Summary
Objective
Brain metastasis occurs in 10–15% of metastatic breast cancer patients and is associated with poor prognosis. This study aims to identify tumor characteristics of primary breast cancer, which are related to brain metastases in Hong Kong Chinese patients.
Methods
A retrospective study of patients with invasive breast cancer receiving treatment in a university hospital from January 2001 to December 2008 was performed. The clinicopathological factors of patients with brain metastases were analyzed and compared with those who had no brain metastasis. Risk factors for brain metastasis were identified by univariate analysis first and then by multivariate analysis.
Results
A total of 912 patients with invasive breast cancer were treated during the study period. Of these, 30 patients were found to have distant metastases to brain. Patients with brain metastases had more breast tumors of higher histological grade (Grade III, 78.9% vs. 30.2%; p = 0.001). Their tumors also had a significantly higher rate of negative estrogen receptors (78.9% vs. 30.2%, p = 0.001). On multivariate analysis, only high tumor grading was found to be predictive of developing brain metastasis.
Conclusion
Chinese breast cancer patients with brain metastasis were more likely to have high-grade tumors and negative estrogen receptor status. A more vigorous surveillance program for the central nervous system should be considered for this group of patients.
Keywords
brain metastasis;breast cancer;HER2;trastuzumab;whole-brain irradiation
1. Introduction
Carcinoma of the breast is by far the most common malignancy in women with growing incidence worldwide.1 It is also the most common malignancy among the Chinese female population in Hong Kong where one in 19 women suffer from the disease. It is the second most common cancer causing brain metastasis,2 with lung cancer at the top. Brain metastasis typically occurs late in metastatic breast disease, usually with preceding lung, liver, or bone metastasis.3 In the past, the central nervous system (CNS) is a relatively rare site of metastasis. The incidence of brain metastasis has previously been reported ranging from 10% to 16%.4 However, due to the improvement in the control of other systematic manifestations of breast cancer, the incidence of brain metastasis is now increasing.5 Prognosis of these patients is poor, with a mean 1-year survival estimated to be around 20%.6 Multiple treatment modalities have been suggested,7 but only the use of whole-brain radiation therapy and surgical resection of solitary tumors in highly selected patients have been shown to improve survival rates. Hence, identifying a subgroup of patients with risk factors for brain metastasis would justify the implementation of a more rigorous surveillance program after primary treatment of breast cancer, which in turn enhances the opportunity for timely intervention. The aim of our study was to identify clinicopathological factors that would predispose to the development of brain metastasis in Chinese female patients.
2. Methods
From January 2001 to December 2008, 912 patients with breast cancer were treated at the Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, Pok Fu Lam, Hong Kong. Of these, 30 patients were found to have either solitary or multiple brain metastases. The clinicopathological data of these patients including age, tumor size, axillary lymph node status, histological grade, estrogen receptor (ER) and progesterone receptor (PR) status, HER2 oncogene expression, and triple-negative disease status were retrospectively reviewed. Patients with synchronous brain metastasis and breast cancer were excluded from this analysis. The time to diagnosis of brain metastasis was defined as the time from histological diagnosis of breast cancer to the time of detection of brain metastasis. Survival analysis was conducted using Kaplan–Meier methods and compared between subgroups using the log-rank test. Continuous variables were expressed by mean with the confidence interval given in parentheses and compared between subgroups using the Student t test. Categorical variables were compared between subgroups using the Chi-square test or Fishers exact test, if appropriate. Risk factors for brain metastasis were first identified by univariate analysis followed by multivariate analysis for the significant factors by Cox proportional hazard model. A p value < 0.05 was taken to be significant. All statistical analyses were performed using SPSS software version 20 (SPSS Inc., Chicago, IL, USA).
3. Results
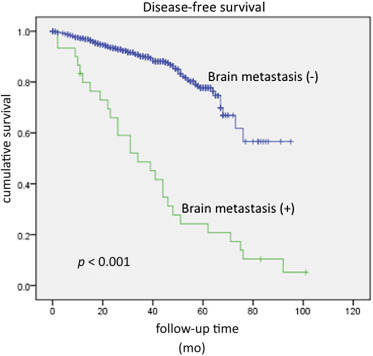
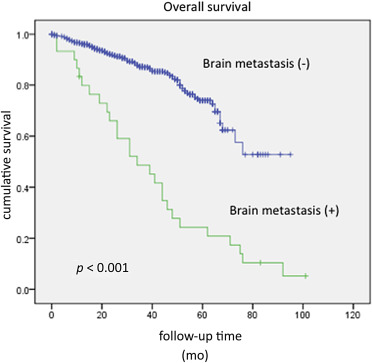
The clinicopathological characteristics of the two groups of patients are presented in Table 1. There was no difference in age and the duration of follow-up between the two groups. The mean time to develop brain metastasis was 29 months. Patients who developed brain metastasis were more likely to have a higher histological grading of primary tumor, a more advanced T stage and N stage, a higher incidence of positivity for ER and PR status, and a lower frequency of triple-negative disease. On multivariable analysis, only histological grading of the primary tumor was identified as the independent risk factor for the development of brain metastasis (odds ratio 6.83, 95% confidence interval 2.04–22.91; p = 0.002). The mean disease-free survivals for patients with and without brain metastasis were 40.2 months and 76.8 months, respectively, and the corresponding 5-year disease-free survival rates were 24% and 78% ( Fig. 1). The mean overall survival rates for patients with and without brain metastasis were 40.2 months and 74.2 months, respectively, and the corresponding 5-year overall survival rates were 24% and 74% (Fig. 2).
| Brain metastasis (n = 30) | No brain metastasis (n = 882) | p | |
|---|---|---|---|
| Age (y) | 53 (28–80) | 52 (10–91) | 0.085 |
| Follow-up (mo) | 33 (2–101) | 34 (0–95) | 0.273 |
| Grade | 0.001 | ||
| I | 1 (5.3) | 117 (19.8) | |
| II | 5 (15.8) | 295 (50.0) | |
| III | 24 (78.9) | 178 (30.2) | |
| T stage | <0.001 | ||
| T0 | 0 | 153 (17.4) | |
| T1 | 5 (18.5) | 427 (48.5) | |
| T2 | 12 (44.4) | 253 (28.7) | |
| T3 | 9 (33.3) | 42 (4.8) | |
| T4 | 1 (3.7) | 6 (0.7) | |
| N stage | 0.026 | ||
| N0 | 8 (44.4) | 562 (63.9) | |
| N1 | 5 (27.8) | 161 (18.3) | |
| N2 | 0 | 95 (10.8) | |
| N3 | 5 (27.8) | 62 (7.0) | |
| ER status | 0.019 | ||
| Positive | 15 (50) | 538 (29.9) | |
| Negative | 15 (50) | 229 (70.1) | |
| PR status | 0.029 | ||
| Positive | 10 (33.3) | 409 (53.7) | |
| Negative | 20 (66.7) | 353 (46.3) | |
| C-erb-2 status | 0.051 | ||
| Score 0–2 | 16 (57.1) | 557 (73.8) | |
| Score 3 | 12 (42.9) | 198 (26.2) | |
| ER/PR/C-erb-2 negative | 0.033 | ||
| Yes | 6 (25.0) | 686 (91.5) | |
| No | 22 (75.0) | 64 (8.5) |
Data are presented as n (%) or n (range).
ER = estrogen receptor; PR = progesterone receptor.
a. On multivariable analysis, only histological grading of the primary tumor was identified to be independent risk factor for the development of brain metastasis (odds ratio 6.83, 95% confidence interval 2.04–22.91; p = 0.002).
|
|
|
Figure 1. Mean disease-free survivals for patients with and without brain metastasis. |
|
|
|
Figure 2. Mean overall survival rates for patients with and without brain metastasis. |
4. Discussion
Breast cancer-associated brain metastasis is a manifestation of advanced disease with dismal prognosis. The median survival from the time of diagnosis varies from 5 weeks to 7 weeks and the estimated 1-year survival rate is ∼20%, depending on the extent of cortical involvement and the choice of treatment selected.6 With improvements in systemic chemotherapy and molecular target therapy, control of extracranial metastatic disease may no longer be the only factor that dictates survival. A study by Bendell et al8 showed that a median survival of 13 months was attainable in patients with metastatic breast cancer treated with trastuzumab.
The results of this study show that patients with higher T stage, N stage, histological grading of tumor, ER-negative, PR-negative, and C-erb-2-positive status were more likely to develop brain metastasis. A drawback of our study is that the Ki-67 proliferative index was not routinely performed by the pathologist in our centre until the recent few years, and therefore the patients in this study could not be stratified into different molecular subtypes for more sophisticated analysis. Among all the factors analyzed, the histological grade of tumor was found to be the most powerful risk factor for the development of brain metastasis. Hence, it is conceivable to propose that a more vigorous surveillance program including brain imaging at regular intervals should be offered to patients with a high-grade tumor after primary treatment of breast cancer. The majority of patients with brain metastasis are often diagnosed late when they become symptomatic, and therefore only very limited choices for further treatment are available. The implementation of a vigilant surveillance program in asymptomatic patients with a propensity to develop brain metastasis based on the pathological risk factors may therefore improve the chance for timely intervention. However, the cost effectiveness of such strategy needs to be elucidated in further studies.
Although statistically not significant, patients with HER2 overexpression seemed to be more likely to develop brain metastasis. The presence of HER2 overexpression has received much interest due to the evolution of targeted therapies in the management of breast cancer. There have been many hypotheses on the molecular mechanisms, which have been proposed to understand the tumor aggressiveness phenotype. 5 In addition, the introduction of trastuzumab has brought good responses in the management of breast tumors with HER2 overexpression. 9 However, although improvement in the control of disseminated disease and prolongation of survival had been reported with trastuzumab, this provided an opportunity to the development of occult brain metastasis, which would otherwise remain clinically silent before the patients' death.10 Furthermore, the poor blood–brain barrier permeability of trastuzumab rendered the CNS a sanctuary site for metastasis. The development of newer ErbB2 inhibitors such as lapatinib, an oral dual tyrosine kinase inhibitor targeting HER1 (also called EGFR gene) and HER2, 11 may be beneficial to this group of patients as it is a smaller molecule that is capable of penetrating the blood–brain barrier.12 Pertuzumab, a new generation humanized anti-HER2 monoclonal antibody, has also shown promising results when used in conjunction with trastuzumab (Clinical Evaluation of Pertuzumab and Trastuzumab trial) in neoadjuvant and metastatic settings, and there is emerging evidence regarding its efficacy in brain metastasis.13
5. Conclusion
Effective treatment options in patients who present late with brain metastasis are often limited. Some prophylactic measures to prevent brain metastasis have been established in other cancers. For example, the use of prophylactic cranial irradiation could decrease CNS involvement and enhance survival14 in patients with small-cell carcinoma of the lung. Hence, further study to evaluate prophylactic therapies in breast cancer patients who are at risk of developing brain metastasis could be worthwhile to improve the overall outlook of such advanced stage disease.
Acknowledgments
No additional funding was required.
References
- 1 F. Kamangar, G.M. Dores, W.F. Anderson; Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world; J Clin Oncol, 24 (2006), pp. 2137–2150
- 2 A.E. Walker, M. Robins, F.D. Weinfeld; Epidemiology of brain tumors: the national survey of intracranial neoplasms; Neurology, 35 (1985), pp. 219–226
- 3 Y.L. Tham, K. Sexton, R. Kramer, S. Hilsenbeck, R. Elledge; Primary breast cancer phenotypes associated with propensity for central nervous system metastases; Cancer, 107 (2006), pp. 696–704
- 4 Y. Tsukada, A. Fouad, J.W. Pickren, W.W. Lane; Central nervous system metastasis from breast carcinoma. Autopsy study; Cancer, 52 (1983), pp. 2349–2354
- 5 X. Cheng, M.-C. Hung; Breast cancer brain metastases; Cancer Metastasis Rev, 26 (2007), pp. 635–643
- 6 N.J. Carty, A. Foggitt, C.R. Hamilton, G.T. Royle, I. Taylor; Patterns of clinical metastasis in breast cancer: an analysis of 100 patients; Eur J Surg Oncol, 21 (1995), pp. 607–608
- 7 N.U. Lin, J.R. Bellon, E.P. Winer; CNS metastases in breast cancer; J Clin Oncol, 22 (2004), pp. 3608–3617
- 8 J.C. Bendell, S.M. Domchek, H.J. Burstein, et al.; Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma; Cancer, 97 (2003), pp. 2972–2977
- 9 F. Montemurro, M. Aglietta; Incorporating trastuzumab into the neoadjuvant treatment of HER2-overexpressing breast cancer; Clin Breast Cancer, 6 (2005), pp. 77–80
- 10 R.J. Weil, D.C. Palmieri, J.L. Bronder, A.M. Stark, P.S. Steeg; Breast cancer metastasis to the central nervous system; Am J Pathol, 167 (2005), pp. 913–920
- 11 D. Bilancia, G. Rosati, A. Dinota, D. Germano, R. Romano, L. Manzione; Lapatinib in breast cancer; Ann Oncol, 18 (2007), pp. vi26–vi30
- 12 S. Barni, F. Petrelli, M. Cabiddu, M.E. Cazzaniga, M. Cremonesi; From the trastuzumab era to new target therapies: beyond revolution; Ann Oncol, 18 (2007), pp. vi1–vi4
- 13 S.M. Swain, J. Baselga, S.B. Kim, et al.; Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer; N Engl J Med, 372 (2015), pp. 724–734
- 14 A. Aupérin, R. Arriagada, J.-P. Pignon, et al.; Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission; N Eng J Med, 341 (1993), pp. 476–484
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?