Summary
Background
Dissecting aneurysms of the intracranial carotid circulation were previously thought to occur primarily in young people presenting with cerebral infarction caused by arterial stenosis and occlusion. The appropriate management of dissecting aneurysms in the anterior circulation remains controversial, especially in patients who also present with cerebral infarction. However, recent studies have reported better outcomes for patients with middle cerebral artery (MCA) dissecting aneurysms involving surgically treated subarachnoid hemorrhage (SAH). The purpose of this study is to describe a case of spontaneous SAH from rupture of a dissecting aneurysm in the M2 segment observed in a 79-year-old man with no sign of an ischemic neurological deficit, and also to review the clinical and radiological features of cases reported since 1990.
Methods
Our review of the literature identified 24 cases of MCA dissecting aneurysms after 1990.
Results
Of the patients in these cases, 15 (63%) presented with pure bleeding and 7 (29%) with ischemia, and two were detected incidentally. Our review also found that the outcome of patients presenting with pure bleeding differed from those with ischemia. Patients with an MCA dissecting aneurysm who presented with pure bleeding showed better outcomes if they had surgery than if they did not. In contrast, the appropriate management of patients with a dissecting aneurysm who present with ischemia remains controversial.
Conclusion
Our review found that the clinical course of patients presenting with ischemia differed from that of patients presenting with pure bleeding. Most of the patients with ischemia underwent progressive deterioration. However, while the outcome for patients with ischemia treated surgically was relatively good, it remained poor compared to the outcome for patients who had been bleeding.
Keywords
aneurysm surgery;MCA dissecting aneurysm;subarachnoid hemorrhage
1. Introduction
It is extremely rare for distal aneurysms to develop secondary to dissection of the intracranial arteries. Most intracranial dissecting aneurysms of the vertebrobasilar system are associated with subarachnoid hemorrhage (SAH), whereas those of the carotid system typically present with cerebral infarction caused by arterial stenosis and occlusion.1 ; 2 It has also been reported that only 14% of intracranial dissecting aneurysms causing hemorrhage occur in the anterior circulation. These observations suggest that local anatomic factors may play a role in the pathogenesis of dissection.3
Most studies have focused on dissecting aneurysms occurring in the vertebrobasilar system, with the pathogenesis, clinical features, neuroradiographic findings, and treatment of these aneurysms now well documented.4; 5; 6; 7 ; 8 Publications concerning dissecting aneurysms of the intracranial carotid circulation have been limited to case reports. Dissecting aneurysms of the intracranial carotid circulation were previously thought to occur primarily in young people presenting with symptoms such as headache, focal neurological deficits, and intracranial hemorrhage.9 However, a number of recent case reports have described SAH from a dissecting aneurysm of the intracranial carotid circulation. These reports indicate that the clinical features and pathogenesis of dissecting aneurysms of the intracranial carotid circulation are not well understood.
Here we present a case of spontaneous SAH from rupture of a dissecting aneurysm in the M2 segment, where there was no sign of an ischemic neurological deficit.
2. Case report
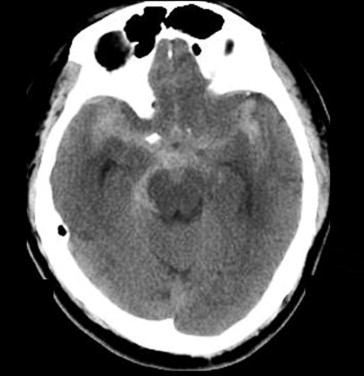
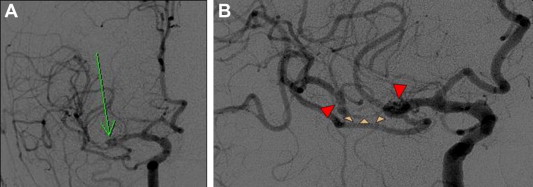
A 79-year-old hypertensive man with abrupt onset of severe headache during defecation was admitted to our hospital on January 17, 2007. There was no initial loss of consciousness, and on admission, his Glasgow Coma Scale score was 15. The patient did not have a clear history of trauma or heart disease. Headache and neck stiffness were reported by the patient but there was no obvious neurological deficit. There were no abnormalities detected with hematological and laboratory examinations, or with electrocardiography, and there were no signs of sepsis. Computed tomography (CT) performed upon admission revealed a SAH (Fig. 1), the Hunt and Hess grade for which was 2. A CT angiogram revealed segmental narrowing with diffuse atherosclerosis and segmental stenosis in the M2 segment of both branches of the MCA (Fig. 2). Angiography also identified a fusiform outpouching in the M2 segment of the right MCA. The diagnosis was of a right M2 dissecting aneurysm, but the etiology of this aneurysm was not clear.
|
|
|
Figure 1. Computer tomography scan of a diffuse subarachnoid hemorrhage over the basal cistern. |
|
|
|
Figure 2. Representative cerebral angiograms on the day of aneurysm onset. (A) Anteroposterior view of the right carotid showing a fusiform dilation (green arrow) and narrow M2 lumen; (B) magnified image showing the dissected segment. Narrow (small arrows) and normal (large arrows) sections of the M2 lumen are indicated. This is so-called the “pearl and string” sign. |
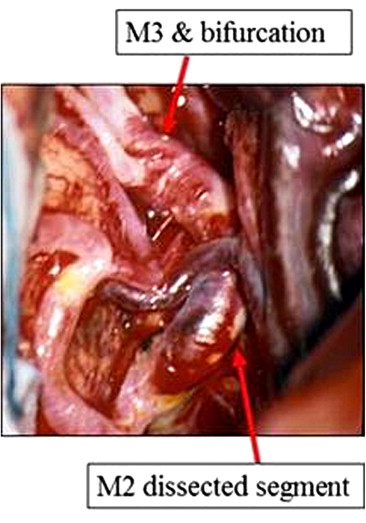
The patient underwent surgery on the day of admission to prevent repeated hemorrhage. A right frontotemporal craniotomy was performed and the distal sylvian fissure was surgically exposed under an operating microscope. A fusiform dilated aneurysm about 2 cm long was visualized in the M2 segment of the MCA (Fig. 3). The aneurysm was wrapped with Teflon mesh. The post-operative course was smooth and uneventful, and the present condition of the patient is stable.
|
|
|
Figure 3. Photographs of the operative view showing the M2 dissected segment. M1/2 and M2/3 bifurcations were identified. |
3. Discussion
Intracranial dissecting aneurysms have been reported with increasing frequency and are recognized as a common cause of stroke. Some reviews and case reports have compared the outcomes of surgical and medical treatments for these aneurysms. Nevertheless, the appropriate management of dissecting aneurysms in the anterior circulation remains controversial, especially in patients who also present with cerebral infarction. In earlier studies on this topic, such cases were typically reported to have a very poor outcome.10 However, recent studies have reported better outcomes for patients with MCA dissecting aneurysms involving surgically treated SAH.
Our review of the literature identified 24 cases of MCA dissecting aneurysms after 1990 (Table 1) 9; 11; 12; 13; 14; 15; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25; 26; 27; 28 ; 29 Of the patients in these cases, 12 (50%) were male and 12 (50%) female; 15 (63%) presented with pure bleeding and seven (29%) with ischemia, and two were detected incidentally. The mean age of all 24 patients was 51.7 years (pure bleeding group: 55.5 ± 15.7 years; ischemia group: 47.6 ± 11.3 years).
| Reference (year) | Region | Age & gender | Initial angiogram findings | Treatment | Prognosis |
|---|---|---|---|---|---|
| Presentation with bleeding | |||||
| Sasaki et al (1991) | R M3 | 41 F | M3 stenosis | Excision and STA-MCA anastomosis | Good recovery |
| Chang et al (1998) | R M1 | 73 M | M1 aneurysmal dilation | No operation | Good recovery |
| Mizutani et al (1998) | M1 | 41 F | M1 stenosis | Wrapping with cotton gauze | Good recovery |
| Abiko et al (1999) | R M1/2 | 59 F | R M1/2 aneurysmal dilation with double lumen sign | Clipping and wrapping with Biobond | Death |
| Bosch et al (1999) | L M1 | 68 M | L M1 aneurysmal dilation | No operation | Death |
| Hashimoto et al (1999) | L M1 | 56 F | M1 aneurysmal dilation and stenosis | Clipping and STA-MCA anastomosis | Severe morbidity |
| Nimura et al (2000) | R M1/2 | 61 F | String sign in M1 segment and M2 occlusion | Coating with muscle | Moderate morbidity (L hemiplegia) |
| Ono et al (2001) | R M1/2 | 68 F | No definitive aneurysm-like finding | No operation | Death |
| Nakashima et al (2001) | R M2/3 | 63 F | No definitive aneurysm-like finding | Clipping | Severe morbidity |
| Nakashima et al (2002) | R M2 | 29 M | No definitive aneurysm-like finding but R M2 occlusion | Trapping | Good recovery |
| Isono et al (2002) | R M1 | 30 M | R M1 aneurysmal dilation | Clipping | Good recovery |
| Ning et al (2003) | L M1 | 50 M | L M1 aneurysmal dilation | Clipping and wrapping | Good recovery |
| Sakamoto et al (2003) | L M3 | 65 F | M3 aneurysmal dilation | Excision and STA-MCA anastomosis | Good recovery |
| Sakamoto et al (2003) | R M2 | 41 M | M2 aneurysmal dilation | Clipping in the first operation. Wrapping and STA-MCA anastomosis as 2ndoperation. (5 months later) | Rebleeding after 5 months, followed by good recovery |
| Shioya et al (2004) | L M2 | 64 M | L M2 occlusion but no definitive aneurysm | Clipping and wrapping with Bemsheets | Good recovery |
| Presentation with ischemia | |||||
| Fu et al (1996) | R M1/2 | 38 M | M1 aneurysmal dilation and occlusion | Intra-arterial fibrinolysis tx | Good recovery |
| Abiko et al (1999) | L M1 | 33 F | L M1 aneurysmal dilation with severe stenosis | No operation | Severe morbidity |
| Presentation with both bleeding and ischemia | |||||
| Kawaguchi et al (1997) | L M2 | 48 M | L M2 occlusion but no definitive aneurysm | Wrapping and STA-MCA anastomosis | Mild morbidity (motor aphasia) |
| Mizutani et al (1998) | M 1/2 | 67 F | M1 aneurysmal stenosis and M2 occlusion | No operation | Death |
| Kurino et al (2002) | R M2 + R A2 | 45 M | R M2 aneurysmal dilation and R A2 segmental dilation | No operation | Good recovery |
| Niikawa et al (2002) | R M1 | 46 F | R M1 aneurysmal dilation | Clipping and wrapping | Severe morbidity |
| Incidental finding | |||||
| Piepgras et al (1994) | R M3 | 56 F | M3 enlarged saccular aneurysm | Excision and STA-MCA anastomosis | Good recovery |
| Fujimura et al (1997) | L M1 | 20 M | Intramural hemorrhage | Clipping | Good recovery |
L = left; MCA = middle cerebral artery; R = right; STA = superficial temporal artery.
Of the seven aneurysm patients presenting with ischemia, four (57%) had a dissection in the M1 segment of the MCA (Table 2). In 10 (67%) of the patients presenting with pure bleeding, the dissection was located more distally, in either the M2 or M3 segment. A review of cases of MCA dissecting aneurysms by Kurino et al11 reported similar findings, with M1 dissection occurring in 69% (9 of 13) of patients presenting with ischemia but in only 30% (3 of 10) of patients presenting with pure bleeding. It was previously thought that dissecting aneurysms tended to occur proximally in the anterior circulation because of the arterial pressure being stronger there than in the distal branches. The difference in the typical segment of the MCA in which dissection occurs suggests a difference in the pathogenesis of dissection between patients presenting with pure bleeding and those presenting with ischemia.11
| Clinical group (n cases) | Location (n) | Treatment (n) | Number of patients per outcome | |||
|---|---|---|---|---|---|---|
| GR | MD | SD | D | |||
| Hemorrhagic (15) | M1(5), M2(7), M3(3) | Surgery with anastomosis (3) | 3 | |||
| Surgery without anastomosis (9) | 6 | 1 | 1 | 1 | ||
| Conservative (3) | 1 | 2 | ||||
| Ischemia (2) | M1(1), M2(1) | Surgery (0) | ||||
| Intra-arterial fibrinolysis (1) | 1 | |||||
| Conservative (1) | 1 | |||||
| Both (5) | M1(3), M2(2) | Surgery with anastomosis (2) | 1 | 1 | ||
| Surgery without anastomosis (1) | 1 | |||||
| Conservativea (2) | 1 | 1 | ||||
| Incidental finding (2) | M1(1), M3(1) | Surgery with anastomosis (1) | 1 | |||
| Surgery without anastomosis (1) | 1 | |||||
GR: good recovery; MD: moderate disability; SD: severe disability; D: death.
a. Includes external decompressive craniotomy.
Our review also found that the outcome of patients presenting with pure bleeding differed from those with ischemia (see Table 2). Most of the patients with pure bleeding had a good outcome, with this being the case for 75% (9 of 12) of those treated surgically. Only two patients with pure bleeding had a poor outcome (Table 3), with one having died and the other presently comatose. However, both these patients were admitted to hospital while deeply comatose. A smaller number of patients were managed conservatively, 66% (2 of 3) of whom died. One of these patients was alert upon admission, but she collapsed 7 days later due to rebleeding.
| Age & gender | Region | Initial status on admission | Treatment | Prognosis | Cause of poor outcome |
|---|---|---|---|---|---|
| Presentation with bleeding | |||||
| 59 F | R M1/2 | Comatose | Clipping and wrapping with Biobond | Death | Initial damage |
| 68 M | L M1 | Comatose | No operation | Death | Initial damage |
| 68 F | R M1/2 | Alert with L homonymous hemianopsia | No operation | Death | Rebleeding on Day 7 |
| 63 F | R M2/3 | Comatose | Clipping | Severe morbidity | Intra-operative bleeding |
| Presentation with ischemia | |||||
| 33 F | L M1 | Alert with R hemiplegia | No operation | Severe morbidity | Progressive infarction |
| Presentation with both bleeding and ischemia | |||||
| 67 F | M1 | Alert | No operation | Death | Rebleeding on Day 8 |
| 56 F | L M1 | Alert with mild R hemiparesis | Clipping and STA-MCA anastomosis | Severe morbidity | Progressive infarction |
| 46 F | R M1 | Somnolent | Clipping and wrapping | Severe morbidity | Progressive infarction |
L = left; MCA = middle cerebral artery; R = right; STA = superficial temporal artery.
Vulnerability to rebleeding has been reported for dissecting aneurysms of the vertebrobasilar circulation, with the rate of rebleeding within the posterior fossa being as high as 30%. Some authors view this information as an indication for emergent surgery within the anterior circulation.8 ; 30 Ohkuma31 has presented a series of cases of dissecting aneurysm in the intracranial carotid circulation with SAH. Rebleeding within 14 days of the aneurysm was reported to have occurred in half of the patients. This suggests that early surgery is critical for preventing rerupture and SAH in cases of dissecting aneurysm of the MCA.
Patients with an MCA dissecting aneurysm who presented with pure bleeding showed better outcomes if they had surgery than if they did not. In contrast, the appropriate management of patients with a dissecting aneurysm who present with ischemia remains controversial. Our review found that 57% (4 of 7) of such patients had a poor final outcome, despite being alert upon arrival at hospital. Two of the four patients received emergent surgery, but progressive infarction resulted in severe morbidity. One patient who was admitted with both bleeding and ischemia and did not undergo surgery collapsed on Day 8 of hospitalization due to rebleeding. The clinical course of patients presenting with ischemia differed from that of patients presenting with pure bleeding. Indeed, most of the patients with ischemia underwent progressive deterioration. In the series of cases reported by Kurino,11 71% (5 of 7) of patients presenting with an MCA dissecting aneurysm and ischemia died under conservative treatment. Kurino emphasized the importance of revascularization surgery in patients experiencing recurrent ischemia. However, while the outcome for patients with ischemia treated surgically was relatively good, it remained poor compared to the outcome for patients with pure bleeding.
Our review identified nine patients with pure bleeding who did not receive revascularization. The majority of these patients had a good outcome; the two patients who did not show good outcomes arrived at the hospital in a comatose state. The patient in our case study received wrapping without revascularization, as there was no evidence of ischemia. No neurological deficit was observed during the course of post-operation follow-up. This suggests that strong evidence of ischemia is required before revascularization is performed. In this regard, it may be prudent to first look for reductions in cerebral blood flow with single photon emission CT.
However, it is still unclear if revascularization surgery can be indicated for patients with a dissecting aneurysm in whom there is no evidence of ischemia. Many believe that revascularization minimizes the risk of cerebral infarction associated with trapping procedures, eliminates the risk of rebleeding by excluding the diseased vessel from circulation, and stops potential longitudinal progression of the dissection.
The treatment in our case did not reduce the risk of further dissection, stenosis or thromboembolic risk.
Indications for the surgical treatment of ruptured dissecting aneurysms of the MCA, as well as guidelines for the timing of any such treatment, have not been established. This is because of the perceived ambiguity in the natural history of ruptured dissecting aneurysms of the MCA. Mizutani et al4 identified a variety of internal elastic lamina and intima lesions in nonatherosclerotic aneurysms unrelated to the branching zones. From this identification, a pathological classification of fusiform aneurysms was proposed, which has been supplemented by pathological material from vertebrobasilar arteries. However, we agree that a strong relationship does exist between the pathological features of an aneurysm and its clinical course.
Recently, a technique of proximal occlusion in which coils or balloons are used has been developed as an endovascular treatment for dissecting aneurysms.32; 33 ; 34 The advances in endovascular surgery have afforded safe treatment of these aneurysms, especially in posterior circulation. However, the report of experience of endovascular treatment in MCA dissecting aneurysm is limited. Fu et al35 found intra-arterial fibrinolysis to be effective in a patient with a major cerebral arterial occlusion caused by an embolus or thrombus arising from a dissecting aneurysm. Despite the risk of hemorrhage, this endovascular technique may be considered in high-risk patients undergoing direct surgery. Innovative technological advances in developments of intra-arterial stents maybe provide a therapeutic option in the future.
In conclusion, we recognize that the indications for surgical treatment of MCA dissecting aneurysm are not well defined. Most studies have focused on dissecting aneurysms occurring in the vertebrobasilar system where the treatment has been open surgery or endovascular surgery. However, it is doubtful that the conclusion reached regarding the vertebrobasilar system could also apply to carotid circulation. In addition, difficulties remain in determining the type of aneurysm present from angiographic studies. Nevertheless, this is the best approach for determining the connections between the pathogenesis, clinical features, neuroradiographic findings, histopathology, and treatment of aneurysms.
References
- 1 A. Yamaura, Y. Watanabe, N. Seki; Dissecting aneurysms of the intracranial vertebral artery; J Neurosurg, 72 (1990), pp. 183–188
- 2 A.H. Friedman, C.G. Drake; Subarachnoid hemorrhage from intracranial dissecting aneurysms; J Neurosurg, 60 (1984), pp. 325–334
- 3 O. Sasaki, T. Koike, S. Takeuchi, R. Tanaka; Serial angiography in a spontaneous dissecting anterior cerebral artery aneurysm; Surg Neurol, 36 (1991), pp. 49–53
- 4 T. Mizutani, Y. Miki, H. Kojima, H. Suzuki; Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysm; Neurosurgery, 45 (1999), pp. 253–260
- 5 O. Sasaki, H. Ogawa, T. Koike, T. Koizumi, R. Tanaka; A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery; J Neurosurg, 75 (1991), pp. 874–882
- 6 T. Hosoya, M. Adachi, K. Yamaguchi, T. Haku, T. Kayama, T. Kato; Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection; Stroke, 30 (1999), pp. 1083–1090
- 7 K. Nakagawa, H. Touho, T. Morisako, et al.; Long-term follow-up study of unruptured vertebral artery dissection: clinical outcomes and serial angiographic findings; J Neurosurg, 93 (2000), pp. 19–25
- 8 C. Kitanaka, T. Sasaki, T. Eguchi, A. Teraoka, M. Nakane, K. Hoya; Intracranial vertebral artery dissections: clinical, radiological features, and surgical consideration; Neurosurgery, 34 (1994), pp. 620–627
- 9 D.G. Piepgras, K.M. McGrail, H.D. Tazelaar; Intracranial dissecting of the distal middle cerebral artery as an uncommon cause of distal cerebral artery aneurysm; J Neurosurg, 80 (1994), pp. 909–913
- 10 O. Sato, J.F. Bascom, J. Logothetis; Intracranial dissecting aneurysm; J Neurosurg, 35 (1971), pp. 483–487
- 11 M. Kurino, S. Yoshioka, Y. Ushio; Spontaneous dissecting aneurysms of anterior and middle cerebral artery associated with brain infarction: a case report and review of the literature; Surg Neurol, 57 (2002), pp. 428–436
- 12 O. Sasaki, T. Koike, R. Tanaka, H. Ogawa; Subarachnoid hemorrhage from a dissecting aneurysm of the middle cerebral artery; J Neurosurg, 74 (1991), pp. 504–507
- 13 M. Fujimura, H. Seki, T. Sugawara, T. Sakuma, Y. Otawara, N. Harata; Giant thrombosed fusiform aneurysm at the branch of the middle cerebral artery presenting with intramural hemorrhage: a case report; No Shinkei Geka, 25 (1997), pp. 151–155 (Japanese with English abstract)
- 14 T. Kawaguchi, T. Kawano, K. Kazekawa, et al.; Dissecting aneurysm of the middle cerebral artery with subarachnoid hemorrhage and brain infarction: a case report; No Shinkei Geka, 25 (1997), pp. 1033–1037 (Japanese with English abstract)
- 15 C.C. Chang, M. Noji, N. Kuwana; Dissecting aneurysm of the middle cerebral artery associated with subarachnoid hemorrhage; J Clin Neurosci, 5 (1998), pp. 361–363
- 16 T. Mizutani; Subarachnoid hemorrhage associated with angiographic “stenotic” or “occlusive” lesions in the carotid circulation; Surg Neurol, 49 (1998), pp. 495–504
- 17 S. Abiko, T. Okamura, Y. Kurokawa, N. Ikeda, M. Ideguchi, K. Watanabe; Diagnosis and treatment of nontraumatic dissecting aneurysm in the middle cerebral artery; No Shinkei Geka, 27 (1999), pp. 743–749 (Japanese with English abstract)
- 18 J. Bosch, A. Mauleon, P. Coscojuela, et al.; Intraventricular hemorrhage due to the rupture of atherosclerotic dissecting aneurysm of the middle cerebral artery; Rev Neurol, 28 (1999), pp. 973–975 (Spanish with English abstract)
- 19 H. Hashimoto, J. Iida, Y. Shin, Y. Hironaka, T. Sakaki; Subarachnoid hemorrhage from intracranial dissecting aneurysms of the anterior circulation: two case reports; Neurol Med Chir (Tokyo), 39 (1999), pp. 442–446
- 20 T. Nimura, T. Oku, N. Narita, H. Higuchi; Dissecting aneurysm of the middle cerebral artery: case report; No Shinkei Geka, 28 (2000), pp. 61–65 (Japanese with English abstract)
- 21 Y. Ono, T. Kawamura, J. Ito, S. Kanayama; Dissecting aneurysm of the middle cerebral artery (M1-2 portion) with subarachnoid hemorrhage: a case report; No Shinkei Geka, 29 (2001), pp. 347–352 (Japanese with English abstract)
- 22 S. Nakashima, S. Shimokawa, S. Nakagawa, et al.; A case of aneurysm of the peripheral middle cerebral artery undetectable on preoperative angiogram; No Shinkei Geka, 29 (2001), pp. 545–549 (Japanese with English abstract)
- 23 S. Nakashima, S. Nomura, M. Tomokiyo, et al.; Dissecting aneurysm of the middle cerebral artery with subarachnoid hemorrhage showing complete occlusion at M2 portion of the middle cerebral artery on preoperative angiograms: case report; No Shinkei Geka, 30 (2002), pp. 541–545 (Japanese with English abstract)
- 24 M. Isono, T. Abe, M. Goda, K. Ishii, H. Kobayashi; Middle cerebral artery dissecting aneurysm causing intracerebral hemorrhage 4 years after the non-hemorrhagic onset: a case report; Surg Neurol, 57 (2002), pp. 346–350
- 25 S. Niikawa, J. Yamada, Y. Sumi, H. Yamakawa; Dissecting aneurysm of middle cerebral artery manifesting as subarachnoid hemorrhage and hemorrhagic infarctions; Neurol Med Chir (Tokyo), 42 (2002), pp. 62–66
- 26 L. Ning, Y. Kato, H. Sano, et al.; Spontaneous dissecting aneurysm of middle cerebral artery: a case report with review of the literature; Minim Invasive Neurosurg, 46 (2003), pp. 357–360
- 27 S. Sakamoto, F. Ikawa, H. Kawamoto, N. Ohbayashi, T. Inagawa; Acute surgery for ruptured dissecting aneurysm of the M3 portion of the middle cerebral artery; Neurol Med Chir (Tokyo), 43 (2003), pp. 188–191
- 28 S. Sakamoto, F. Ikawa, H. Kawamoto, N. Ohbayashi, T. Inagawa; Subsequent rupture after clip on wrap method for ruptured dissecting aneurysm of the distal middle cerebral artery: a case report and review of the literature; Hiroshima J Med Sci, 52 (2003), pp. 15–19
- 29 H. Shioya, K. Kikuchi, Y. Suda, H. Shoji, K. Shindo; Dissecting aneurysm of the middle cerebral artery with subarachnoid hemorrhage undetectable on preoperative neuroradiological findings: case report; No To Shinkei, 56 (2004), pp. 965–970 (Japanese with English abstract)
- 30 N. Aoki, T. Sakai; Rebleeding from intracranial dissecting aneurysm in the vertebral artery; Stroke, 21 (1990), pp. 1628–1631
- 31 H. Ohkuma, S. Suzuki, N. Shimamura, T. Nakano; Dissecting aneurysms of the middle cerebral artery: neuroradiological and clinical features; Neuroradiology, 45 (2003), pp. 143–148
- 32 J.D. Rabinov, F.R. Hellinger, P.P. Morris, C.S. Ogilvy, C.M. Putman; Endovascular management of vertebrobasilar dissecting aneurysms; AJNR, 24 (2003), pp. 1421–1428
- 33 K. Iihara, N. Sakai, K. Murao; Dissecting aneurysms of the vertebral artery: a management strategy; J Neurosurg, 97 (2002), pp. 259–267
- 34 A. Kurata, T. Ohmomo, Y. Miyasaka, K. Fujii, S. Kan, T. Kitahara; Coil embolization for the treatment of ruptured dissecting vertebral aneurysms; AJNR, 22 (2001), pp. 11–18
- 35 Y. Fu, M. Komiyana, T. Inoue, K. Ohata, Y. Matsuoka, A. Hakuba; A case of middle cerebral artery occlusion caused by dissecting aneurysm; No Shinkei Geka, 24 (1996), pp. 955–959 (Japanese with English abstract)
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?