Summary
Objective
To evaluate the long-term results of tumorectomy and concomitant bilateral oncoplastic reduction mammoplasty (ORM) for early stage breast cancer patients with macromastia in terms of local disease control and long-term oncological results.
Patients and method
Data of 82 patients with macromastia undergoing ORM for breast cancer between 1996 and 2011 were retrospectively examined and evaluated with regard to oncological results.
Results
The median age was 50 years. The median follow-up was 121 months (range 28–212 months). The median breast volume was 1402 cm3 and the median weight of excised breast material was 679 g. The median surgical margin was 16 mm. Ten-year local recurrence rate was 8.7%. The 10-year overall survival rate was 82.2% and the disease-free survival rate was 73.2%. Early and late complication rates were 12.2% and 14.6%, respectively.
Conclusions
From the standpoint of local disease control and long-term observation, ORM can be considered a very safe and acceptable treatment for early stage breast cancer in women with macromastia.
Keywords
breast cancer;cosmesis;macromastia;oncoplastic breast reduction;satisfaction
1. Introduction
Breast-conserving surgery (BCS) is the standard treatment for early stage breast cancer, but this procedure is associated with certain oncological and cosmetic problems, such as large breast size, positive margins, tumor/breast volume ratio, problems associated with radiotherapy (RT), and patient dissatisfaction. The frequency of macromastia in breast cancer patients undergoing BCS is 40%.1 In Losken et als meta-analysis,2 the rate of positive margins after BCS was 20.6%. Some problems have been reported with RT dose homogeneity in post-BCS patients with large breasts,3 and aesthetic concerns in post-BCS patients have reached 30%.4 Indeed, postoperative RT problems, aesthetic concerns, and overall patient satisfaction rate are considered relative contraindications for choosing BCS in breast cancer cases with macromastia.
Bilateral oncoplastic reduction mammoplasty (ORM) combines the techniques of tumorectomy and bilateral breast reduction. Thus, a tumor can be excised with wider margins, and the effectiveness of RT on a reduced breast increases.5 Because screening programs and adjuvant therapies indicate that patients with breast cancer have a longer life expectancy, breast aesthetics and quality of life have become more critical. Bilateral reduction mammoplasty improves quality of life.6
Despite the increase in the number of ORM studies, there are no data showing long-term oncological results for ORM in patients with macromastia, although this is by far the more common procedure. Therefore, we examined the 10-year results of women with macromastia undergoing ORM for early stage breast cancer in terms of oncological results. The principal aim of this study was to evaluate the efficacy of long-term oncological local control in ORM. This was gauged by positive margins, close margins, and ipsilateral recurrence. Regional recurrence was not considered. The secondary aim was to determine the impact of ORM on 10-year overall survival (OS) and disease-free survival (DFS) rates.
2. Patients and methods
A retrospective review of the medical records of consecutive 82 patients with macromastia undergoing concomitant ORM between January 1996 and May 2011 was carried out. According to the 2010 American Joint Committee on Cancer/Union for International Cancer Control breast cancer staging system, patients with Stages 1 and 2 were included in this study. Patients with in situ Stage 3 breast cancer, or breast volume less than 1000 cm3 were excluded. Eight patients who underwent ORM withdrew from observations and were excluded from the study. Macromastia was defined as breast volume more than 1000 cm3. The cases were examined for demographics, macromastia, operative and oncologic outcomes, complications, and adjuvant therapy. All cases were discussed and treatment options were initially planned in multidisciplinary weekly meetings. Written informed consent was obtained for the surgical procedure. This study was approved by the local ethics committee.
2.1. Patient evaluation and operative techniques
Routine preoperative oncological screening was carried out in all patients diagnosed with breast cancer. Wire localization was used on nonpalpable breast lesions during ultrasound and/or mammography. Preoperative magnetic resonance imaging (MRI) was performed in nine cases. Breast volume of all patients was measured using a Grossman–Roudner device.7 Breast asymmetry was accepted as a disparity if breast volume was over 10%.
During the preoperative evaluation, we determined the tumor quadrants to be excised, the choice of nipple areola complex (NAC) flap, access to the axilla, choice of skin incision, and the estimated volume of breast tissue to be removed. Similar decisions were made for the contralateral breast. Tumors were excised with a minimum margin of 1.5 cm. In the final pathological evaluation, any margin less than 2 mm was accepted as a positive margin. Intraoperative margin control was achieved using frozen sections with specimen mammography for multifocal tumors, and all re-excisions were performed immediately. The only skin removed included biopsy-incision scars and skin-covering tumors closer than 1 cm to the surface. Nipple resection was performed in tumors closer than 2 mm to the nipple. Metal clips were placed in the tumor bed as a guide for RT, and the orientation of the excised specimen was marked. Similar procedures were carried out simultaneously on the contralateral breast to achieve symmetry. The ipsilateral breast was left 10% larger to allow for shrinkage during RT. At least two members of the strong five-member surgical team were present at each operation.
The Wise pattern incision was chosen for its ease of axillary access, flap alternatives, and ease of breast reconstruction. We preferred the vertical incision in cases of macromastia less than 1300 cm3 to minimize the incision. Our choice of NAC carrying the pedicle was based on forming a pedicle in the breast section furthest from the tumor. A free nipple graft was used in cases where the NAC distance was more than 35 cm. In cases of nipple involvement, we performed a central resection, followed by a Grisotti flap reconstruction. Sentinel lymph node biopsy (SLNB) or four to eight lymph node sampling was implemented in clinically node-negative patients, and axillary dissection (AD; Levels 1 and 2) was performed in node-positive cases. Complications were recorded as early (<2 months) and late (>2 months).
Standard RT was applied 3 weeks postoperatively with 50 Gy to the whole breast and a 10-Gy boost to the tumor bed. Of the total patients in this study, 24 were administered chemotherapy (CT), 25 were administered both CT and hormone therapy (HT), and 33 received only HT. The CT regimen was fluorouracil, epirubicin, and cyclophosphamide (FEC) in 21 patients; cyclophosphamide, methotrexate, and fluorouracil in nine; adriamycin (doxorubicin) and cyclophosphamide (AC) in seven; FEC + taxane in seven; and AC + taxane in five patients. Tamoxifen was used for HT in 47 cases, and aromatase inhibitors were used in 16 cases. In addition, 16 Cerb-B2-positive cases were treated with trastuzumab. Patients were followed by surgeons and medical oncologists every 4 months for the first 2 years, every 6 months for the following 3 years, and then on an annual basis.
Patients were also asked to complete a short two-question survey.
- Would you be prepared to undergo the same operation again, if necessary?
- Yes (B) No (C) No comment
- Would you recommend this operation to other women with similar problems (macromastia and breast cancer)?
- Yes (B) No (C) No comment
2.2. Statistics
Data were analyzed using SPSS version 15 (SPSS Inc., Chicago, IL, USA). DFS, locoregional, metastasis-free, and OS rates were estimated using Kaplan–Meier estimates, and the patient and treatment groups were compared using the log-rank test. Multivariate analyses were performed with Cox proportional hazard models to test the differences between groups. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Patient data
The median age was 50 years (range, 31–70 years). Median body mass index was 28.9 kg/m2. At the time of diagnosis, 65 patients (79.3%) were postmenopausal and 17 patients (20.7%) were premenopausal. As much as 12 (14.6%) patients had a family history of breast cancer, 11 patients had diabetes mellitus, 10 had hypertension, and 14 (17%) were smokers. The median length of the postoperative hospital stay was 3.4 days, and the tumor was on the right side in 42 patients (53.6%). The median follow-up period was 121 months (range, 28–212 months).
3.2. Operative findings
The mean operation time was 124 minutes ± 25 minutes. Quadrantectomy followed by oncoplastic reduction was performed in the first 27 cases, and the tumor area was excised en bloc in the next 55 cases. The Wise pattern was implemented for the skin incision in 60 cases, and a vertical incision was used in 22 cases. Most tumors were located in the upper outer quadrant (n = 25).
For reconstruction, 48 inferior, 17 superior-medial, seven superior, four free nipple, four superior-lateral, and three Grisotti flaps were used. Similar techniques were used concomitantly on the contralateral breast. Intraoperative frozen section and specimen radiography were conducted in 48 (58%) and 43 (52%) cases, respectively. Two patients underwent re-excision because of positive margins seen during the frozen-section examination. Clips were placed in the tumor bed in 56 cases. Fifty patients underwent an SLNB intraoperatively before ORM for axillary surgery. AD was performed in nine cases because of SLNB positivity. Axillary samples were taken in eight cases. AD was performed in 24 preoperative clinically node-positive cases.
3.3. Histopathological evaluation
The final pathological evaluation is summarized in Table 1. The mean tumor size was 26 mm (range, 4–47 mm). The median tumor margin distance measured in 61 patients was 16 mm (range, 8–46 mm). Multifocality was found in 12 cases and vascular invasion was found in 11 cases. We found that 17 patients were Grade (G1), 27 were G2, and 12 were G3. Positive margins were found in three cases (3.7%). Because of positive margins in three cases, re-excisions were performed, and a frozen section was evaluated. A close margin was found in one patient (1.2%), in whom re-excision was performed, but no tumor occurred in the final histopathological evaluation. Two (2.4%) invasive (ductal and lobular) cancers, four (4.9%) in situ cancers, and six (7.4%) atypical ductal hyperplasia cases were recorded during the histological evaluation of contralateral breasts.
| Biology | n (%) |
|---|---|
| Tumor type | |
| Ductal | 59 (72) |
| Ductal + DCIS | 16 (19.5) |
| Lobular | 5 (6.1) |
| Lobular + DCIS | 2 (2.4) |
| Tumor size, mm | |
| 0–5 | 4 (4.9) |
| 6–10 | 9 (11.0) |
| 11–20 | 29 (35.4) |
| 21–50 | 40 (48.8) |
| Axilla | |
| No | 45 (54.9) |
| N mic | 7 (8.5) |
| N1 (1–3) | 30 (36.6) |
| ER or PR | |
| Positive | 56 (68.3) |
| Negative | 14 (17.1) |
| Unknown | 12 (14.6) |
| HER2/neu | |
| Positive | 16 |
| Negative | 30 |
| Unknown | 36 |
DCIS = ductal carcinoma in situ; ER = estrogen receptor; PR = progesterone receptor.
3.4. Complications
A total of 10 cases (12.2%) had early complications, including suture-line dehiscence (n = 4), seroma (n = 3), wound-site infection (n = 2), and areola necrosis (n = 1). Adjuvant treatment was postponed for 10–20 days in the four (4.9%) cases of incision opening and areola necrosis. Late complications were seen in 12 cases (14.6%), including the following: skin problems (color, scarring); fat necrosis and fibrosis (re-excision was performed); impaired breast shape (dog ear correction); breast hypertrophy (treated with re-reduction), and chronic mastalgia. MRI was used two times on average (range, 1–3) for 11 patients (13.4%) with irregular breast symptoms. Fine-needle biopsy was performed once or twice on eight patients (9.6%) with clinically suspicious areas, and all were benign.
3.5. Oncology
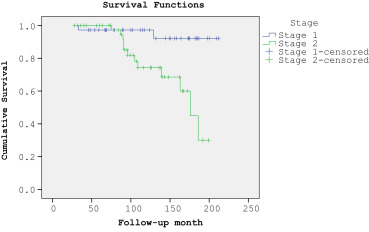
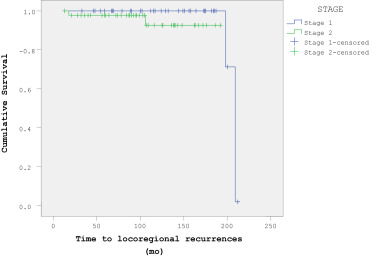
Oncological results and survival rates are shown in Table 2 ; Table 3 and Figure 1 ; Figure 2. Four cases (4.9%) of isolated breast recurrence were observed in the original cohort. The median interval between the primary breast cancer diagnosis and axillary recurrence was 43 months (range, 18–106 months). Breast recurrence was seen 19 months postoperatively in a patient whose positive margin led us to re-excise the upper outer quadrant. Mastectomy and late-stage transverse rectus abdominis myocutaneous reconstruction was performed with the help of a plastic surgeon. A wide re-excision was performed at the 21-month postoperative follow-up visit, and a frozen section was obtained for breast recurrence in a patient whose primary tumor had been in the lower inner quadrant. One patient, who needed axillary node sampling, developed an axillary recurrence 18 months postoperatively and underwent axillary clearance. This patient had missed a dose of RT. Another patient who had undergone axillary node sampling developed an axillary recurrence after 18 months, and axillary clearance was performed. Two incidences of ipsilateral recurrence 59 months and 106 months after the operation revealed lobular carcinoma. In the first case, the recurrence was in the same quadrant as the primary tumor, and concomitant distant metastases developed. This patient received wide local excision with CT and additional HT. In the second case, the 72-year-old patient underwent wide excision of a mass, and a frozen section was obtained with additional HT. Distant metastases were diagnosed in 17 patients (20.7%) over a median period of 83 months. Metastases were located in the bone (n = 5), bone and liver (n = 5), bone and lungs (n = 3), liver (n = 3), lungs (n = 2), and brain (n = 1). A total of 14 patients received CT, eight patients received HT (in addition to the first 5 years), and eight patients received palliative RT for metastatic bone involvement. Fourteen patients (17.1%) died after a mean observation period of 108.1 months (range, 33–186 months); four of the five cases that developed locoregional recurrence developed distant metastases, and three subsequently died. Thirteen patients with metastatic cancer died because of tumor dissemination, and one patient died in a traffic accident.
| Stage | N | LR (%) | RR (%) | DFS (%) | OS (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Year 5 | Year 10 | Year 5 | Year 10 | Year 5 | Year 10 | Year 5 | Year 10 | ||
| I | 36 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 96.5 | 88.8 | 98.4 | 94.4 |
| II | 46 | 2 (4.1) | 4 (8.7) | 1 (2.2) | 1 (2.2) | 81.1 | 65.2 | 93.9 | 73.9 |
| I + II | 82 | 2 (4.1) | 4 (8.7) | 1 (1.2) | 1 (1.2) | 86.4 | 73.2 | 97.1 | 82.2 |
DFS = disease-free survival; LR = local recurrence, OS = overall survival; RR = regional recurrence.
| LRR | Metastasis | Death | |
|---|---|---|---|
| N | 5 | 17 | 14 |
| Tumor size (mm) | |||
| 1–10 | — | 1 | 1 |
| 11–20 | 1 | 3 | 2 |
| >20 | 4 | 13 | 9 |
| ER/PR | |||
| Positive | 1 | 8 | 6 |
| Negative | 2 | 6 | 5 |
| Unknown | 2 | 3 | 3 |
| HER2/neu | |||
| Positive | 2 | 5 | 3 |
| Negative | 2 | 7 | 7 |
| Unknown | 1 | 5 | 5 |
| Surgical margin | |||
| Positive | 1 | 2 | 2 |
| Negative | 4 | 15 | 12 |
ER = estrogen receptor; LR = locoregional recurrence; PR = progesterone receptor.
|
|
|
Figure 1. Overall patient survival analysis (Stages 1 and 2). |
|
|
|
Figure 2. Disease-free survival analysis (Stages 1 and 2). |
3.6. Macromastia
The mean weight of breast tissue removed was 678 g. Because four patients died and three patients refused breast volume measurements, the final breast volume evaluation was based on 65 patients. Table 4 summarizes the macromastia data.
| Characteristics | Preoperative | Final evaluation | p |
|---|---|---|---|
| N | 82 | 65 | |
| Median BMI, kg/m2 | 28.9 (22–36) | 32.1 (24–41) | |
| Median breast volume, cm3 | |||
| Right breast | 1380 (100–1900) | 890 (650–960) | |
| Left breast | 1420 (1050–2000) | 875 (67–940) | |
| Asymmetry (%) | 14 (17.1) | 6 (9.2) | 0.04 |
BMI = body mass index.
Responses to the short survey questions showed that 57 patients (93.4%) would choose this procedure again, two patients (3.3%) would not, and two patients (3.3%) did not comment. In addition, 59 patients (96.7%) would recommend this procedure to a patient in a similar situation, whereas two patients (3.3%) would not.
4. Discussion
The main finding of this study was that the choice of concomitant ORM BCS led to improved oncologic and satisfaction outcomes in women with early breast cancer and macromastia in terms of long-term results. This procedure was considered favorable by a large proportion of the patient cohort.
4.1. Oncology
The use of ORM is highly suitable for treating breast cancer in women with macromastia, and is efficient for local disease control. Similar studies of ORM for treating breast cancer in women with macromastia reported positive margins of 2.7–7.3% with breast recurrence rates of 0–4.9% (Table 5).9; 10; 11; 12 ; 13 However, these studies, with a median dissection material weight of 370–766 g, comprised fewer cases and a shorter follow-up time. In a meta-analysis, Losken et al2 reported positive margins in 12.4% of ORM cases without macromastia, 12.2% for oncoplastic flaps, and 20.6% for BCS alone. Local recurrence was reported as 4.7% for ORM, 3.6% for oncoplastic flap, and 6.7% for BCS alone. In an oncoplastic review, Haloua et al8 reported positive margins and recurrence rates for the oncoplastic flap procedure of 7–20% and 0–7%, respectively. The National Cancer Institute (NCI) reported a local recurrence rate of 18% over a 10-year period in patients operated on with clean margins for Stage I and Stage II breast cancer after BCS.14 Tanis et al15 reported a 10.1-year locoregional recurrence rate of 11% after BCS for cancer Stages I–III. In our study, positive margins occurred in 3.7% of cases, the breast recurrence rate was 4.9%, and the rate of locoregional recurrence was 6.1%. We believe that these low rates stemmed from the wide excision of nerves, tumors, and breast parenchyma en bloc, as well as from the use of intraoperative frozen section and specimen radiography. Our data show that ORM with macromastia provided better local control than BCS, oncoplastic breast surgery, or oncoplastic reduction (OR) alone. In this study, two cases of breast recurrence appeared at a delayed stage; it was interesting that both cases were invasive lobular cancer. The most important factor affecting local recurrence was a positive margin. In situ cancer, multifocality, and an extensive intraductal component were biological features associated with recurrence. The type of procedure used was not alone responsible for the outcome. Removing a large volume of breast material together with the tumor was associated with a better oncologic prognosis. Critics of oncoplastic surgery suggest that as the tumor bed location changes during breast reconstruction, there is a change in location for RT, and consequently this may lead to the need for larger RT doses. 8 ; 16 One study reported that breast recurrence mainly developed at the primary tumor site in patients undergoing ORM; therefore, RT should be directed to that area.17 In our study, three of the four recurrence incidents were in the primary tumor region, which would support additional RT being applied to that area. Although there are no reports of significant problems with postoperative imaging of OMC patients, we found that additional imaging and biopsy were necessary. 16 ; 18
| Reference | n | Follow-up, mo | Stage/tumor size, mm | Specimen, g | Positive margin, % | Recurrence, % | Survival, % | Complication, % |
|---|---|---|---|---|---|---|---|---|
| Newman et al9 | 28 | 24 | 15 | 766 | 7.1 | 0.0 | 96.0 | 35.7 |
| Chang et al10 | 37 | — | Ι, ΙΙ | 653 | 2.7 | 0.0 | 100.0 | 16.2 |
| Munhoz et al11 | 74 | 22 | — | 610 | 2.7 | 0.0 | 100.0 | 32.4 |
| Kronowitz et al12 | 41 | 36 | — | 626 | 7.3 | 4.9 | 100.0 | 34.1 |
| Currie et al13 | 20 | 34 | Ι, ΙΙ | 370 | 5.9 | 0.0 | — | 25.0 |
| This study | 82 | 121 | Ι, ΙΙ | 679 | 3.7 | 4.9 | 82.2 | 26.6 |
ORM = oncoplastic reduction mammoplasty.
Our study established good reliability for the long-term survival of women with macromastia undergoing ORM for breast cancer. We did not compare survival rates with similar studies and short follow-up periods (Table 5), but we did compare them with long-term survival rates reported in BCS procedures for breast cancer. An NCI study reported a 77% overall 10-year survival rate for Stage I and Stage II breast cancers that received BCS with an additional RT dose (15–20 Gy).14 The European Organization for Research and Treatment reported an overall 10-year survival rate of 65% after BCS treatment for early stage breast cancer with a 5-cm tumor.19 Rietjens et al20 reported a 93% survival rate over 74 months in a series of 148 cases with a median tumor size of 22 mm. Our survival rates were similar to these studies. We consider that there are no long-term oncological problems for ORM in patients with breast cancer and macromastia.
The histopathological evaluation of the contralateral breast is crucial. Freedman et al21 reported a hidden cancer rate of 0.3% in specimens from patients undergoing reduction surgery for macromastia, and a 3.6% rate in the contralateral breast in patients with cancer and macromastia. In our study, this rate was 6.1%. No suspicious areas were detected on the preoperative imaging of these patients. Because breast cancer cannot always be detected on preoperative imaging, great care should be taken during the histopathological evaluation of a specimen from the contralateral breast.
4.2. Macromastia: Complications
It was found that women with macromastia and breast cancer were very pleased with ORM outcomes. Because a very large amount of breast tissue was removed in this technique, complaints from patients regarding this technique may be decreased; in addition to this, the cancer treatment was provided simultaneously. Chadbourne et al22 noted that macromastia problems were resolved in more than half of the cases by reduction mammoplasty.22 This is the most attractive aspect of the procedure, as ORM surgery allows for wide excision of the tumor and provides a solution to the patients chronic problem. Furthermore, concomitant reconstructive surgery is cheaper than two stand-alone surgeries.
Our study highlights the need to improve complications rates for ORM. The frequency of complications during ORM treatment in patients with macromastia is 16.5–35.7% (Table 5). Complications due to breast reduction in noncancerous women with macromastia are as high as one third of cases in a series.23 The morbidity for this procedure alone is high. Complications from ORM in patients with breast cancer and macromastia do delay adjuvant therapy.18 For example, adjuvant therapy was delayed in four patients because of complications. We noticed that most complications in our series occurred during early follow-up. Most complications can be reduced with good patient selection, good planning, and experience. There are many arguments for the benefits and disadvantages of oncoplastic breast reduction surgery. However, we recommend ORM for patients with early stage breast cancer and macromastia.
4.3. Study limitation
ORM should not be assessed without the functional results, but this study focused only on the oncological results. Functional and aesthetic results are the subject of another study.
5. Conclusions
Long-term observations have demonstrated that ORM is a very safe and appropriate treatment for early stage breast cancer in women with macromastia, with regard to local control and metastasis formation. The breast cancer and macromastia problems of women should be considered in conjunction, rather than treating the breast cancer alone. For further results, larger and multisite studies are needed.
References
- 1 K.L. Dundas, J. Atyeo, J. Cox; What is a large breast? Measuring and categorizing breast size for tangential breast radiation therapy; Australas Radiol, 51 (2007), pp. 589–593
- 2 A. Losken, C.S. Dugal, T.M. Styblo, G.W. Carlson; A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique; Ann Plast Surg, 72 (2014), pp. 145–149
- 3 A.M. Moody, W.P. Mayles, J.M. Bliss, et al.; The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity; Radiother Oncol, 33 (1994), pp. 106–112
- 4 K.B. Clough, J. Cuminet, A. Fitoussi, C. Nos, V. Mosseri; Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction; Ann Plast Surg, 41 (1998), pp. 471–481
- 5 N. Kaur, J.Y. Petit, M. Rietjens, et al.; Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer; Ann Surg Oncol, 12 (2005), pp. 539–545
- 6 L. Blomqvist, A. Eriksson, Y. Brandberg; Reduction mammaplasty provides long-term improvement in health status and quality of life; Plast Reconstr Surg, 106 (2000), pp. 991–997
- 7 R. Kayar, S. Civelek, M. Cobanoglu, O. Gungor, H. Catal, M. Emiroglu; Five methods of breast volume measurement: a comparative study of measurements of specimen volume in 30 mastectomy cases; Breast Cancer (Auckl), 5 (2011), pp. 43–52
- 8 L.A. Newman, H.M. Kuerer, M.D. McNeese, et al.; Reduction mammoplasty improves breast conservation therapy in patients with macromastia; Am J Surg, 181 (2001), pp. 215–220
- 9 E. Chang, N. Johnson, B. Webber, et al.; Bilateral reduction mammoplasty in combination with lumpectomy for treatment of breast cancer in patients with macromastia; Am J Surg, 187 (2004), pp. 647–650
- 10 A.M. Munhoz, E. Montag, E.G. Arruda, et al.; Critical analysis of reduction mammaplasty techniques in combination with conservative breast surgery for early breast cancer treatment; Plast Reconstr Surg, 117 (2006), pp. 1091–1103
- 11 S.J. Kronowitz, K.K. Hunt, H.M. Kuerer, et al.; Practical guidelines for repair of partial mastectomy defects using the breast reduction technique in patients undergoing breast conservation therapy; Plast Reconstr Surg, 120 (2007), pp. 1755–1768
- 12 A. Currie, K. Chong, G.L. Davies; Using therapeutic mammoplasty to extend the role of breast-conserving surgery in women with larger or ptotic breasts; Ann R Coll Surg Engl, 95 (2013), pp. 192–195
- 13 M.H. Haloua, N.M. Krekel, H.A. Winters, et al.; A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects; Ann Surg, 257 (2013), pp. 609–620
- 14 J.A. Jacobson, D.N. Danforth, K.H. Cowan, et al.; Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer; N Engl J Med, 332 (1995), pp. 907–911
- 15 E. Tanis, C.J. van de Velde, H. Bartelink, M.J. van de Vijver, H. Putter, J.A. van der Hage; Locoregional recurrence after breast-conserving therapy remains an independent prognostic factor even after an event free interval of 10 years in early stage breast cancer; Eur J Cancer, 48 (2012), pp. 1751–1756
- 16 J.M. Roberts, C.J. Clark, M.J. Campbell, et al.; Incidence of abnormal mammograms after reduction mammoplasty: implications for oncoplastic closure; Am J Surg, 201 (2011), pp. 611–614
- 17 B.R. Eaton, A. Losken, D. Okwan-Duodu, et al.; Local recurrence patterns in breast cancer patients treated with oncoplastic reduction mammaplasty and radiotherapy; Ann Surg Oncol, 21 (2014), pp. 93–99
- 18 M.A. Gulcelik, L. Dogan, M. Camlibel, et al.; Early complications of a reduction mammoplasty technique in the treatment of macromastia with or without breast cancer; Clin Breast Cancer, 11 (2011), pp. 395–399
- 19 Effects of radiotherapy and surgery in early breast cancer; An overview of the randomized trials. Early Breast Cancer Trialists' Collaborative Group; N Engl J Med, 333 (1995), pp. 1444–1455
- 20 M. Rietjens, C.A. Urban, P.C. Rey, et al.; Long-term oncological results of breast conservative treatment with oncoplastic surgery; Breast, 16 (2007), pp. 387–395
- 21 B.C. Freedman, S.M. Smith, A. Estabrook, J. Balderacchi, P.I. Tartter; Incidence of occult carcinoma and high-risk lesions in mammaplasty specimens; Int J Breast Cancer, 2012 (2012), p. 145630
- 22 E.B. Chadbourne, S. Zhang, M.J. Gordon; Clinical outcomes in reduction mammaplasty: a systematic review and meta-analysis of published studies; Mayo Clin Proc, 76 (2001), pp. 503–510
- 23 O. Wolf, M. Westreich, A. Shalom; Trends in breast reduction technique; Isr Med Assoc J, 14 (2012), pp. 304–306
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?