. The cooling procedure is fast so that the product remains as lime and carbonation, which is uptake of carbon dioxide from the atmosphere, is minimal.
Oxyfuel combustion in rotary kiln lime production
Oxyfuel combustion aims to concentrate carbon dioxide in the flue gases enough for utilization or storage. Replacing air (79% nitrogen and 21% oxygen) with pure oxygen can decrease the volume of flue gases from the process. This also increases the carbon dioxide concentration since no nitrogen is added to the system. Combustion in elevated oxygen concentrations increases the flame temperature. This increase in flame temperature changes the heat load of the kiln combustion area. Thus, flame temperature needs to be controlled. This is achieved by recirculating the flue gas back to the lower end of the kiln. This work studies the effect of recirculation levels 50%, 60%, 70%, and 80%. The recirculated flue gas has a high carbon dioxide concentration. The increased carbon dioxide concentration increases the calcination temperature of the raw material. This will change the heat balances of the kiln. The basic recirculation scenario applied to a lime kiln is illustrated in Figure 1.
Carbon dioxide emissions from lime production
Within the European Union Emission Trade Schemes (EU ETS) 2008–2012 and 2013–2020, the lime process has two types of carbon dioxide emissions. Combustion emissions relate to combustion of carbon-based fuels. The carbon dioxide released from the raw material during production is referred to as process emissions. The process emissions are the main source of carbon dioxide emissions. They constitute 60–75% of the total emissions. Their portion varies with kiln type and quality requirements of fuels, raw materials, and products. The combustion emissions are defined as fossil and nonfossil. The process emissions are defined as fossil. All fossil carbon dioxide emissions are monitored and reported and the corresponding amount of emission allowances are surrendered annually. The nonfossil carbon dioxide emissions are monitored and reported but no emission allowances need to be surrendered. The product benchmark in the EU ETS 2013–2020 is 0.954 ton carbon dioxide per ton product. EU operational data show that lime plants with low specific carbon dioxide emissions usually are low-carbon fuel firing shaft kilns, around 1.0 ton carbon dioxide per ton product. At around 1.4 ton carbon dioxide per ton of product, the rotary kiln technology is not as emission efficient as the shaft kiln technology. The advantage of the shaft kiln lies in the high-energy efficiency of the technology.
The energy consumption of the kiln as fuel for calcination, studied in this work, varies mainly with kiln technology, process setup, raw material properties, and product properties. The variations are large. Shaft kiln operations are reported at 3.2–4.9 GJ per ton product and rotary kilns at 5.1–9.2 GJ per ton product [3].
The benefits of the rotary kiln are, e.g., higher utilization of quarried raw material, different product quality, and higher fuel flexibility.
The combustion emissions from lime production can be reduced by [1, 4]:
- Increased energy efficiency
- Fuel switch to low-carbon fuels
- Fuel switch to nonfossil fuels
- Precombustion capture of carbon dioxide with storage or utilization
- Postcombustion capture of carbon dioxide with storage or utilization
- Capture of carbon dioxide through oxyfuel combustion with storage or utilization
The process emissions from lime production can be reduced by:
- Postcombustion capture of carbon dioxide with storage or utilization
- Capture of carbon dioxide through oxyfuel combustion with storage or utilization
The only two technologies that can allow a near-zero carbon dioxide emission lime plant are postcombustion capture and capture through oxyfuel combustion. Both technologies require storage or utilization of carbon dioxide to achieve the emissions' reduction potential. Post-combustion capture of carbon dioxide with application on lime production was reviewed by Eriksson and Backman in 2009 [4] and this paper studies oxyfuel combustion in lime production.
Literature review
There are no known lime kilns operating in an oxyfuel mode. No publications on oxyfuel lime kilns have been found.
There are publications on oxygen enrichment in lime production [5, 6]. Oxygen enrichment usually aim at e.g., higher production capacity, increased fuel efficiency, or increased fuel flexibility, but as such the technology still operates with high nitrogen levels in the process and has no relevance as carbon dioxide capture. Further work is needed to optimize the oxygen feed to the lime kiln in an oxyfuel process but this is outside the scope of this study. The pulp lime recovery kiln operates at similar though different conditions. Some oxygen enrichment publications on lime recovery kilns in the pulp industry are available [7, 8].
There are publications on oxyfuel in other industrial processes, e.g., cement production [9-12], iron and steel production [13, 14], and power production [15, 16]. The technology has been demonstrated, e.g., for power production, but there are no full-scale plants in operation. Of these three processes, cement production is the closest to lime production but still significantly different. The results published on oxyfuel in cement production cannot be transferred as such to lime production.
Method
Through chemical thermodynamic modeling utilizing Gibbs free energy minimization, one can predict the chemical composition of a mixture of species at a given temperature and pressure. A predictive simulation tool has been developed by the Energy Technology and Thermal Process Chemistry Research Group at Umeå University in cooperation with Nordkalk Oy and Cementa AB.
Predictive simulation tool
The predictive simulation tool utilized in this paper is a combination of commercially available software: AspenPlus™ V7.1 [17] and ChemApp™ [18]. In this work, Aspen Plus™ is used for drawing the process scheme and iteratively solving the mass and energy balances. ChemApp™ is used to calculate the equilibrium chemistry. In addition, FactSage V6.1 [19, 20] is used as a source of thermochemical data for the system. The reasons for choosing ChemApp™ over the Aspen Plus™ are several. ChemApp™ makes it possible to utilize user-defined component data and the thermodynamic data from FACT. ChemApp™ also makes it possible to calculate solution phases not available in AspenPlus™. The predictive simulation tool has been validated for an operational lime plant and an operational cement plant [21-24]. The system components are aluminum, carbon, calcium, chloride, iron, hydrogen, potassium, magnesium, nitrogen, sodium, oxygen, phosphorus, sulfur, silica, titanium, and zinc. There is one gas phase and two pure liquids, 173 pure solid phases, seven solid solution phases, and two liquid solution phases. The system is further described by Hökfors et al. [22].
The model produces a vast amount of data. Not all components are critical when interpreting the results. Therefore, only data for selected compounds are included in the discussion. The selection criterion varies and is stated in the discussion. Some presented data are statistically normalized so that the selected data comprise 100 percentages.
This selection method is used to support the purpose of this article. A more comprehensive study on each specific retrofit or green field project is of course needed.
Generic lime kiln model
The generic lime kiln model is simplified from a more extensive model of an operational lime plant of Nordkalk Oy. The full plant model has been validated against the operational data. The validated full plant model was reduced to a more generic rotary kiln lime process setup and equipped with a flue gas circulation option. The generic model reference case was validated against operational data. This generic model is designed to be easier and faster to operate than the full plant model. The result should also be easier to export and apply to other lime plants. The generic model utilizes the same compounds present in the database as the full model. The full model is further described by Hökfors et al. [22].
In this work, eight different recirculation cases are compared with the validated reference case.
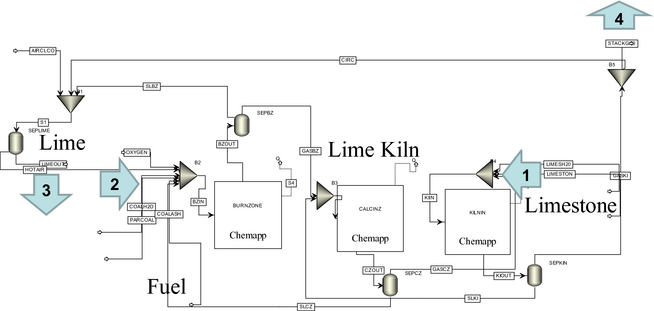
All of the chemical equilibria are calculated by defining the pressures and the enthalpy changes in the systems. The model allows for setting either the equilibrium temperatures or the enthalpy changes. When setting enthalpy changes, one can observe changes in temperature related to different fuels or process setups. This means that the model is controlled by energy losses. The simulated temperatures are calculated at a constant energy-loss profile. The energy-loss profile is calculated from kiln surface temperature measurements as part of the model validation. The total energy loss is set at 5.5 MJ/s. In the model the rotary kiln is described by three ChemApp™ equilibrium blocks. The Aspen Plus™ schematic of the generic model with recirculation can be seen in Figure 2. The main streams are indicated as streams 1–4 in Figure 2. Stream 1 is the input data of fuel and stream 2 is the input data for limestone. The chemical composition of the input streams is presented in Table 1. The simulation results, the output data, are streams 3 and 4. Stream 3 is the condensed phase, the lime product, and stream 4 the noncondensed phase, the stack gas. The recirculation gas has the identical equilibrium composition and properties as the stack gas. The composition of the model output streams, the simulation results, are presented and discussed below.
| Fuel | Ash | Limestone | |||
|---|---|---|---|---|---|
| C | 75.84 | CaO | 6.90 | CaCO3 | 97.41 |
| H | 5.00 | SiO2 | 39.00 | SiO2 | 1.25 |
| O | 8.80 | Al2O3 | 43.90 | Al2O3 | 0.58 |
| N | 1.20 | Fe2O3 | 7.90 | Fe2O3 | 0.14 |
| S | 0.76 | K2O | 2.30 | FeSO4 | 0.33 |
| Cl | 0.00 | K2O | 0.24 | ||
| Ash | 8.40 | CaCl2 | 0.05 | ||
| 100.00 | 100.00 | 100.00 | |||
|
|
|
Figure 2. ASPEN schematic of generic lime model. |
Results and Discussion
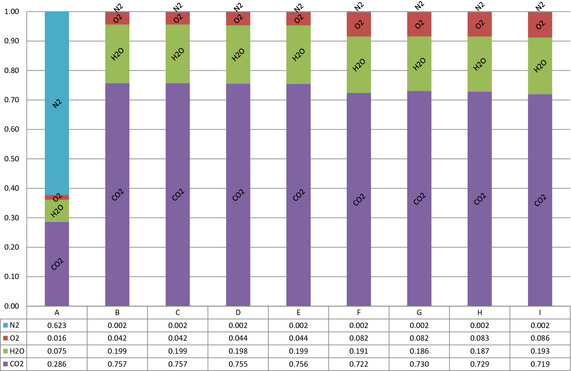
To study the impact of process changes nine cases were simulated. The simulations are referred to as cases A–I. Reference case A is the conventional lime process. The cases B–I are oxyfuel processes varied by energy input and flue gas circulation. The simulation matrix is seen in Table 2. The results show that we have achieved to simulate oxyfuel combustion. In Figure 3, cases B to I show that composition of the gas recirculated for combustion with pure oxygen is mainly oxygen, carbon dioxide, and water, which is the expected stack gas composition from oxyfuel combustion. The recirculation gas is identical to stream 4 in Figure 2, the stack gas. Case A is the conventional air combustion process with high nitrogen.
| Case | Fuel in | Oxygen in | Recirculation % | |||||
|---|---|---|---|---|---|---|---|---|
| Energy % | Coal kg/h | Ash kg/h | Tot fuel in kg/h | % of ref | kg/h | O2/coal | ||
| A | 100% | 2167 | 199 | 2366 | 100% | 5610 | 2.59 | 0% |
| B | 100% | 2167 | 199 | 2366 | 100% | 5610 | 2.59 | 50% |
| C | 100% | 2167 | 199 | 2366 | 100% | 5610 | 2.59 | 60% |
| D | 100% | 2167 | 199 | 2366 | 100% | 5610 | 2.59 | 70% |
| E | 100% | 2167 | 199 | 2366 | 100% | 5610 | 2.59 | 80% |
| F | 90% | 1951 | 179 | 2129 | 90% | 5049 | 2.59 | 50% |
| G | 90% | 1951 | 179 | 2129 | 90% | 5049 | 2.59 | 60% |
| H | 90% | 1951 | 179 | 2129 | 90% | 5049 | 2.59 | 70% |
| I | 90% | 1951 | 179 | 2129 | 90% | 5049 | 2.59 | 80% |
|
|
|
Figure 3. Gas compositions in mole fraction for the recirculation gases in cases B–I and case A kiln exit gas. |
The reference case modeled in this study has an energy consumption of ~7 GJ/t lime produced. The energy consumption for oxygen production is reported at 400 kJ/mole of oxygen, as O2 [9]. This corresponds to <0.5% of the energy as fuel consumed for calcination. The energy consumption of oxygen production is not significant and is, therefore, excluded from this study. It can be noted that oxygen production consumes energy as electricity which is more expensive than the low-grade fuels used for calcination, so of course, a more detailed study will be required to establish impact of oxyfuel on the cost of production.
Influence on product quality, energy efficiency, and implications to the production process
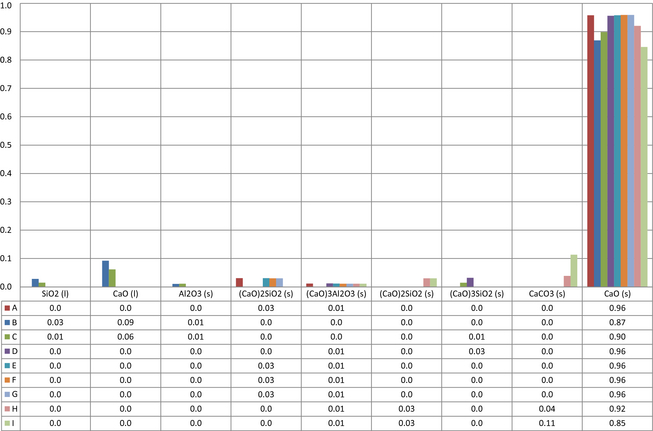
The main component of the product is calcium oxide. The main quality value is the amount calcium oxide readily available for reaction in industrial processes. This is here described as free calcium oxide. In the product calcium oxide is also found as e.g., carbonates, oxide melts, and salts. Stream 3 in Figure 2 is the output data for the condensed phase, the lime product. The selected product compositions with mole fractions equal to or >0.01 can be seen in Figure 4.
|
|
|
Figure 4. Mole fractions for all components in product. |
The calculated free lime is here used as product quality indicator. The results show that the amount of free calcium oxide in the product ranges from 0.894 to 0.952 mole fraction, meaning that there is a loss in product quality in some of the cases. This is a wide range taking into consideration the identical raw material and fuel.
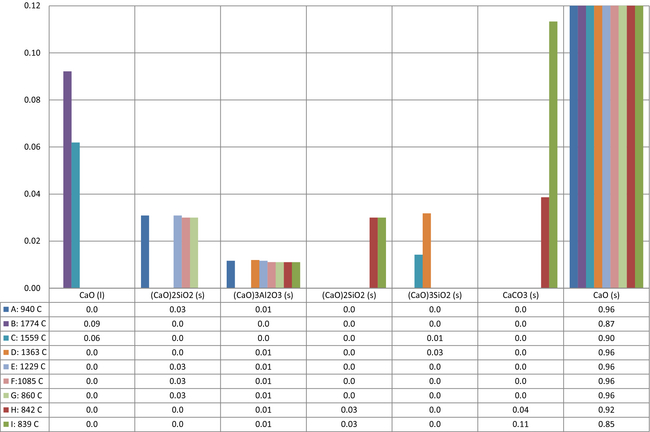
Being the single most important compound in the product, calcium was investigated in more detail. The distribution of calcium species in Figure 5 shows why several cases suffer high quality losses. The data are selected at equal to or >0.01 mole fraction. In cases B and C, high temperatures increase the oxide melt phase increasing the amount of calcium oxide dissolved. Calcium also forms solid solutions with e.g., silica as di-calcium silicates and tri-calcium silicates. These types of solutions lower the amount of free calcium oxide reducing product quality. In cases H and I, changes in the gas phase, presented later in Figure 9, and the low temperature results in high amounts of calcium as carbonate.
|
|
|
Figure 5. Distribution of calcium species in the product in mole fraction and maximum equilibrium temperature in °C. |
None of the cases increased product quality. The cases D, E, F, and G show equal free calcium oxide levels as in the reference case A. These are high-quality products.
Energy efficiency is an important operational result. To investigate whether any significant energy efficiency improvements can be achieved, cases F–I were run with only 90% energy input.
Of the five high-quality products two; F and G, used 10% less energy. Although requiring further study this is a significant result. A 10% reduction in energy consumption while maintaining product quality reduces carbon dioxide emissions and production costs.
The results show that the heat balances of the kiln have moved in a beneficial direction. Recirculating hot flue gases (from preheating zone to burn zone) will conserve energy compared to the air-fired conventional reference case A. In case F, the temperatures rise over the length of the kiln although energy input is lowered by 10%. The temperature profiles can be seen in Table 3.
| Case | Energy input | Recirculation | Burnzone [C] | Calcination zone [C] | Preheating zone [C] |
|---|---|---|---|---|---|
| A | 100% | 0% | 940 | 784 | 532 |
| B | 100% | 50% | 1774 | 979 | 660 |
| C | 100% | 60% | 1559 | 987 | 709 |
| D | 100% | 70% | 1363 | 967 | 742 |
| E | 100% | 80% | 1229 | 936 | 783 |
| F | 90% | 50% | 1085 | 842 | 556 |
| G | 90% | 60% | 860 | 753 | 538 |
| H | 90% | 70% | 842 | 760 | 572 |
| I | 90% | 80% | 839 | 796 | 647 |
The high temperature of the process causes melting of the product. The total weight percent melt is seen in Figure 6. The conventional lime kiln is not equipped to handle such high temperatures and large amount of melt. Case B and C do not achieve full product quality because of the high amount of calcium oxide in the melt.
|
|
|
Figure 6. Weight percent melt in product and equilibrium temperature. |
Influence on stack gas composition and implications to the production process
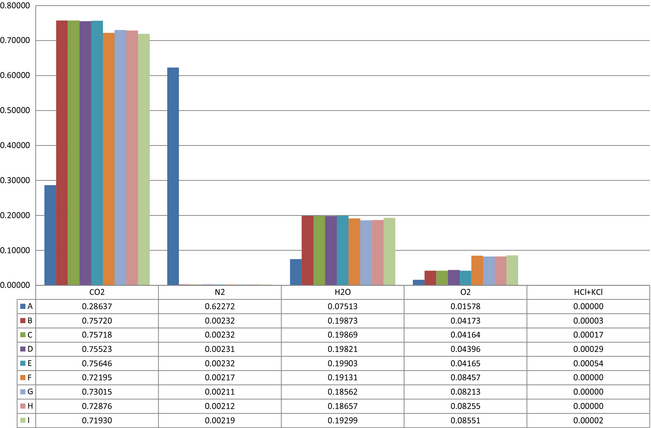
The results show significant changes in stack gas composition. Stream 4 in Figure 2 is the output data for the noncondensed phase. Figure 7 show selected concentrations equal to or higher than 0.00001 mole fractions of all compounds in the stack gas. The selection criterion is set so that hydrochloride is present in the gas. The hydrochloride concentration in the flue gases is environmentally and technically nonbeneficial. Some components are present at concentrations below the selection criteria and are, therefore, excluded from this study. The results show 28.6% carbon dioxide in the reference case. With circulation the carbon dioxide concentration increases to a maximum of 75.7% at full fuel load and 73.0% at reduced fuel load. Nitrogen levels decrease from 62.3% to 0.2%. The water content of the stack gas is increased from 7.5% to 19.9% when the circulation is introduced. The changes in level of circulation and fuel load show only small variations on the water content of the stack gas. The oxygen levels increase with recirculation.
|
|
|
Figure 7. Stack gas compositions in mole fraction. |
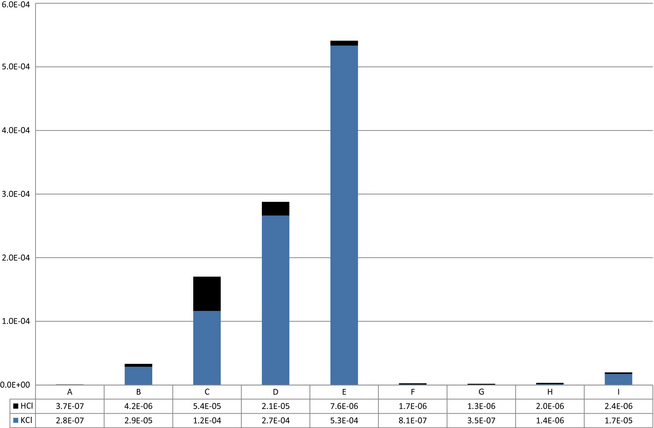
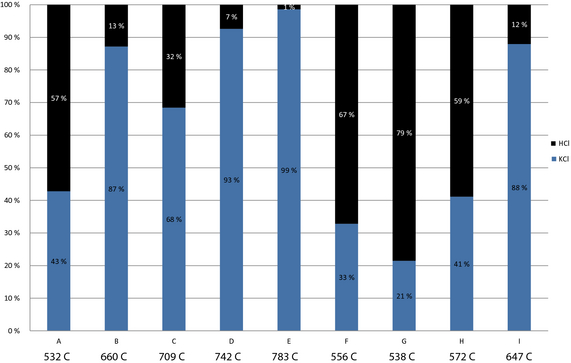
Chloride enters the system with the limestone raw material feed. As seen in Figure 8, the circulation levels and fuel loads influence the levels and compounds of chloride present in the kiln exit gas. All in all the chloride levels remain low, peaking at 0.054% for circulation case E. In the full fuel recycle scenarios, B–F, the chloride containing compounds increase with the recirculation level. Even if the overall chloride levels are very low the results show a distinct accumulation of chlorides that might be significant for operations. Increasing chloride concentrations with increasing recirculation rates can be described as an accumulative phenomenon. The accumulative effect of chloride is not as significant at reduced fuel level. The chloride is present as hydrochloride and potassium chloride. The distribution is seen in Figure 9. Hydrogen and potassium varies widely between the cases. This might also be significant for operations. The variations in chloride component distribution can be described as a function of temperature. At high temperatures, potassium evaporates increasing potassium concentration in the gas phase.
|
|
|
Figure 8. Total chlorides and distribution in mole fraction of total stack gas. |
|
|
|
Figure 9. Distribution of chloride compounds in stack gas. |
Influence on carbon dioxide emissions and implications on Carbon Capture and Storage
The use of oxyfuel combustion enables high carbon dioxide concentrations in the lime kiln flue gas. Very high carbon dioxide content opens up opportunities for carbon utilization and storage processes. Recirculation of hot flue gases offers a potential to reduce the operational fuel energy per ton of product ratio. If an oxyfuel lime plant is operated with carbon neutral fuels, e.g., biofuels, and equipped with carbon dioxide storage or utilization facilities, it also allows for “below zero emission” operations storing separated biogenic carbon away from the atmosphere. With sustainable nonfossil fuel production, this could enable a reduction of carbon dioxide in the atmosphere.
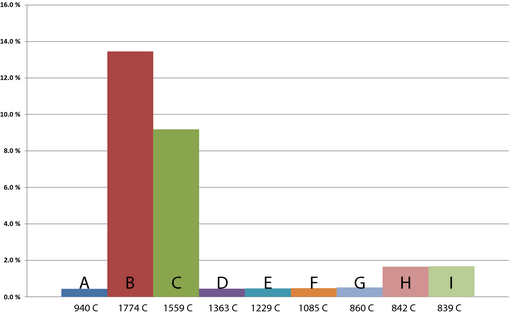
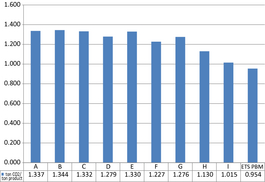
Even though carbon dioxide capture and storage are not employed in this model, the results show decreased carbon dioxide emissions in the oxyfuel cases in comparison to the reference case. The ton carbon dioxide per ton product ratio for the cases is shown in Figure 10. The reference case A has a ratio of 1.34 ton carbon dioxide per ton product. The lowest emitting case with acceptable product quality is F at 1.23 ton carbon dioxide per ton product. Cases H and I have low-quality products containing high amounts of calcium carbonate. The product benchmark for free allocation of emission allowances within the European Union Emissions trade scheme, ETS PMB, as decided by the European Commission [25] is seen in the last column. The benchmark is set at 0.954 on carbon dioxide per ton lime product. The Commission has reserved the right to cut free allocation to comprise only a certain, yet undefined, quota of the benchmark. It is clearly shown that the free allocation is not sufficient to cover rotary kiln lime production completely at the given fuel mixtures.
|
|
|
Figure 10. Ton carbon dioxide per ton of product ratio. |
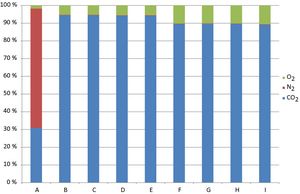
From a carbon capture view, the dry stack gas composition is of interest. Drying of the gas can be easily achieved. The result in Figure 11 shows high carbon dioxide concentrations in the dry gas. Reference case A has a carbon dioxide concentration of 31.0%. Introducing oxyfuel combustion increases the concentration to the range between 89.1% and 94.5% the rest being mainly oxygen. These levels seem attractive for compression and transportation. The feasibility of reducing the oxygen concentration, e.g., by injection of carbon carrying fuels, resulting in even higher carbon dioxide purity in the processed gas, should be further assessed.
|
|
|
Figure 11. Dry stack gas concentrations. |
Conclusions
Oxyfuel combustion in rotary kiln lime production was studied. A validated predictive simulation tool has been applied to lime production.
The results show that an oxyfuel process could produce a high-quality lime product at reduced specific energy consumption. The new process would have a lower carbon dioxide emission per ton of product. The results show that to avoid a low-quality product, the process energy balances the need to be optimized. If the kiln runs too warm we will experience melting of oxide and salt phases in the product, and if the kiln runs too cold there is not enough energy to complete the calcination.
If dried the flue gas from the new process could be suitable for carbon dioxide transport and storage or utilization. The new process would have different energy and material balances compared to a conventional process. To exemplify the method the chloride balances were studied. The results show accumulation of chloride and variation in chloride species in the oxyfuel process.
In this work recirculation cases F and G, at 50% and 60% recirculation, show full product quality with reduced fuel consumption. Both cases show low chloride accumulation and high carbon dioxide content in the flue gas. Having lower specific carbon dioxide emissions than G, F at 1.227 ton carbon dioxide per ton product is considered the best case simulated in this work.
It is the conclusion that lime production with an oxyfuel process seems feasible but still requires further studies in order to reach a technical application level. A more comprehensive study on each specific retrofit or green field project is of course needed.
Acknowledgments
The authors would like to acknowledge Kjell Dahlberg at Nordkalk Oy Ab, Bo-Erik Eriksson, Thomas Lind, Anders Lyberg, Stefan Sandelin, and Erik Viggh at Cementa, Jan Bida, and Marianne Thomaeus at MinFo (Swedish Mineral Processing Research Association). The Swedish Energy Agency (No. 2006-06679. Project 30527-1) and the National (Swedish) Strategic Research Program Bio4Energy for financial support.
Conflict of Interest
None declared.
References
- IPCC. 2005. Capture of CO2 . P. 442inB. Metz, O. Davidson, H. C. de Coninck, M. Loos, and L. A. Meyer, eds. IPCC special report on carbon dioxide capture and storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom; New York, NY.
- Oates, J. O. H. 1998. Lime and limestone: chemistry and technology, production and use. Wiley-VCH, Weinheim, ISBN 3-527-29527-5.
- Industrial Emissions Directive 2010/75/EU, European IPPC Bureau, Best Available Techniques (BAT) Reference Document for the Production of Cement, Lime and Magnesium Oxide, JOINT RESEARCH CENTRE, Institute for Prospective Technological Studies Sustainable Production and Consumption Unit, 2013.
- Eriksson, M. 2009. Post combustion CO2 capture in the Swedish lime and cement industries. Licentiate thesis, Umeå university, ETPC Report 09-05, ISSN 1653-0551, ISBN 978-91-7264-922-4.
- Wrampe, P., H. C. Rolseth. 1976. The effect of oxygen upon the rotary Kilns production and fuel efficiency: theory and practice. IEEE Trans. Ind. Appl.IA-12:568–573.
- Kudrina, A. P., V. N. Andryushchenko, and L. P. Kushchenko. 1979. Use of oxygen for limestone burning in rotary Kilns. Heat Eng.19:353–357.
- Garrido, G. F., A. S. Perkins, and J. R. Ayton. 1982. Upgrading lime recovery with oxygen enrichment. Pulp Paper Canada83:T11–15, 67th Annual Meeting of Technical Section, CPPA, at Montreal 1981.
- Watkinson, A. P., and J. K. Bricombe. 1983. Oxygen Enrichment in roatry lime kilns. Can. J. Chem. Eng.61:842–849.
- Zeman, F., K. Lackner. 2008. The reduced emission oxygen Kiln. The Earth Institute at Columbia University, New York, NY.
- ECRA, European Cement Research Academy. Phase I report: 2007 Phase II report: 2009 Phase III report: 2012. CCS project, phase I-III reports. Available at http://www.ecra-online.org/226/ (accessed 28 March 2014).
- Hökfors, B., E. Viggh, and M. Eriksson. 2013. Simulation of oxy-fuel combustion in cement clinker manufacturing. Adv. Cement Res.25:1–8.
- Zeman, F.2009. Oxygen combustion in cement production. Energy Procedia1:187–194.
- ULCOS. Ultra low CO2 steelmaking. Available at http://www.ulcos.org/en/press.php# (accessed 28 March 2014).
- Danloy, G., Berthelemot, A., M. Grant, J. Borlée, D. Sert, and J. van der Stel, et al. 2009. ULCOS - pilot testing of the Low-CO2 Blast Furnace process at the experimental BF in Luleå. Revue de Métallurgie106:1–8. doi: 10.1051/metal/2009008
- Wilkinson, M, Boden, J., R. Panesar, and R. Allam et al. 2001. CO2 capture via oxyfuel firing: optimisation of a retrofit design concept for a refinery power station boilerin USA First National Conference on Carbon Sequestration, Washington, DC, 15–17 May 2001.
- Strömborg, L., Lindgren, G., J. Jacoby, R. Giering, M. Anheden, and U. Burchhardt et al. 2009. Update on Vattenfalls 30 MWth oxyfuel pilot plant in Schwarze Pumpe, Proceedings of the 9th International Conference on Greenhouse Gas Control Technologies (GHGT-9), 16–20 November 2008, Washington DC, Energy Procedia 1:581–589, Greenhouse Gas Control Technologies 9.
- AspenPlus. Conceptual design of chemical processes, 1994–2011. Available at http://www.aspentech.com/core/aspen-plus.aspx (accessed 1 August 2011).
- ChemApp. 2011. The Thermochemistry Library for Your Software. Available at http://www.gtt-technologies.de (accessed 1 August 2011).
- Bale, C. W., P. Chartrand, S. Degterov, G. Eriksson, K. Hack, R. Mahfoud, et al. 2002. FactSage thermochemical software and databases. CALPHAD26:189–228.
- FactSage. 2011. Interactive programs for computational thermochemistry. Available at http://www.gtt-technologies.de (accessed 1 August 2011).
- Wilhelmson Hökfors, B., E. Viggh, and R. Backman. 2008. A predictive chemistry model for the cement process, Zement-kalk-gips, 61:60–70, no 7, [Note(s): 60-70 [8 p.]].
- Hökfors, B., M. Eriksson, and R. Backman. 2012. Improved process modeling for a lime rotary kiln using equilibrium chemistry, J. Eng. Technol.29:8–18.
- Eriksson, M. 2009. An industrial perspective on modelling of a rotary kiln for lump lime productionin Proceedings at the NAFEMS NORDIC Seminar: Multi-Disciplinary Simulation in Engineering analysis, Helsinki, Finland, 21–22 April, 2009.
- Hökfors, B., M. Eriksson, and E. Viggh. 2013. Modelling the cement process and cement clinker quality, Advances in Cement Research. Available at http://dx.doi.org/10.1680/adcr. 13.00050 (accessed 22 September 2014).
- EU EEC 2011/278/EU, COMMISSION DECISION, 27 April 2011. 2011. Determining transitional Union-wide rules for harmonized free allocation of emission allowances pursuant to Article 10a of Directive 2003/87/EC of the European Parliament and of the Council, notified under document C(2011) 2772, 2011.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?