Abstract
Objective
The aim of this study was to investigate the effects of short-term, high-magnitude whole-body vibration (WBV) on serum type I collagen turnover in immobilized rats.
Materials and Methods
Thirty Wistar albino rats were randomly divided into the following 5 groups: immobilization (IS), immobilization + remobilization (IR), immobilization + WBV (IV), control (C), and WBV control (CV). Immobilization was achieved by casting from the crista iliaca anterior superior to the lower part of the foot for 2 weeks. The applied WBV protocol involved a frequency of 45 Hz and amplitude of 3 mm for 7 days starting a day after the end of the immobilization period. Serum type I collagen turnover markers were measured by using ELISA kits.
Results
Serum NH2-terminal propeptide of type I collagen (PINP) levels were significantly lower in the immobilization groups (p < 0.02) compared with the control groups. Although WBV improved PINP levels in the control groups, there were no differences in PINP levels among the immobilization groups. Similarly, serum COOH-terminal telopeptide of type I collagen (CTX) levels were higher in the WBV controls than their own controls (p < 0,05). Immobilization led to deterioration of tendon tissue, as observed by histopathological analysis with a transmission electron microscope.
Conclusion
Although 1 week of WBV had a positive effect on type I collagen turnover in controls, it is not an efficient method for repairing tissue damage in the early stage following immobilization.
Keywords
Rehabilitation ; Immobilization ; Whole-body vibration ; Collagen ; PINP
Introduction
Despite its detrimental effects on the musculoskeletal system, immobilization is still an imperative and frequently used treatment protocol in sports injuries, particularly in those involving the lower extremities. It is a known fact that immobilization results in muscle atrophy, ultrastructural deterioration of the tendons, bone degeneration, joint stiffness, and functional limitations of the musculoskeletal system.1 ; 2 ; 3 Therefore, providing the optimal treatment and rehabilitation post immobilization is essential in minimizing the harmful effects of immobilization on the components of the muscle-tendon unit.4 ; 5
Recently, research has drawn attention to the therapeutic effects of vibration techniques.6 Whole-body vibration (WBV) in particular has been shown to possess great potential in the treatment of several musculoskeletal system pathologies. Mechanical stimulation similar to that applied by WBV appears to be beneficial for the maintenance and/or enhancement of the trabecular bone volume and skeletal mass in individuals with low bone mineral density.7 ; 8 ; 9 Experimental evidence indicates that rehabilitation protocols involving WBV may be useful in restoring muscle strength, balance, and mobility in the elderly and in patients with musculoskeletal problems.10 ; 11
In light of these findings, WBV was thought to be an alternative treatment option for accelerated rehabilitation of athletes following immobilization. To show the effects of WBV on the musculoskeletal system, the levels of serum Type 1 collagen turnover, a major collagen in the bone and tendon tissue was evaluated. It was hypothesized that immobilization would result in the reduction of collagen synthesis and an increase in collagen degradation and that the application of WBV would have a positive effect on collagen turnover. The serum N-terminal propeptide of Type 1 collagen (PINP) level was used as a collagen Type 1 formation marker and the serum C-terminal telopeptide of Type 1 collagen (CTX) level was used as an indirect marker of collagen Type 1 degradation.1 ; 12
Our objective was to evaluate how Type 1 collagen turnover was affected by immobilization and subsequent vibration.
Materials and Methods
Study design
Thirty Wistar albino female rats aged 4–6 months (mean weight: 230.9 ± 23.3 g, range: 200–250 g) were included in the study. The rats were housed for one week in an accredited animal facility room with controlled temperature (22 °C), humidity (50 ± 10%), and light (12-hr light/dark cycle) in order to adapt with the environmental conditions. They were then randomly divided into the IS, IR, IV, C and CV groups (Table 1 ), each consisting of six animals. Animals in each group were sacrificed at the end of the protocol.
| Day | 1–14 days | 15 day | 16–21 days | 22 day |
|---|---|---|---|---|
|
Casting immobilization | S | ||
|
Casting immobilization | Remobilization | S | |
|
Casting immobilization | WBV | S | |
|
– | S | ||
|
– | WBV | S | |
Casting
Casts were applied from the anterior superior iliac crest to the lower part of the foot (pelvipedal cast). The animals were anesthetized with short-acting anesthesia just prior to the casting procedure (90 mg/kg ketamine + 10 mg/kg xylazine i.p.). While anesthetized, the hindlimbs were immobilized with the hip and knee joints fixed at approximately 160° and 180°, respectively, as previously described.13 To induce deterioration in the Achilles tendon, the ankle joints were immobilized at 25°–30° of plantar flexion. To minimize movement in the casts, slight pressure was applied when wrapping the plaster on the body. Care was taken to minimize the weight of the cast, with a target weight of 90–120 g, and standardization in each group. The rats were checked on daily basis for chewed plaster, abrasions, venous occlusion, and fecal clearance. The rats were free to move using their forelimbs, and they ate and drank ad libitum . The casts were removed after two weeks. The length of the casting period was chosen to simulate the immobilization period after a sports injury or after a period of hospitalization.
WBV protocol
To evaluate the immediate effects of vibration after two weeks of immobilization (IV group) and effects of vibration on control subjects (CV group), WBV with a frequency of 45 Hz and an amplitude of 3 mm was applied. The WBV platform, which directly provided mechanical stimulation on the animals' feet, was designed by the mechanical engineers of Hacettepe University, School of Sports Sciences and Technology. For the animals to adapt, intermittent vibration was used with cycles of vibration and rest periods. The WBV application was initiated with a duration of 15 min on the first day and then was gradually increased by 5 min each day until it reached a maximum of 30-min application a day. A detailed explanation of the WBV protocol is provided in Table 2 . Animals in the IR group were kept on the vibration platform for the same duration as those in the IV and CV groups, but they did not undergo WBV.
| Day | Frequency | Type of application | Total time |
|---|---|---|---|
| 1 | 45 Hz | 1 min. WBV–1 min. rest | 15 min |
| 2 | 45 Hz | 2 min. WBV–1 min. rest | 20 min |
| 3 | 45 Hz | 2 min. WBV–1 min. rest | 25 min |
| 4 | 45 Hz | 2 min. WBV–1 min. rest | 30 min |
| 5 | 45 Hz | 2 min. WBV–1 min. rest | 30 min |
| 6 | 45 Hz | 2 min. WBV–1 min. rest | 30 min |
| 7 | 45 Hz | 2 min. WBV–1 min. rest | 30 min |
Measurement of serum Type 1 collagen turnover
Just before the animals were sacrificed, blood samples (2 ml) were collected from the aorta, following a high-dose of anesthesia. Serum samples were centrifuged for 10 min at 3500 g in a frigofric centrifuge and stored at −80 °C until analysis. PINP (CSB-E12774r; Cusabio Biotech Co., Wuhan, Hubei Province, China) and CTX (CSB-E12776r; Cusabio Biotech Co.) were measured using a commercial sandwich ELISA assay kit, following the manufacturers instructions.12 The enzyme–substrate reaction was terminated by the addition of 50 μl stop solution, and the optical density and color change of each well was measured using a microplate reader spectrophotometer (SpectraMax Plus 384 Microplate Reader; Molecular Devices, LLC, Sunnyvale, CA, USA) at a wavelength of 450 ± 2 nm. The PINP and CTX levels in the samples were determined by comparing the optical density of the samples with the standard curve. The detection ranges were 62.5–4000 pg/ml for PINP and 0.47–30 ng/ml for CTX.
Histopathology
The Achilles tendon was dissected from the calcaneus and musculotendinous junction via an incision over 2 cm proximal to the Achilles tendon insertion for histopathological analysis under a transmission electron microscope (TEM). Tissue samples taken from the Achilles tendons of both limbs were fixed in 2.5% glutaraldehyde for 24 h, washed in Sørensens phosphate buffer (pH 7.4), and postfixed in 1% osmium tetroxide in a phosphate buffer (pH 7.4) for one hour. Then, a third fixative, 10% formaldehyde, was applied to the samples for one hour before they were dehydrated in increasing concentrations of alcohol (25%, 50%, 75%, and pure alcohol). After this procedure, the samples were washed with propylene oxide and embedded in an epoxy-resin-containing media. Semi-thin sections of approximately 2 μm thickness and ultra-thin sections of approximately 60 nm thickness were cut with a glass knife on an LKB Nova ultramicrotome (LKB, Bromma, Sweden). Ultra-thin sections were collected on copper grids, stained with uranyl acetate and lead citrate, and examined with JEM-1200EX TEM (JEOL Ltd., Tokyo, Japan).14
In addition to these evaluations, the soleus muscles (which can be easily differentiated from the tendon tissue) were carefully dissected from the proximal and distal musculotendinous complexes from both hindlimbs, and the wet weights were calculated using a Sartorius CP225D scale (d = 0.01 mg, max = 220 g). The soleus muscles of the right hindlimbs were selected to determine whether casting may cause atrophy and WBV may improve muscle mass.
Statistics
The parameters were compared among groups with non-parametric methods. Statistical evaluation of intergroup comparisons was performed using the non-parametric Kruskal–Wallis test. The Mann–Whitney U test was employed for pairwise comparisons between IS-IR, IS-IV, IR-IV, IS-C, IV-C, and C-CV groups when a significant difference was present. The Bonferroni correction was used when multiple comparison tests were performed, and the level of statistical significance was set at p < 0.05.
Results
Weight parameters
Having equal cast weights among the rats was critical for the standardization of the casting effects. There were no statistically significant differences among the groups in terms of cast weight; IS (103.5 ± 16.4 g), IR (112.9 ± 11.9 g), and IV (100.4 ± 17.2 g). In addition, body weight loss between the baseline and final (just prior to sacrifice) time points was measured and was found to have a significant difference between the IS (−26.5 ± 12.5 g) and IV (−10.53 ± 7.64 g) (p = 0.01) groups. However, no difference was detected between the IS and IR (−17.05 ± 9.08 g) (p = 0.1) groups. This may indicate that post-immobilization WBV therapy enhances body weight gain compared to the resting strategy.
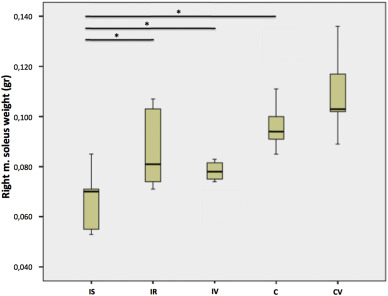
The soleus muscle weight measurements revealed that immobilization decreased the soleus mass in the control groups, but one week of WBV application did not enhance the soleus mass significantly in the IV group when compared with the IS and IR groups (p < 0.001) (Table 3 ).
|
| ||||||||||||
|
Table 3. Right soleus muscle weights. |
Serum protein levels
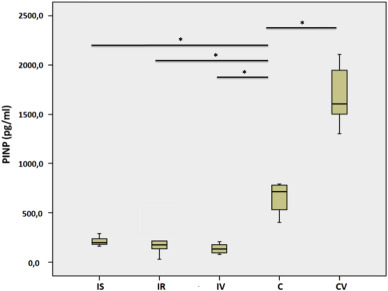
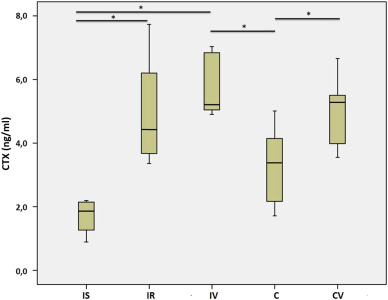
In the immobilized rats, PINP was significantly decreased when compared with those in the control groups (p < 0.02). Although WBV significantly increased the PINP levels in the control subjects (CV vs. C, p = 0.01), no significant effect of WBV on immobilized rats was shown (Table 4 ). CTX was significantly increased in the immobilized rats when compared with the control rats. However, there were no significant differences between the immobilized groups (IS vs. IR or IR vs. IV). As was the case for PINP, WBV increased collagen degradation markers more in the CV group than the C group (Table 5 ).
|
| ||||||||||||
|
Table 4. Serum PINP levels. |
|
| ||||||||||||
|
Table 5. Serum CTX levels. |
Histopathology
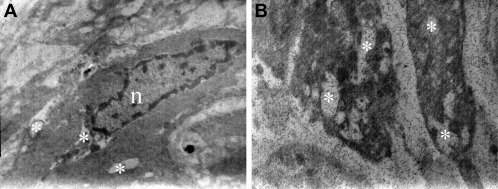
Viewing the Achilles tendons of the immobilized rats (IS, IR, and IV groups) under a TEM revealed widespread and regular placement of collagen fibers. A few tenocytes and fewer tenoblasts were detected between collagen fiber bundles. Pathological evidence among collagen bundles was not found and the ultrastructural composition of the collagen fibers was regular. The nuclei of the tenocytes and tenoblasts, cell membranes, and intracytoplasmic organelles were completely normal. However, under a TEM, vacuoles in the cytoplasm of the tenocytes and tenoblasts were observed on the entire tissue surface in each sample (Fig. 1 ). On the other hand, histopathological results of the control groups (C and CV) were normal (Fig. 2 ).
|
|
|
Fig. 1. Electron microscope images of the immobilized animals (IS, IR, IV groups) showing loosened collagen structure, disruption and splitting of the tendon fibers and vacuoles (Ж) in the cytoplasm of (a) a tenoblast (×10,000) and (b) a tenocyte (×7500). |
|
|
|
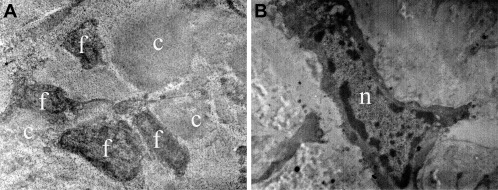
Fig. 2. Electron microscope images from the (a) C and (b) CV groups. Tenocytes (f), collagen fibers (c) (×5000), the nuclei of a tenocyte (n) (×10,000) and ultrastructure of the Achilles tendon (×7500) look completely normal. |
Discussion
Our histopathological analyses have shown that immobilization resulted in damage of the tendon tissue and caused soleus muscle atrophy. Although WBV played a positive role in Type 1 collagen levels in the control subjects, there were no effects of WBV on tissue healing or collagen turnover in the IV group when compared with the remobilization group (IR).
Effects of immobilization on muscle and tendon structure
To simulate the daily medical practice of immobilization, a hindlimb casting model was applied. The length of the immobilization period (14 days) was chosen to simulate the average duration of treatment after a sports injury.
Fixation of a muscle in a slackened state reduces muscular protein synthesis and increases protein degradation, resulting in a net weight loss of the immobilized muscle.15 ; 16 Since the casting of hindlimbs in this study was applied to the ankle in a shortened position of 25°–30° plantar flexion, soleus muscle atrophy was observed as expected. WBV may augment skeletal mass through direct mechanical perturbation or modulation of tissue perfusion; however, this augmentation may also develop from vibration-induced fluctuations in hormones capable of modulating bone cell activity.11 Xie et al reported an increase in total cross-sectional area of the soleus muscle in mice after six weeks of WBV application.17 In line with this studys findings, we observed an increase in soleus muscle wet weight in our control subjects, although the difference was not significant (p = 0.125). In contrast to previous studies, however, our results showed that WBV was not able to reverse soleus muscle atrophy over the course of a week. In fact, we now know that remobilization and rehabilitation of any component of the musculoskeletal tissue requires much more time than what is needed to cause the immobilization atrophy in the first place, and our study showed that there was no significant difference in soleus muscle wet weights between the IR and IV groups.3 ; 18 It is possible that prolonged application of WBV may enhance muscle mass; thus, future studies should be designed with longer periods of WBV administration.
Immobilization alters the ultrastructure of the muscle in a negative way and the biochemical and biomechanical properties of the tendon are affected.19 Immobilization causes atrophy of the tendon, but due to low metabolic rate and vascularity, the effects are gradual and not as dramatic as those in muscle tissues.3 Tensile strength, elastic stiffness, and total weight of the tendon decrease with a prolonged reduction in activity. On a microscopic level, collagen fibers become thinner and disorientated, and longitudinal splitting and abnormal angulation of the fibers is observed. In our study, although pathological evidence among collagen bundles was not found and the ultrastructural compositions of the collagen fibers were regular in the tendon samples as, vacuoles in the cytoplasm of the tenocytes and tenoblasts were observed on the entire tissue surface in each sample. These findings support that the duration of immobilization was long enough to generate adverse effects on the tendon tissue. Although Christensen et al published MRI reports of the cross-sectional areas of the Achilles tendon in humans and suggested that tendon tissue was resistant to short-term (two-week) immobilization,1 we observed immobilization-induced harmful effects on the tendon tissues after one week, using histopathological analysis. These conflicting results may be due to differences in species used in the respective studies, i.e., differences between humans and rats. However, it could also mean that in Christensen et al.’s study, the ankle joint was immobilized in a neutral position whereas in our study it was immobilized at 25°–30° plantar flexion in the slack position (shortened). It is worth noting that if the tendon is stretched during immobilization (lengthened position), the tendon tends to be more resistant to atrophy.1 This may reflect a length-associated adaptive response, where the tendon reacts to a chronically lengthened position by elongation. Indeed, our results were comparable to the findings of Nakagawa et al who reported a decrease in the thickness, as well as a reduction in the average diameter of collagen fibrils of the Achilles tendons immobilized at a similar angle.20
It is well known that remobilization and rehabilitation of any component of the musculoskeletal tissues requires much more time than that needed to cause the immobilization atrophy in the first place. In addition, it has been shown that, compared to the muscle tissues, the metabolic turnover of the tendon tissue is much slower due to poorer vascularity and circulation.3 The adaptive response of the tendons to remobilization and mechanical stimuli are, therefore, slower than that of the muscles. Nevertheless, we investigated whether vibration therapy would hasten the proliferative phase (fibroblast proliferation and fibrillogenesis, from two days up to six weeks) following immobilization and an inflammatory period. Our results have shown that seven days of WBV appear to be insufficient to heal acute hypoxic degenerative signs in tenocytes such as alterations in the size and shape of the mitochondria and nuclei, hypoxic and lipid vacuoles, and immobilization-associated necrosis. TEM revealed no vacuoles or any signs of hypoxia in animals in the CV group, which may indicate that exposure to one week of vibration does not cause any deterioration in the tendon structure. Further studies should be designed with various durations of WBV to include the maturation and remodeling phases of tendon healing to determine whether WBV can reverse histopathological deterioration in the Achilles tendon faster than remobilization alone.
Effects of immobilization and WBV on collagen turnover
In recent years, vibration therapies have been a topic of much interest for musculoskeletal disorders and many studies have been designed to clarify their effects. To assess whether WBV could be an alternative treatment option for accelerated rehabilitation and improved tissue healing, we investigated the effects of short-term, high-magnitude WBV on serum Type 1 collagen turnover. To our knowledge, this is the first study to analyze the effects of mechanical stimuli following immobilization on serum collagen turnover.
Collagen is the major protein of the musculotendinous connective tissue matrix and tendons consist almost entirely of Type 1 collagen fibrils.15 Moreover, Type 1 collagen is the predominant collagen solubilized from skeletal muscle tissue. Over the past decade, an array of immunoassays has been launched for in vitro , in vivo , and ex vivo investigations into collagen turnover indicators for immobilization studies. PINP is the N-terminal propeptide that is removed by enzymatic cleavage from the procollagen molecule, a necessary prerequisite for collagen fiber formation. 21 The removal of these propeptides indicates something about the rate of collagen synthesis. CTX and C-terminal telopeptide region of Type 1 collagen (ICTP) are both telopeptides that are cleaved from the collagen molecule during resorption and can thus be used as indirect markers of collagen Type 1 degradation.22
The hypothesis of the present study was that collagen synthesis would decrease with immobilization and increase with mechanical stimuli. As expected, PINP was significantly reduced in response to immobilization (p < 0.05) and mechanical stimulation significantly improved the serum PINP levels in control subjects (p = 0.01). However, in contrast to our predictions, serum PINP levels remained unchanged after one week of WBV application (IV group) or remobilization (IR group) following two weeks of immobilization in rats. Few studies have investigated serum Type 1 collagen synthesis markers in immobilized subjects and the results are controversial. Christensen et al evaluated the systemic PINP levels in healthy young men that were immobilized for two weeks and reported that they remained unchanged throughout the study.1 This finding agrees with the study of Baecker et al, which showed that PINP and bone alkaline phosphatase (BAP) were not affected by 14 days of bed rest.7 The discrepancy between these studies and the present one may be related to differences between species, i.e., differences between humans and rats. Similar results to our study have been reported with the findings of Karpakka et al and Savolainen et al, assessing the collagen synthesis in peritendinous tissue of immobilized subjects are consistent. The authors reported a decrease in the activity of enzymes involved in the biosynthesis of Type 1 collagen (prolyl 4-hydroxylase and galactosylhydroxylysyl glucosyltransferase) in rat tendons in response to immobilization, but only when the Achilles tendon was immobilized in a shortened position.23 ; 24 However, a decrease in the activity of enzymes involved in the biosynthesis of Type 1 collagen following immobilization has been shown in animal studies. The findings from these animal studies were supported by those of deBoer et al who designed the first human study about the effects of immobilization on collagen synthesis and demonstrated that local collagen synthesis in the patellar tendon was decreased with 21 days of immobilization.2
Previous studies have tended to measure urine CTX levels as a collagen degradation marker; therefore, investigations into the effects of CTX on the healing response are scarce. We found that CTX levels were significantly lower in the serum of the rats sacrificed after two weeks of casting (IS group) (p < 0.05). This finding is opposite to our expectation and needs to be clarified with further studies. On the other hand, WBV significantly increased the CTX level in the CV group in comparison to that of Group C (p < 0.05). This is in accordance with the result of increased collagen synthesis with WBV in control subjects.
In conclusion, we assert that two weeks of immobilization is sufficient to deteriorate the musculotendinous tissue and that a short-term, high-magnitude WBV treatment applied for a week is unable to repair this damage. However, WBV may improve the collagen turnover in mobile subjects. The use of WBV for therapeutic purposes seems to be far from being standardized and the effects of WBV on the tendon system remain to be clarified.
References
- 1 B. Christensen, E. Dyrberg, P. Aagaard, M. Kjaer, H. Langberg; Short-term immobilization and recovery affect skeletal muscle but not collagen tissue turnover in humans; J Appl Physiol, 105 (6) (2008), pp. 1845–1851
- 2 M.D. de Boer, A. Selby, P. Atherton, et al.; The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse; J Physiol, 585 (2007), pp. 241–251
- 3 P. Kannus, L. Józsa, A. Natri, M. Järvinen; Effects of training, immobilization and remobilization on tendons; Scand J Med Sci Sports, 7 (2) (1997), pp. 67–71
- 4 M. Majewski, S. Schaeren, U. Kohlhaas, P.E. Ochsner; Postoperative rehabilitation after percutaneous Achilles tendon repair: early functional therapy versus cast immobilization; Disabil Rehabil, 30 (20-22) (2008), pp. 1726–1732
- 5 M. Vioreanu, S. Dudeney, B. Hurson, E. Kelly, K. O'Rourke, W. Quinlan; Early mobilization in a removable cast compared with immobilization in a cast after operative treatment of ankle fractures: a prospective randomized study; Foot Ankle Int, 28 (1) (2007), pp. 13–19
- 6 K. Chanou, V. Gerodimos, K. Karatrantou, A. Jamurtas; Whole-body vibration and rehabilitation of chronic diseases: a review of the literature; J Sports Sci Med, 11 (2) (2012), pp. 187–200
- 7 N. Baecker, P. Frings-Meuthen, M. Heer, J. Mester, A.M. Liphardt; Effects of vibration training on bone metabolism: results from a short-term bed rest study; Eur J Appl Physiol, 112 (5) (2012), pp. 1741–1750
- 8 B. Christiansen, M.J. Silva; The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice; Ann Biomed Eng, 34 (7) (2006), pp. 1149–1156
- 9 B.V. Keller, M.L. Davis, W.R. Thompson, L.E. Dahners, P.S. Weinhold; Varying whole body vibration amplitude differentially affects tendon and ligament structural and material properties; J Biomech, 46 (9) (2013), pp. 1496–1500
- 10 P.J. Marín, A. Martín-López, D. Vicente-Campos, et al.; Effects of vibration training and detraining on balance and muscle strength in older adults; J Sports Sci Med, 10 (3) (2011), pp. 559–564
- 11 R.D. Prisby, M.H. Lafage-Proust, L. Malaval, A. Belli, L. Vico; Effects of whole body vibration on the skeleton and other organ systems in man and animal models: what we know and what we need to know; Ageing Res Rev, 7 (4) (2008), pp. 319–329
- 12 O. Orum, M. Hansen, C.H. Jensen, et al.; Procollagen type I N-terminal propeptide (PINP) as an indicator of type I collagen metabolism: ELISA development, reference interval, and hypovitaminosis D induced hyperparathyroidism; Bone, 19 (1996), pp. 157–163
- 13 T.N. Frimel, F. Kapadia, G.S. Gaidosh, Y. Li, G.A. Walter, K. Vandenborne; A model of muscle atrophy using cast immobilization in mice; Muscle Nerve, 32 (5) (2005), pp. 672–674
- 14 O.A. Atay, M. Pekmezci, M.N. Doral, M.F. Sargon, M. Ayvaz, D.L. Johnson; Discoid meniscus: an ultrastructural study with transmission electron microscopy; Am J Sports Med, 35 (3) (2007), pp. 475–478
- 15 J. Savolainen, R. Myllyla, V. Vihko, K. V’daniinen, T.E.S. Takala; Effects of denervation and immobilization on collagen synthesis in rat skeletal muscle and tendon; Am J Physiol, 254 (1988), pp. R897–R902
- 16 D.F. Goldspink; The influence of immobilization and stretch on protein turnover of rat skeletal muscle; J Physiol Land, 264 (1977), pp. 267–282
- 17 L. Xie, C. Rubin, S. Judex; Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations; J Appl Physiol, 104 (4) (2008), pp. 1056–1062
- 18 N.C. Pathare, J.E. Stevens, G.A. Walter, et al.; Deficit in human muscle strength with cast immobilization: contribution of inorganic phosphate; Eur J Appl Physiol, 98 (2006), pp. 71–78
- 19 L. Stehno-Bittel, G.K. Reddy, S. Gum, C.S. Enwemeka; Biochemistry and biomechanics of healing tendon. I. Effects of rigid plaster casts and functional casts; Med Sci Sports Exerc, 30 (1998), pp. 788–793
- 20 Y. Nakagawa, M. Totsuka, T. Sato, Y. Fukuda, K. Hirota; Effect of disuse on the ultrastructure of the achilles tendon in rats; Eur J Appl Physiol, 59 (1989), pp. 239–242
- 21 M. Miyahara, F.K. Njieha, D.J. Prockop; Formation of collagen fibrils in vitro by cleavage of procollagen with procollagen proteinases; J Biol Chem, 257 (1982), pp. 8442–8448
- 22 P. Garnero, M. Ferreras, M.A. Karsdal, et al.; The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation; J Bone Min Res, 18 (2003), pp. 859–867
- 23 J. Karpakka, K. Väänänen, P. Virtanen, J. Savolainen, S. Orava, T.E.S. Takala; The effects of remobilization and exercise on collagen biosynthesis in rat tendon; Physiol Scdnd, 139 (1990), pp. 139–145
- 24 J. Savolainen, K. Väänänen, V. Vihko, J. Puranen, T.E. Takala; Effect of immobilization on collagen synthesis in rat skeletal muscles; Am J Physiol, 252 (5 Pt 2) (1987), pp. R883–R888
Document information
Published on 31/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?