Summary
Gastrointestinal stromal tumors are nonepithelial neoplasms of the gastrointestinal tract and have been increasingly recognized in recent years. In contrast, stromal tumor outside the gastrointestinal tract is not frequently found. Here, we present a 57-year-old male patient who had abdominal fullness for several months. It was caused by a 23-cm heterogeneous tumor mass that was successfully removed from the left upper abdominal cavity. The tumor adhered tightly to adjacent organs but postoperative histopathological analysis revealed no direct connection to the stomach, liver, pancreas, spleen, or kidneys. Immunohistochemical examination of the tumor revealed proliferative spindle-shaped cells stained positive for CD117 and CD34 and negative for smooth muscle actin and S-100. The patient received regular follow up. A suspected recurrent liver metastatic lesion was noted 2 years later and radiofrequency ablation of the liver tumor was performed followed by oral imatinib treatment. No tumor recurrence was detected at 3 years after radiofrequency ablation. This case reminds us that extragastrointestinal stromal tumors should be considered in the differential diagnosis when a large heterogeneous mass is present in the abdominal cavity. The characteristics of extragastrointestinal stromal tumor are described and the literature is reviewed in this report.
Keywords
Extragastrointestinal stromal tumors ; Gastrointestinal stromal tumors ; CD117
Introduction
Gastrointestinal stromal tumor (GIST) is a nonepithelial tumor in the digestive tract. The diagnosis and treatment of gastrointestinal stromal tumors have increased in recent years with the help of advanced immunohistochemical methods. However, stromal tumors developing outside the gastrointestinal tract, termed extragastrointestinal stromal tumor (EGIST), are rarer than GIST. Here, we report a patient with a large tumor that grew outside the gastrointestinal tract in the abdominal cavity, with features of stromal cells. It was diagnosed as EGIST. The characteristics of this EGIST are described and the literature is reviewed and discussed.
Case report
A 57-year-old man had a history of essential hypertension, type 2 diabetic mellitus, and chronic constipation for several years. He experienced epigastric discomfort in 2006. The sensation of the epigastric discomfort was dull and compressive, and lasted approximately 30 minutes. At that time, there was no dysphagia, odynophagia, nausea, vomiting, heartburn, hunger pain, acid regurgitation, or easy satiety. There was no overt precipitating, aggravating, or alleviating factor. He took some antacids, and rapid improvement was seen initially. However, after intermittent treatment for 9 months, the same symptoms recurred and became worse despite continued treatment. Occasionally epigastric cramps and fullness followed. His appetite was fair, but easy satiety and body weight loss of ∼5 kg were found. A fecal occult blood test was negative in two separate studies.
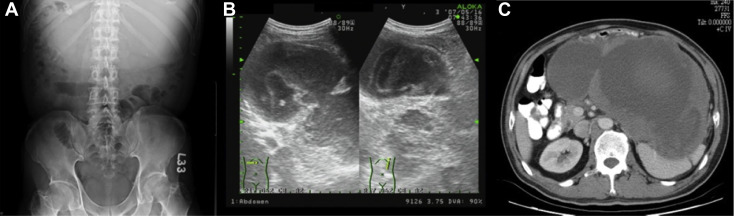
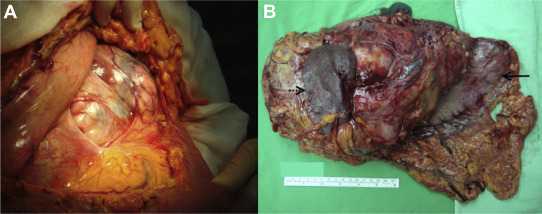
On admission, a physical examination found that the abdomen was flat without tenderness or rebounding pain but there was a palpable soft tissue mass over the epigastric region. The patient did not notice the epigastric mass at all. Plain abdominal film showed an increased soft tissue density over the upper abdomen (Fig. 1 A). Whole abdominal sonography disclosed that it was a cyst-like lesion, with some blood–mucous like material inside. It was >20 cm in diameter (Fig. 1 B). Upper gastrointestinal endoscopy showed an external compression sign over the posterior wall of the gastric body with normal gastric mucosa. Computed tomography of the upper abdomen revealed that there was a large mass of heterogeneous density, measuring 23 cm × 13 cm × 20 cm, with possible central necrosis and peripheral enhancement, and the spleen and stomach were compressed by the mass (Fig. 1 C). The patient soon received surgical intervention. Because the tumor was involved extensively, total gastrectomy, Roux-en-Y esophagojejunostomy, splenectomy, distal subtotal pancreatectomy, cholecystectomy, and segmental colectomy with anastomosis were performed smoothly (Figs. 2 A and 2B).
|
|
|
Figure 1. (A) Kidney, Ureter, Bladder (KUB) X ray found increased soft tissue density over the epigastric region with downward displacement of transverse colon gas; (B) sonographic finding of transverse and longitudinal view. There was a large cystic lesion with septation and mucous-like content in the epigastric and left upper quadrant of the abdomen. The pancreas was compressed downward by the tumor at the head and body; (C) computed tomography showed a large mass, approximately 23 cm × 13 cm × 20 cm, with low density at the central region and peripheral enhancement. The spleen and stomach were heavily compressed by the tumor mass. |
|
|
|
Figure 2. (A) The tumor was seen beneath the colon and omentum when the abdominal cavity was opened; (B) surgical specimen showing the resected portion of the stomach (arrow) and spleen (dot arrow). The ruler is 15 cm in length. |
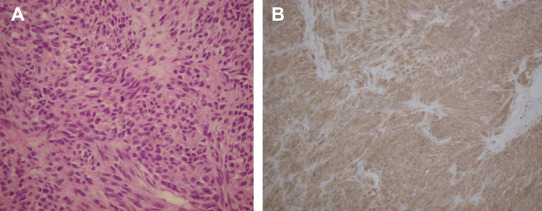
Grossly, there was no connecting stalk between the tumor and adjacent organs. Serial microscopic surveys showed that the stomach, pancreas, and spleen were compressed by the stromal cell tumor. There was fibrosis of the adjacent pancreatic tissue. There was no lymph node metastasis. The microscopic study of the tumor part showed spindle-shaped neoplastic cells with uniform and syncytial-appearing eosinophilic cytoplasm (Fig. 3 A). The mitotic count was 12/50 high-power field (HPF). The MIB-1 test showed an increased proliferation index > 10%. Immunohistochemical study of the tumor cells showed positive for CD117 and CD34, and negative for smooth muscle actin and S-100 (Fig. 3 B).
|
|
|
Figure 3. (A) The tumor specimen appeared spindle-shaped and arranged in a bundle (hematoxylin and eosin stain, 400×); (B) immunohistochemical study showed positive CD117 staining; smooth muscle stain was negative. |
The postoperative course was smooth and uneventful. Two years later, abdominal computed tomography showed a metastatic nodule in the lateral segment of the liver. Radiofrequency ablation of the tumor was performed, followed by oral imatinib treatment. The patient remained free from tumor recurrence in the subsequent 3 years and is still under follow up.
Discussion
GIST constitutes only 1% of the total primary digestive tract cancer. The annual incidence of GIST is 15–20 cases per million population [1] ; [2] ; [3] ; [4] . Due to the improvement of diagnostic immunohistochemical techniques, the numbers of GISTs have increased in recent years. GIST occurs in and throughout the whole gastrointestinal tract. The most common sites of origin are the stomach (60%), followed by jejunum and ileum (30%), duodenum (5%), and colorectum (<5%). Only a small number of cases (<1%) have been reported in the esophagus and appendix. By contrast, EGISTs are relatively rarer compared with GISTs.
The cellular origin of GIST is presumed to be the interstitial cells of Cajal, while the origins of EGIST remain inconclusive. Interstitial cells of Cajal are pacemaker cells present throughout the wall of the gastrointestinal tract to regulate motility. They express CD117 antigen, which is the c-Kit receptor tyrosine kinase. Approximately 95% of GISTs are positive for CD117 [5] ; [6] ; [7] . However, primary stromal cells developed in nongastrointestinal tract tissues are possible. Recently, the existence of interstitial cells of Cajal in the exocrine pancreas has been discovered and reported to have a phenotype similar to that of enteric Cajal cells [8] ; [9] . There are several reports of cases of primary stromal tumors originating in the pancreas [10] ; [11] ; [12] , urinary bladder [13] , prostate [14] , and scrotum [15] . Moreover, one case report showed that a cirrhotic patient developed a subcutaneous EGIST nodule in the perineum after liver transplantation [16] . More recently, a primary intrathoracic EGIST arising in the pleura has been reported [17] . It was unusual in our case that this EGIST grew in the abdominal cavity and showed no histological evidence of any connection to the adjacent organs. Besides, there was no other GIST detected in this patient. Thus, it could be a primary EGIST that had developed from the peritoneum or other tissues in the abdominal cavity. Although it has been proposed that EGISTs could be due to metastasis or simply GISTs detached from the primary origin [2] ; [18] ; [19] , the clinical and histological evidence of our case appears to be against the above possibilities.
There are no specific clinical characteristics of EGIST within the abdominal cavity. The common clinical presentations of GIST such as gastrointestinal tract bleeding and obstruction are usually absent in EGIST. EGIST lacks mucosal involvement, therefore, intraabdominal EGIST is often asymptomatic and may grow to a relatively large size when it is initially diagnosed. Bai et al [20] presented one retrospective study of 30 cases of intraabdominal EGIST with a median size of 12.5 cm. Kobayashi et al [21] have reported one giant EGIST 18 cm in size. In our case report, the tumor had grown to >20 cm.
There are several parameters to evaluate the prognosis of GIST. In a retrospective analysis, Langer et al [22] found that tumor size > 5 cm, mitotic count > 2/HPF, and proliferation index > 10% were markedly associated with a shorter recurrence-free survival rate. As for EGIST, Yamamoto et al [18] reviewed 39 cases and concluded that tumors with high mitotic counts (≥ 5/50 HPF), or a high Ki-67 labeling index (≥ 10%), were significantly correlated with worse prognosis. In our case, the mitotic count was 12/50 HPF. Overall, the tumor size, mitotic count, and proliferation index were compatible with the high-risk criteria of the previous study.
Currently, surgery and imatinib are two powerful methods for treatment of GIST. For primary resectable GIST, only complete surgical resection offers a possibility of cure. The main goal of surgery is to remove the tumor with negative margins. A 2-cm margin should be sought. Regional lymphadenectomy is generally not required because GIST rarely (<5%) metastasizes to lymph nodes [23] . The tyrosine kinase inhibitors (TKIs) such as imatinib and sunitinib are used primarily for metastatic, recurrent, and unresectable GIST. The phase III trial ACOSOG Z9001 demonstrated that imatinib can be considered as GIST patients recover from surgical procedures, especially in those with a significant risk of recurrence (tumor size > 10 cm, >5 mitoses/50 HPF, rupture, intraperitoneal hemorrhage, and multifocal GIST). However, the impact of this approach on long-term survival remains unclear [23] . In our case, TKI was prescribed after the metastatic lesion developed and it appeared to be beneficial and effective in tumor control.
In summary, we report here the case of a man with a 20-cm EGIST in the abdominal cavity who received resection, initially, then Radiofrequency ablation for metastasis. Under TKI treatment, the patient remained recurrence free for 3 years. An EGIST of large size and high mitotic rate should be followed up closely. Local ablation combined with TKI may be used to control tumor metastasis and recurrence.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] M. Miettinen, J. Lasota; Gastrointestinal stromal tumors – definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis; Virchows Arch, 438 (2001), pp. 1–12
- [2] M. Miettinen, M. Sarlomo-Rikala, J. Lasota; Gastrointestinal stromal tumors: recent advances in understanding of their biology; Hum Pathol, 30 (1999), pp. 1213–1220
- [3] C.D. Fletcher, J.J. Berman, C. Corless, F. Gorstein, J. Lasota, B.J. Longley, et al.; Diagnosis of gastrointestinal stromal tumors: a consensus approach; Hum Pathol, 33 (2002), pp. 459–465
- [4] P. Bumming, H. Ahlman, J. Andersson, J.M. Meis-Kindblom, L.G. Kindblom, B. Nilsson; Population-based study of the diagnosis and treatment of gastrointestinal stromal tumours; Br J Surg, 93 (2006), pp. 836–843
- [5] B.P. Rubin; Gastrointestinal stromal tumours: an update; Histopathology, 48 (2006), pp. 83–96
- [6] S.M. van der Zwan, R.P. DeMatteo; Gastrointestinal stromal tumor: 5 years later; Cancer, 104 (2005), pp. 1781–1788
- [7] Y. Shinomura, K. Kinoshita, S. Tsutsui, S. Hirota; Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors; J Gastroenterol, 40 (2005), pp. 775–780
- [8] L.G. Kindblom, H.E. Remotti, F. Aldenborg, J.M. Meis-Kindblom; Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal; Am J Pathol, 152 (1998), pp. 1259–1269
- [9] K.W. Min, M. Leabu; Interstitial cells of Cajal (ICC) and gastrointestinal stromal tumor (GIST): facts, speculations, and myths; J Cell Mol Med, 10 (2006), pp. 995–1013
- [10] S. Padhi, R. Kongara, S.G. Uppin, M.S. Uppin, A.K. Prayaga, S. Challa, et al.; Extragastrointestinal stromal tumor arising in the pancreas: a case report with a review of the literature; JOP, 11 (2010), pp. 244–248
- [11] O. Daum, J. Klecka, J. Ferda, V. Treska, T. Vanecek, R. Sima, et al.; Gastrointestinal stromal tumor of the pancreas: case report with documentation of KIT gene mutation; Virchows Arch, 446 (2005), pp. 470–472
- [12] S.L. Showalter, J.M. Lloyd, D.T. Glassman, A.C. Berger; Extra-gastrointestinal stromal tumor of the pancreas: case report and a review of the literature; Arch Surg, 143 (2008), pp. 305–308
- [13] A. Mekni, I. Chelly, H. Azzouz, I. Ben Ghorbel, S. Bellil, S. Haouet, et al.; Extragastrointestinal stromal tumor of the urinary wall bladder: case report and review of the literature; Pathologica, 100 (2008), pp. 173–175
- [14] S. Yinghao, Y. Bo, G. Xiaofeng; Extragastrointestinal stromal tumor possibly originating from the prostate; Int J Urol, 14 (2007), pp. 869–871
- [15] S.H. Kang, M.J. Kim, M.G. Park, H.S. Park, D.G. Moon, D.J. Sung, et al.; Extragastrointestinal stromal tumor presenting as a scrotal mass: an unusual case; Asian J Androl, 9 (2007), pp. 275–279
- [16] M.A. Camargo, I. Boin, J.P. Mainnardi, M. de Lourdes, S. Ayrizono, C.S. Coy, et al.; Extragastrointestinal stromal tumor and liver transplantation: case report and review; Transplant Proc, 40 (2008), pp. 3781–3783
- [17] K.B. Long, J.E. Butrynski, S.D. Blank, K.S. Ebrahim, D.M. Dressel, M.C. Heinrich, et al.; Primary extragastrointestinal stromal tumor of the pleura: report of a unique case with genetic confirmation; Am J Surg Pathol, 34 (2010), pp. 907–912
- [18] H. Yamamoto, Y. Oda, K. Kawaguchi, N. Nakamura, T. Takahira, S. Tamiya, et al.; c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue); Am J Surg Pathol, 28 (2004), pp. 479–488
- [19] J.D. Reith, J.R. Goldblum, R.H. Lyles, S.W. Weiss; Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome; Mod Pathol, 13 (2000), pp. 577–585
- [20] Y.K. Bai, Y.F. Shao, S.S. Shi, Y.N. Gao, Y.T. Sun, S.J. Cheng, et al.; Multivariate analysis of prognosis in gastrointestinal stromal tumor; Zhonghua Zhong Liu Za Zhi, 27 (2005), pp. 598–601 (in Chinese)
- [21] T. Kobayashi, M. Teruya, S. Shimizu, Y. Nishio, A. Ito, K. Kobayashi, et al.; Giant extragastrointestinal stromal tumor; Am J Surg, 188 (2004), pp. 191–192
- [22] C. Langer, B. Gunawan, P. Schuler, W. Huber, L. Füzesi, H. Becker; Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours; Br J Surg, 90 (2003), pp. 332–339
- [23] I. Deshaies, J. Cherenfant, N.J. Gusani, Y. Jiang, H.A. Harvey, E.T. Kimchi, et al.; Gastrointestinal stromal tumor (GIST) recurrence following surgery: review of the clinical utility of imatinib treatment; Ther Clin Risk Manag, 6 (2010), pp. 453–458
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?