Summary
Background/Objective
Defective defecation function, also known as low anterior resection syndrome (LARS), is a common problem after surgical treatment of rectal cancer that has a detrimental effect on quality of life. This study aimed to look for the incidence of LARS in patients whose native rectum could not be kept and determine factors influencing major LARS.
Methods
Rectal cancer patients who underwent tumor removal with mesorectal excision and colorectal anastomosis by a colorectal surgeon during the years 2004–2013 were asked to participate a structured interview using the verified version of the Low Anterior Resection Score questionnaire. Clinical parameters were analyzed against the incidence of major LARS. The cut-off anastomotic level that corresponded to the risk of major LARS was calculated by using a receiver operating characteristic curve. Anorectal physiology was compared between those with major LARS and those without LARS by anorectal manometry.
Results
This study included 129 patients (67 men and 62 women). Incidences of minor LARS (LAR score 21–29) and major LARS (LARS score ≥ 30) score 21een those with major LARS and those univariate analysis, factors associated with major LARS were extent of operation, presence of temporary ostomy, and chemoradiation therapy. Major LARS was found at 28.2% in those who underwent low anterior resection, which was significantly higher than the incidence of 5.2% in the anterior resection group (p < 0.01). Radiation therapy was the only factor independently associated with major LARS at an odds ratio of 6.55 (95% confidence interval: 2.37–18.15). The receiver operating characteristic curve plot between sensitivity and specificity of the anastomotic level in determining major LARS showed an area under the curve of 0.73. The cut-off anastomotic level that best predicted major LARS was at 5 cm, which gave a negative predictive value of 89%. Individual defecation symptoms that were significantly associated with major LARS included pain on defecation, difficulty holding stool, and needing to use a pad. Anorectal manometry showed a significant difference in the resting anal pressure and squeeze pressure, which suggests that derangement in sphincteric function caused by surgery and postoperative adjuvant treatment may contribute to the LARS.
Conclusion
LARS is a significant problem found in about one third of rectal cancer patients after colorectal anastomosis. Symptoms of concern include pain on defecation and decreased ability to hold. Risk of having major LARS increases with adjuvant treatment and lower anastomotic level.
Keywords
continence;low anterior resection syndrome;rectal cancer
1. Introduction
Colorectal cancer (CRC) is one of the leading cancers worldwide. In Thailand, the cancer ranks within the top five and is responsible for 15% of all cancers in men and 11% in women.1 Survival of CRC has markedly improved recently with an overall 5-year survival rate at ∼70%.2 In Songklanagarind Hospital, the major tertiary care unit and referral center in southern Thailand, a recent study reported that the 5-year survival rate of Stage I–III CRC was 73%.3
Low anterior resection (LAR) with a total mesorectal excision (TME) is the current gold standard surgical technique that is generally used for the mid and some lower level rectal cancers.4 ; 5 Recent advances in surgical techniques and neoadjuvant therapy have reduced the tumor recurrence rate after resection and, at the same time, provided a better chance to preserve the sphincter in rectal cancer patients whose tumor is situated in the lower rectum.6 Unfortunately, anatomical preservation of the sphincter does not always mean perfect restoration of anorectal functions, as many patients who undergo a LAR for rectal cancer suffer major defecation dysfunction, including incontinence, urgency, and clustering of stools. Such symptoms are defined as LAR syndrome (LARS) or anterior resection syndrome7 ; 8 and are usually associated with a negative impact on long term quality of life.9
A variety of studies have reported an incidence of LARS of between 19% and 52% in patients receiving a LAR, depending on syndrome classification as well as the period and intensity of follow-up.10; 11; 12 ; 13 Recent studies have addressed factors determining LARS, such as age, sex, surgical technique (mesorectal excision, intersphinteric resection, and temporary stoma), type of anastomosis, adjuvant therapy, neoadjuvant therapy, and postoperative complications (e.g., anastomosis leakage).6 ; 7 However, no consensus has yet been drawn regarding the major risk factors for LARS. Some studies have attempted to identify LARS risk factors, but have not indicated the statistical significance of the identified factors. Some recent studies have suggested that the level of anastomosis could be a crucial factor determining poor continence outcome. However, most studies have been limited in various ways and no significant conclusion could be reached.11; 13 ; 14 It is generally agreed that a low level of anastomosis tends to increase the risk of a worse outcome, which can be explained by the disturbance of normal physiology of rectal capacity and reduced rectal compliance after a LAR. However, there has been to date no study examining the relationship between anastomosis cut-off level and good continence.
In this study, we aimed to evaluate functional outcomes after LAR and anterior resection (AR) in patients with rectal cancer and address the incidence of LARS by using a standard questionnaire. Factors determining significant LARS, especially anastomosis level, were analyzed. In addition, anorectal manometric profiles were compared between the major LARS group and the normal one, in order to determine the pathophysiology of LARS.
2. Methods
Patients who were diagnosed with rectal cancer and had undergone a tumor resection with mesorectal excision in either AR or LAR method at our institution between 2004 and 2013 who met the inclusion criteria were asked to participate in the study. For analysis, LARs in this study were subgrouped into: conventional LAR (LAR, those with colorectal anastomosis); and extended LAR (ELAR: those with coloanal anastomosis). The indication for colostomy in this study was the preference of the attending surgeon, which generally depended on difficulty of the anastomosis as determined by height of the anastomosis from the anorectal ring, type of pelvis, and body build of the patient. All included patients had a postoperative follow-up period of at least 12 months, had completed their adjuvant treatment and had had their protective ostomy closed. Patients with a distant metastasis at the time of diagnosis, who had not had their ostomy closed prior to being considered for the study and/or had local recurrence after surgery and had neoadjuvant therapy were not included. All participants consented to a structured interview wherein they completed a questionnaire to assess their defecation functions. The questionnaire consisted of two parts, an LAR scoring part and an additional “stool diary” to look for any abnormal stooling behaviors (stool diary created by one of the authors, K.T.). The LAR score used the translated-to-Thai version of the questionnaire proposed by Emmertsen and Laurberg.15 Validation of the translated questionnaire was done before the study.
Clinical profiles were reviewed regarding each patient before the study and consisted of tumor, stage of disease (American Joint Committee on Cancer (AJCC) staging 7th edition), operative details, anastomosis level, adjuvant treatment and any postoperative surgical complications. Anastomotic levels were re-examined postoperatively by a flexible sigmoidoscopy or colonoscopy. Access to electronic medical records was approved by the Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand.
2.1. Anorectal manometry
After completing the questionnaire, patients were grouped according to their LAR score.16 Those with a LAR score of ≥30 were regarded as having major LARS while those scoring 21–29 were categorized as having minor LARS. Patients with no LARS and those with major LARS were invited to agree to anorectal manometry. On the day of the manometric study, the patients were asked to complete the questionnaire again in order to confirm that they remained in that group. A flexible sigmoidoscopy was performed before the manometric study in order to determine the anastomotic level.
The manometric study used a 10F UniTip solid state four-channel microtransducer (Unisensor AG, Attikon, Switzerland). The patient was placed in the left lateral position with knees flexed, the lubricated probe was introduced into the rectum and taped in place in order to locate the pressure sensors at 2 cm, 3 cm, 4 cm, and 9 cm from the anal verge. A 5-cm latex balloon was tied to the end of the probe, which was connected to a computer. Analysis was performed by Medtronic Polygram net software version 4.1.1322.287 (Medtronic, Solna, Sweden).
After a 10-minute run-in period, resting pressures of the anus and rectum were measured. The patient was then asked to perform sphincter squeezing and artificial defecation. Rectoanal inhibitory reflex (RAIR) readings were taken when the rectal balloon was inflated sequentially at 20 mL, 40 mL, 60 mL, 80 mL, 100 mL, 120 mL, and 150 mL. Reduction of the anal pressure of at least 50% of the resting pressure was deemed a positive rectoanal inhibitory reflex. Rectal sensation was tested by sequential balloon insufflation again and the patient was asked to tell the examiner when they felt the balloon in the rectum for the first time, desire to defecate, and felt an urgent need to defecate. Each test was performed at least in triplicate. Normal anorectal manometry profiles in Thai used the previous work by Kritasampan et al.16
2.2. Statistical analysis
Demographic data are presented as mean and standard deviation. The LAR score were stratified into three levels: no LARS (score 0–20), minor LARS (score 21–29), and major LARS (score 30–42). Analyzing for factors correlating with major LARS used either Chi-square test or Fishers exact test as appropriate. Further univariate and multivariate analyses for associations between those factors and major LARS used logistic regression.
Student t test was used to compare LAR scores among various levels of resection. Correlations between the cut-off anastomotic levels and their prediction of major LARS were evaluated by constructing a receiver operating characteristic (ROC) curve. The optimum cut-off point that specifically predicted a high risk of major LARS was determined by the crossing over point of the sensitivity and specificity curves plotted over different anastomotic levels. The statistical package Stata Release 14.0 (Stata, College Station, TX, USA) was used for statistical calculations.
3. Results
3.1. Demographic data
The inclusion criteria were met by 129 patients (67 men and 62 women) who agreed to participate in the study. The average age of the patients was 60.2 years (range 25–85 years). Eighty cases (62.0%) had localized disease (AJCC Stages I–II) and 49 cases (38.0%) had regional metastasis (Stage III) at the time of diagnosis. Of 102 cases whose tumor location was recorded, the primary tumor was situated <5 cm from the anal verge in 29 cases (28.4%), 5–10 cm in 31 cases (30.4%) and >10 cm in 42 cases (41.2%). Tumor resection was done with an anterior resection (AR) in 58 cases (44.9%), low anterior resection (LAR) in 33 cases (25.6%), and ELAR in 38 cases (29.5%). Among those who underwent ELAR, the anastomosis was constructed using a stapling device in 27 cases and hand-sewn technique in 11 cases. The anastomosis level ranged from 2–16 cm with an average level at 7.6 cm. On linear regression analysis, the anastomosis level was significantly correlated with the tumor level (p < 0.01, r2 = 0.49). A temporary ileostomy was performed in 41 cases (31.8%) and the median time from the operation to its closure was 308 days (average 318 days and range 105–830 days). A synchronous pelvic operation was performed in 20 cases (15.5%), in which hysterectomy with bilateral oophorectomy was the most common procedure.
Anastomotic complication occurred in six patients (4.7%), consisting of five leakages and one case of anastomotic disruption. Adjuvant chemoradiation therapy was given in 84 cases (65.1%), of which 35 cases received chemotherapy alone while three patients received radiation therapy only.
3.2. Anorectal symptoms and low anterior resection score
The mean interval between the primary operation and the interview date was 1458 days (median, 1159 days; range, 356–3584 days). The mean LARS score from these interviews was 15.4 (range, 0–41). When the LAR scores were stratified into three levels, 84 patients (65.2%) had no LARS, 22 (17.0%) had minor LARS, and 23 (17.8%) had major LARS. On analyzing factors that might be associated with major LARS, it was found that the extent of operation, presence of protective ostomy, chemotherapy, and radiation therapy were factors that were significantly associated with major LARS (Table 1). Extent of operation had the highest odds ratio (OR) as a risk factor for major LARS (Table 2). However, on multivariate analysis, radiation therapy was the only factor associated with major LARS (OR 6.5, 95% confidence interval 2.37–18.15).
| No. of cases | No/minor LARS | Major LARS | p | |||
|---|---|---|---|---|---|---|
| cases | (%) | cases | (%) | |||
| All | 129 | 106 | −82.2 | 23 | −17.8 | |
| Sex | 0.18 | |||||
| Male | 67 | 58 | −86.6 | 9 | −13.4 | |
| Female | 62 | 48 | −77.4 | 14 | −22.6 | |
| Age | 0.72 | |||||
| <60 years | 63 | 51 | −81 | 12 | −19 | |
| >60 years | 66 | 55 | −83.3 | 11 | −16.7 | |
| Extent of operation | <0.01 | |||||
| AR | 58 | 55 | −94.8 | 3 | −5.2 | |
| LAR/ELAR | 71 | 51 | −71.8 | 20 | −28.2 | |
| Temporary ostomy | 0.02 | |||||
| No | 88 | 77 | −87.5 | 11 | −12.5 | |
| Yes | 41 | 29 | −70.7 | 12 | −29.3 | |
| Operative complications | 0.31 | |||||
| No | 123 | 102 | −82.9 | 21 | −17.1 | |
| Yes | 6 | 4 | −66.7 | 2 | −33.3 | |
| Additional pelvic operation | 0.32 | |||||
| No | 109 | 88 | −80.7 | 21 | −19.3 | |
| Yes | 20 | 18 | −90 | 2 | −10 | |
| AJCC stage | 0.28 | |||||
| Stage 1–2 | 80 | 68 | −85 | 12 | −15 | |
| Stage 3 | 49 | 38 | −77.6 | 11 | −22.4 | |
| Follow–up duration | 0.98 | |||||
| <3 years | 62 | 51 | −82.3 | 11 | −17.7 | |
| >3 years | 67 | 55 | −82.1 | 12 | −17.9 | |
| Chemotherapy | 0.03 | |||||
| No | 48 | 44 | −91.7 | 4 | −8.3 | |
| Yes | 81 | 62 | −76.5 | 19 | −23.5 | |
| Radiation therapy | <0.01 | |||||
| No | 80 | 74 | −92.5 | 6 | −7.5 | |
| Yes | 49 | 32 | −65.3 | 17 | −34.7 | |
AR = anterior resection; ELAR = extended low anterior resection, LAR = low anterior resection.
| OR | 95% CI | p | |
|---|---|---|---|
| Temporary ostomy | 2.9 | (1.15–7.28) | 0.024 |
| Chemotherapy | 3.37 | (1.07–10.60) | 0.038 |
| Radiation therapy | 6.55 | (2.37–18.15) | <0.001 |
| Operation (ELAR + LAR/AR) | 7.18 | (2.01–25.60) | 0.002 |
AR = anterior resection; CI = confidence interval; ELAR = extended low anterior resection; LAR = low anterior resection; OR = odds ratio.
Considering correlations between individual defecation symptoms and major LARS, pain on defecation, difficulty holding, pampers dependent, and inability to discriminate between stool and flatus were the symptoms most likely to be associated with major LARS (Table 3).
| Symptom category | Frequency of major LARS/number of patients in each severity | p-value | |||
|---|---|---|---|---|---|
| No symptoms | Mild | Moderate | Severe | ||
| I. Quality of defecation | |||||
| Pain on defecation | 14/103 (13.6%) | 7/21 (33.3%) | 2/5 (40.0%) | – | 0.04 |
| Strain on defecation | 9/59 (15.3%) | 9/45 (20.0%) | 3/19 (15.8%) | 2/6 (33.3%) | 0.69 |
| Stool clustering in large amount | 9/58 (15.5%) | 7/50 (14.0%) | 7/21 (33.3%) | – | 0.11 |
| Hard stool | 12/63 (19.0%) | 7/51 (13.7%) | 4/15 (26.7%) | – | 0.48 |
| Need stool evacuation | 19/113 (17.0%) | 3/13 (23.1%) | 1/3 (33.3%) | – | 0.68 |
| Need laxative | 22/100 (22.0%) | 1/25 (4.0%) | 0/4 (0%) | – | 0.07 |
| Need enema | 23/125 (18.4%) | 0/2 (0%) | 0/2 (0%) | – | 0.64 |
| II. Continence | Had problem with; | ||||
| Never | Flatus | Loose stool | Formed stool | ||
| Difficulty holding | 12/101 (11.7%) | 2/15 (13.3%) | 5/14 (35.7%) | 4/9 (44.4%) | <0.01 |
| No | Yes | ||||
| Need to use pad/pampers | 18/119 (15.1%) | 5/10 (50.0%) | <0.01 | ||
| Inability to discriminate between flatus/stool | 18/117 (15.4%) | 5/12 (41.7%) | 0.02 | ||
3.3. Correlation between colorectal anastomotic level and LARS
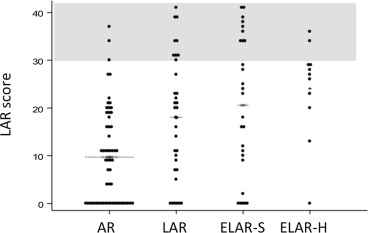
On comparing LARS scores between different levels of colorectal anastomosis, we found that those who had LAR/ELAR had a significantly higher score than AR (Figure 1). It seemed that a hand-sewn ELAR procedure was associated with a higher LARS scores than those closed with the stapling device, although the difference was not statistically significant.
|
|
|
Figure 1. Distribution of low anterior resection scores according to type of operation. AR = anterior resection; ELAR-S = extended low anterior resection using a stapling device; ELAR-H = extended low anterior resection using a hand-sewn technique; LAR = low anterior resection. |
3.4. Defining the cut-off anastomotic level that best indicates the probability of developing major LARS
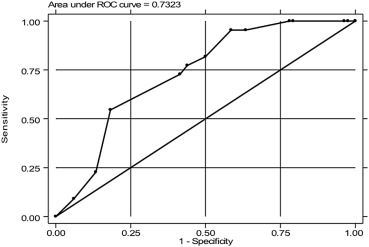
In order to evaluate the correlation between level of anastomosis and later development of major LARS, an ROC curve was constructed. The area under the curve was 0.73 (Figure 2). The crossing between sensitivity (percent of cases with anastomosis below the level of major LARS) and specificity (percent of cases with anastomosis above the level in cases without major LARS) the curve was between the anastomotic levels of 4 cm and 5 cm. Based on these data, if 5 cm was chosen as the optimum cut-off, the sensitivity was 72.7% and the specificity was 58.8%. The positive predictive value was 32.0% and the negative predictive value was 88.9%. Those with an anastomotic level <5 cm had a higher risk of having major LARS at the crude OR of 3.76 (95% confidence interval 1.34–10.61).
|
|
|
Figure 2. The receiver operating characteristic (ROC) curve plot between sensitivity and 1-specificity of anastomotic level in defining major low anterior resection syndrome. |
3.5. Correlation between anorectal manometry profile and LARS
The anorectal manometry profiles showed that mean resting anal pressure, mean anorectal pressure gradient, and maximum squeeze pressure in those with major LARS were significantly lower than in the patients who had good functional outcome (Table 4). Mean resting rectal pressure was not significantly different between the two groups. Notably, positive of rectoanal inhibitory reflex was found in only one patient in the major LARS group (11.1%), while a positive reflex was found in 42.8% of the no LARS patients (p = 0.22). Regarding sensory perception and rectal capacity, there were no statistically significant differences between the two groups.
| Parameter | No LARS (n = 16) | Major LARS (n = 12) | p |
|---|---|---|---|
| Mean resting rectal pressure (mmHg) | 24.2 | 22.8 | 0.71 |
| Mean resting anal pressure (mmHg) | 49.6 | 34.5 | 0.03 |
| Mean anorectal pressure gradient (mmHg) | 51.4 | 22.5 | 0.03 |
| Maximum squeeze pressure (mmHg) | 119.2 | 75.4 | 0.03 |
| Positive RAIRa | 6/16 (37.5%) | 1/12 (8.3%) | 0.22b |
| First sensation (mL) | 27.5 | 25 | 0.5 |
| Desire to defecate sensation (mL) | 57.5 | 53.3 | 0.72 |
| Urgency sensation (mL) | 80 | 80 | 0.18 |
RAIR = rectoanal inhibitory reflex.
a. Proportion of patients whose anal pressure drop > 50% when rectal balloon was adequately insufflated.
b. Fishers exact test for proportion difference.
4. Discussion
With improving surgical techniques of colorectal anastomosis, operations involving removal of CRC tumors with adequate surgical margin plus mesorectal excision followed by colorectal anastomosis have become the current surgical standard for rectal cancer.4 ; 5 However, anatomical restoration of rectal continuity does not always guarantee satisfactory defecation function. Even considering varying definitions, difficulty defecation syndrome has been reported in 19–52% of patients undergoing this procedure and the problem has been proven to affect long-term quality of life of rectal cancer patients.10; 11; 12; 13 ; 17 Using an internationally validated symptom-based scoring system, our finding of an overall incidence of major LARS at 28.2% in patients who underwent low anterior resection was comparable with a European study that used the same scoring system and reported 52% incidence.13 Interestingly, major LARS occurred not only in rectal cancer cases who underwent low anterior resection, but also in patients with a high-lying tumor who had anterior resection, although at a lower frequency. Understanding the pathophysiology of such failures of functional recovery may help in surgical decision making and anorectal rehabilitation for rectal cancer patients.
Mechanical injury to the sphincter and its related innervation have been suggested as the cause of LARS,12 and various other factors have been reported as possibly associated with LARS including female sex,17 techniques used in rectal reconstruction,12 adjuvant radiation therapy,18 presence of stoma, postoperative complications,19 and level of anastomosis.20 Among these factors, adjuvant radiation therapy has been the most extensively studied. In our study, we found no significant difference of the major LARS incidence between sexes. Consistent with previous reports,2 ; 21 our study showed that radiation therapy was the only factor independently associated with major LARS. In a manometric study by Lewis and colleagues,22 rectal capacity and high-pressure length were affected by postoperative irradiation. In addition, a study using endoanal ultrasound demonstrated significantly increased scarring in the anal sphincter in irradiated patients.23
Other factors found to correlate with functional outcomes that should be considered include the use of protective ostomy and the level of anastomosis. As with a recent study,17 our study found an association between a temporary ostomy and major LARS. Normally, the surgeon chooses to open a proximal stoma when they feel that the anastomosis is not secure enough. For this reason, the use of a protective ostomy itself might not have a direct causal relationship with anal physiology, but rather is a covariant of the anastomotic level. The anastomotic levels that were reported to be associated with major LARS ranged from <2 cm to 12 cm.10; 14 ; 20 However, the experimental foundation of those reported levels have never been clearly stated before and in our study, using serial analysis by ROC curve, we found that the cut-off that best correlated with a higher risk of having major LARS was 5 cm, which corresponded to a tumor level of around 7 cm from the anal verge.
Our study found decreased mean resting anal pressure in the major LARS group, which may explain the difficulty holding problem. Extensive surgical resection and radiation therapy probably contributed to this physiologic change. Alternative anastomotic techniques such as colonic pouch construction, use of the descending colon and coloplasty have been used in some centers.7 Recent meta-analyses have found that a pouch reconstruction gave better medium-term (8–18 months) functional outcomes in terms of stool frequency and need for antidiarrheal medications, although the best technique for optimal long-term outcomes remains unclear.24 ; 25
In conclusion, we used a standard questionnaire to survey the problem of LARS in Thai patients. The study found a rather high incidence of major LARS at 28.2% after low anterior resection. Radiation therapy and level of anastomosis <5 cm were the factors indicating higher risk of major LARS.
Acknowledgments
The authors thank the staff of the NKC institute of Gastroenterology and Hepatology for their cooperation in conducting this research. Dave Patterson helped editing the English Language in the manuscript.
References
- 1 P. Attasara, H. Sriplung; Cancer incidence in Thailand. National Cancer Institute of Thailand Volume VII, 2007–2009; (2013), pp. 31–33
- 2 B. Glimelius, H. Gronberg, J. Jarhult, A. Wallgren, E. Cavallin-Ståhl; A systematic overview of radiation therapy effects in rectal cancer; Acta Oncol, 42 (2003), pp. 476–492
- 3 A. Kritsanasakul, T. Boonpipattanapong, W. Wanitsuwan, M. Phukaoloun, P. Prechawittayakul, S. Sangkhathat; Impact of lymph node retrieval on surgical outcomes in colorectal cancers; J Surg Oncol, 106 (2012), pp. 238–242
- 4 G. Palmer, A. Martling, B. Cedermark, T. Holm; A population-based study on the management and outcome in patients with locally recurrent rectal cancer; Ann Surg Oncol, 14 (2007), pp. 447–454
- 5 C.J. van de Velde, P.G. Boelens, P.J. Tanis, et al.; Experts reviews of the multidisciplinary consensus conference colon and rectal cancer 2012: science, opinions and experiences from the experts of surgery; Eur J Surg Oncol, 40 (2014), pp. 454–468
- 6 K.J. Emmertsen, S. Laurberg; Bowel dysfunction after treatment for rectal cancer; Acta Oncol, 47 (2008), pp. 994–1003
- 7 Y.H. Ho; Techniques for restoring bowel continuity and function after rectal cancer surgery; World J Gastroenterol, 12 (2006), pp. 6252–6260
- 8 R. Kakodkar, S. Gupta, S. Nundy; Low anterior resection with total mesorectal excision for rectal cancer: functional assessment and factors affecting outcome; Colorectal Dis, 8 (2006), pp. 650–656
- 9 J. Camilleri-Brennan, D.A. Ruta, R.J. Steele; Patient generated index: new instrument for measuring quality of life in patients with rectal cancer; World J Surg, 26 (2002), pp. 1354–1359
- 10 A.S. Scheer, R.P. Boushey, S. Liang, S. Doucette, A.M. O'Connor, D. Moher; The long-term gastrointestinal functional outcomes following curative anterior resection in adults with rectal cancer: a systematic review and meta-analysis; Dis Colon Rectum, 54 (2011), pp. 1589–1597
- 11 F. Pucciani; A review on functional results of sphincter-saving surgery for rectal cancer: the anterior resection syndrome; Updat Surg, 65 (2013), pp. 257–263
- 12 Y. Ziv, A. Zbar, Y. Bar-Shavit, I. Igov; Low anterior resection syndrome (LARS): cause and effect and reconstructive considerations; Tech Coloproctol, 17 (2013), pp. 151–162
- 13 T. Juul, M. Ahlberg, S. Biondo, et al.; Low anterior resection syndrome and quality of life: an international multicenter study; Dis Colon Rectum, 57 (2014), pp. 585–591
- 14 O.O. Rasmussen, I.K. Petersen, J. Christiansen; Anorectal function following low anterior resection; Colorectal Dis, 5 (2003), pp. 258–261
- 15 K.J. Emmertsen, S. Laurberg; Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer; Ann Surg, 255 (2012), pp. 922–928
- 16 P. Kritasampan, S. Lohsiriwat, S. Leelakusolvong; Manometric tests of anorectal function in healthy adult Thai subjects; J Med Assoc Thai, 87 (2004), pp. 536–842
- 17 K.J. Emmertsen, S. Laurberg; Rectal Cancer Function Study Group. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer; Br J Surg, 100 (2013), pp. 1377–1387
- 18 S. Bregendahl, K.J. Emmertsen, J. Lous, S. Laurberg; Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study; Colorectal Dis, 15 (2013), pp. 1130–1209
- 19 C. Coco, V. Valentini, A. Manno, et al.; Functional results after radiochemotherapy and total mesorectal excision for rectal cancer; Int J Colorectal Dis, 22 (2007), pp. 903–910
- 20 Q. Denost, C. Laurent, M. Capdepont, F. Zerbib, E. Rullier; Risk factors for fecal incontinence after intersphincteric resection for rectal cancer; Dis Colon Rectum, 54 (2011), pp. 963–968
- 21 M.M. Lange, M. den Dulk, E.R. Bossema, et al.; Risk factors for faecal incontinence after rectal cancer treatment; Br J Surg, 94 (2007), pp. 1278–1284
- 22 W.G. Lewis, I.G. Martin, M.E. Williamson, et al.; Why do some patients experience poor functional results after anterior resection of the rectum for carcinoma?; Dis Colon Rectum, 38 (1995), pp. 259–263
- 23 J. Pollack, T. Holm, B. Cedermark, N.K. Holmstr, A. Mellgren; Long-term effect of preoperative radiation therapy on anorectal function; Dis Colon Rectum, 49 (2006), pp. 345–352
- 24 F.J. Hüttner, S. Tenckhoff, K. Jensen, et al.; Meta-analysis of reconstruction techniques after low anterior resection for rectal cancer; Br J Surg, 102 (2015), pp. 735–745
- 25 C.J. Brown, D.S. Fenech, R.S. McLeod; Reconstructive techniques after rectal resection for rectal cancer; Cochrane Database Syst Rev, 16 (2008), p. CD006040
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?