Abstract
Objective
To investigate whether women presenting with suspected angina would show less severe coronary artery disease in than men as determined by the extent score.
Methods
We examined 994 participants of the Australian Heart Eye Study presenting for coronary angiography in the investigation of chest pain from June 2009 to February 2012. People were excluded if there was a history of coronary artery bypass surgery, previous stenting procedure or incomplete angiogram scoring. An extent and vessel score was calculated using invasive coronary angiography. Normal coronary arteries were defined as having no luminal irregularity (Extent score = 0). Obstructive coronary artery disease was defined as a luminal narrowing of greater than 50%.
Results
Women compared to men without infarction had a lower burden of CAD with up to 50% having normal coronary arteries in the 30–44 year group and 40% in the 45–59 year group. Compared to men, women with chest pain had lower mean extent scores (19.6 vs 36.8; P < 0.0001) and lower vessel scores (0.7 v 1.3; P < 0.0001). Although the mean extent score was lower in women than men with myocardial infarction, this was not statistically significant (34.8 vs 41.6 respectively; P = 0.18).
Conclusion
There is a marked difference in coronary artery disease severity and burden between females and males presenting for the investigation of suspected angina. Women are more likely to have normal coronary arteries or less severe disease than age-matched men, particularly if they do not present with myocardial infarction.
Keywords
Coronary artery disease;Gender;Extent score;Coronary angiography;Chest pain
1. Introduction
Cardiovascular disease, in particular coronary disease, is the leading cause of mortality in women. In fact, women are more likely to die following a myocardial infarct than men [1]. Despite this, the pattern of coronary artery disease (CAD) is substantially different between men and women [2]. Up to 60% of women and 30% of men who present with angina have either normal arteries or non-obstructive lesions [3]; [4] ; [5]. Women present with less obstructive epicardial stenoses and more diffuse atherosclerosis and microvascular dysfunction [6]. Women can display evidence of ischemia on functional assessment (such as pressure wire studies, myocardial perfusion imaging and magnetic resonance imaging) without obstructive epicardial coronary disease. This may be unrelated to the presence of Framingham risk factors [7]; [8] ; [9]. Women also demonstrate a higher prevalence of atherosclerosis with positive remodeling and preserved lumen size as demonstrated by intravascular ultrasound [9] ; [10].
The Australian Heart Eye Study (AHES) was a large cross-sectional study of patients presenting for coronary angiography in the investigation of chest pain at Westmead Hospital, Sydney, Australia. It was developed to study the relationship between gender and both retinal vascular disease and CAD. We investigated the anatomical relationship between angiographically proven CAD and gender in this population using the extent score. The extent score reflects the proportion of the coronary tree with angiographically detectable atherosclerotic disease, scaled according to the functional significance of the involved artery [11]. There are no studies to our knowledge that use the extent score to describe the anatomical burden of CAD in men compared to women. We hypothesized that women with chest pain would show less severe CAD in all age groups than men.
2. Methods
2.1. Study population
The Australian Heart Eye Study (AHES) recruited patients presenting to Westmead Hospital for the assessment of suspected CAD between June 2009 and January 2012. Patients were both internally and externally referred for investigation and the decision to pursue angiography was made by the referrer who was unaware of any subsequent participation in the study. Participants were consented to the study prior to or following invasive coronary angiography, and included 1680 patients, who were interviewed to obtain data on demographic characteristics, medical history and behavioral habits. The presence of risk factors was determined either by physician diagnosis or treatment for the medical condition. Medical records were reviewed to obtain medication use and confirm medical history. Of the 1680 examined in this study, a total of 398 participants were excluded because they had a previous history of coronary artery bypass grafting (n = 191) and/or previous coronary artery stent (n = 298). If there was incomplete background information or if an extent score was not able to measured due to suboptimal angiogram quality, these participants were also excluded. A total of 994 participated (712 men and 282 women). Of those presenting with chest pain, 221 (187 men and 34 women) were diagnosed with myocardial infarction. This was based on symptoms of ischemia, characteristic electrocardiographic findings, elevation in cardiac troponin enzyme.
The study protocol conforms to the ethical guidelines of the 1975 declaration of Helsinki and ethical approval was obtained from the Western Sydney Local Health Network Human Research Ethics Committee (Westmead).
2.2. Coronary angiography image acquisition and analysis
Diagnostic coronary angiography was performed via a femoral or radial approach using a catheter of known dimension. The technique employed was determined by vascular accessibility of the patient and operator preference. Selective coronary injections were filmed in standard projections with a Siemens Bi-Plane radiographic unit (Siemens Healthcare, Germany). All angiograms were filmed at 15 frames/s and cine runs were stored at the time of acquisition in DICOM format.
All angiograms were masked to patient name and diagnosis. Analysis was performed offline by a cardiologist (author J.C.) blinded to the results of the adjunctive investigations. Two orthogonal views were examined in end-diastole to maximize contrast enhancement and vessel diameter. The image with the most severe stenosis was used for each evaluated segment of the coronary artery tree. For each segment, the severity of obstruction was documented using several grades: normal, 1–25%, 25–50%, 50–74%, 75–99% and 100% (occluded). Each lesion that was visually scored as greater than 50% luminal obstruction in a vessel that was ≥ 1.5 mm diameter, was further analyzed using validated computerized edge-detection software (QCAPLUS, Sanders data Systems, Palo Alto, California, USA) to allow more accurate assessment and classification of lesion severity. Catheters of known diameter were used for calibration. Coronary angiograms were scored according to two methods. The analyzing cardiologist was masked to patient medical history and investigation results.
- Vessel score: A vessel score was calculated based on the number of vessels with significant obstructive coronary disease. The 2011 American College of Cardiology (ACC) taskforce definition uses 50% stenosis to define significant vessel disease [12]. This definition was used for the left main coronary artery, right coronary, left anterior descending and left circumflex arteries. Scores ranged from 0 to 4, depending on a finding of vessels with greater than 50% stenosis [13]. Left main artery stenosis was scored as double vessel disease.
- Extent score: This score, proposed by Sullivan et al. defines the proportion of the coronary artery tree involved by angiographically detectable coronary atheroma [11]. The proportion of each vessel involved by atheroma, identified by lumen irregularity, was multiplied by a factor for each vessel, related to the length of that vessel. The scores for each vessel were added to give a total score out of 100. This percentage represents the proportion of the coronary intimal surface area containing coronary atheroma [11].
To assess the inter-observer and intra-observer variation in the coronary angiography analysis, 40 random cases were selected. None of the observers participated in the selection of the angiograms and were masked to patient name and diagnosis. We used the Bland Altman method to evaluate reproducibility and inter-observer reliability of the extent score [14]. The bias was 0.15 (limit of agreement − 1.77 to 2.06) for intra-observer reproducibility. For inter-observer difference the bias was 0.076 (limit of agreement − 1.74 to 1.25).
2.3. Statistical analysis
Continuous variables are presented as means (SD or SE) and categorical variables are presented as proportions (%). SAS statistical software (SAS Institute, Cary NC) version 9.2 was used for analyses. The Bland Altman method was performed to evaluate the inter- and intra-variability of the Extent score grading. Analyses performed included t-tests, chi-squared (χ2)-tests and generalized linear models, binary and generalized logistic regression models. Pearsons correlation coefficients were used to correlate the discontinuous and non-normally distributed data. We performed a Kolmogorov–Smirnov test for extent scores that showed a significant difference between gender (P < 0.0001) with women having lower scores than men. We chose to stratify all analyses by sex and age for this study. Both continuous and categorical data were used to present the extent score. Multivariable logistic regression models (binary and generalized) were used to assess the association between gender, age and extent scores. Extent scores were expressed as ordinal categories ranging from 0 (no disease) to 1, 2, and 3 (corresponding to the first, second and third tertiles among those who had disease). The tertile ranges for extent score were 0.3 to 10.7, 11.1 to 33.6 and 33.7 to 100 for the female population and 0.7 to 25.5, 25.7 to 59.6 and 59.6 to 100 in the males. Independent t-tests were used to compare the extent and vessel scores between infarct and non-infarct groups and in our models, data were adjusted for the potential influence of significant covariates: age, mean arterial blood pressure, body mass index, dyslipidemia, smoking, hypertension, presence of type 2 diabetes and treatment with calcium-channel blockers and nitrates. P-values < 0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
The study cohort was predominantly male (73%) with an average age of 60.3 years and Caucasian background (Table 1). Men were more likely to give a history of smoking. In contrast, more women had a history of dyslipidemia, hypertension and diabetes. Body mass index and waist to height ratio were both greater in women (P < 0.0001).
| Characteristics | Women (n = 282) | Men (n = 712) | P-value |
|---|---|---|---|
| Sex (%) | 28 | 72 | < 0.0001 |
| Age, yrs | 63.8 (10.9) | 60.3 (11.9) | < 0.0001 |
| Ethnicity | |||
| Caucasian | 253 (72.1) | 651 (66.6) | 0.0164 |
| East Asian | 18 (5.1) | 64 (6.6) | |
| Southeast Asian | 20 (5.7) | 90 (9.2) | |
| Middle Eastern | 25 (7.1) | 104 (1.6) | |
| Others | 35 (10) | 68 (7) | |
| Body Height, m | 1.58 (0.1) | 1.71 (0.1) | < 0.0001 |
| Body Weight, kg | 75.9 (16.4) | 86.7 (18.5) | < 0.0001 |
| BMI, kg/m2 | 30.3 (6.5) | 29.4 (5.7) | 0.03 |
| Waist:height ratio | 62.3 (9.6) | 59.3 (8.2) | < 0.0001 |
| Blood Pressure (mm Hg) | |||
| Systolic | 125.9 (21.8) | 128.3 (19.2) | 0.0749 |
| Diastolic | 71.9 (12.3) | 73.9 (12.1) | 0.0108 |
| Mean arterial pressure | 89.9 (13.8) | 92.0 (13.0) | 0.0111 |
| Ever Smoked (%) | 44.1 | 74.7 | < 0.0001 |
| History of Hypercholesterolemia (%) | 86.2 | 80.7 | 0.0281 |
| History of hypertension (%) | 74.6 | 65.7 | 0.0021 |
| History of diabetes | 36.5 | 31.9 | 0.1188 |
| Current medications (%) | |||
| Aspirin | 36.5 | 39.1 | 0.385 |
| Clopidogrel/Prasugrel/Ticagrelor | 15.4 | 13.3 | 0.336 |
| Nitrate | 13.4 | 8.5 | 0.0081 |
| Beta-blocker | 25.4 | 21.6 | 0.1486 |
| Calcium channel blocker | 21.4 | 16.9 | 0.0614 |
| ACE-inhibitor | 21.9 | 19.8 | 0.4068 |
| Angiotensin II receptor antagonist | 29.3 | 21.6 | 0.0034 |
| alpha-Blocker | 2.3 | 2.6 | 0.7728 |
| Statin | 57.6 | 42.2 | < 0.0001 |
| Other antianginal agent | 3.7 | 2.6 | 0.2698 |
Data are presented as mean (± SD) or n (%).
3.2. Angiographic scoring and correlations by gender
The mean extent and vessel scores were higher in men than women (Table 2). Correlation analysis showed a strong positive linear relationship between each of the scoring systems used. The correlation was higher in men (r = 0.78–0.94, P < 0.0001) than in women (r = 0.67–0.9, P < 0.0001). Compared to women, men had significantly increased the odds of having an extent score not equal to zero (Table 3).
| Scoring system | Male | Female | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Extent score | 712 | 36.8 (32.4)⁎a | 282 | 19.6 (26.2)⁎b |
| Vessel score | 712 | 1.3 (1.1)#a | 282 | 0.7 (0.9)#b |
Data are presented as mean (± SD) or n (%).
⁎# Males vs females P < 0.0001.
a. Pearson correlation coefficient for extent vs vessel score in males r = 0.78, P < 0.0001.
b. Pearson correlation coefficient for extent vs vessel score in females r = 0.74, P < 0.0001.
| Gender | Extent score (n = 994) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| n (%) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Female | 282 (28) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |
| Male | 712 (72) | 1 (reference) | 2.45 (1.59,3.78) | 2.81 (1.81,4.38) | 3.13 (1.98,4.93) | |
Generalized logistic model. Adjusted for age, ethnicity, mean arterial blood pressure, body mass index, dyslipidemia, smoking, hypertension, presence of type 2 diabetes and nitrate medication.
Chi-square for raw data P < 0.0001.
OR, odds ratio; CI, confidence interval.
3.3. Extent scores and relationship to gender and age
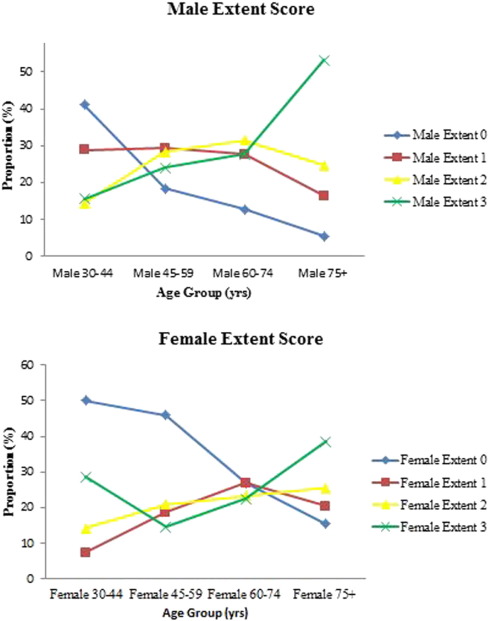
Fig. 1 is a graphical representation of the extent scores by age and gender. There were proportionally more women with normal coronary arteries than men and a slow tapering with age. By contrast, men had a lower proportion of normal arteries and had significant coronary disease at an earlier age than women. Further, the proportion of men with significant coronary disease was higher for all age groups but particularly from ages 45–59 years. The proportion of women with significant disease remained low until 60 years. Among men, age increased the odds of having an extent score greater than zero, a consistent relationship found for all age groups (χ2 P-value < 0.0001). Although the trend was similar for women, the odds approached significance in the oldest group only (data not shown).
|
|
|
Fig. 1. Distribution of extent score by age group and gender for females and males. |
Table 4 shows the relationship between extent and vessel scores with gender in the infarct and non-infarct group. There was no significant difference in the extent score between men and women who presented with myocardial infarction (P = 0.179). The vessel score however, was lower for the women compared to men (1.2 vs 1.6, respectively, P = 0.005). Extent and vessel scores were significantly higher for men in the non-infarct group. Further, women without infarction had lower extent and vessel scores than those with infarction (P < 0.0001 for both). Extent and vessel scores were significantly higher in the infarct group for both sexes.
| Total | Male | Female | r | P | |
|---|---|---|---|---|---|
| Extent score (0–100) | |||||
| Total (n = 994) | 31.9 (31.7) | 36.8 (32.4) | 19.6 (26.2) | 0.254 | < 0.0001 |
| Infarct (n = 221) | 40.6 (26.9) | 41.6 (26.9)⁎% | 34.8 (26.5)#% | 0.091 | 0.179 |
| No infarct (n = 773) | 29.5 (32.6) | 35.2 (33.9)⁎$ | 17.6 (25.6)#$ | 0.252 | < 0.0001 |
| Vessel score (0–3) | |||||
| Total (n = 994) | 1.2 (1.1) | 1.3 (1.1) | 0.8 (0.9) | 0.261 | < 0.0001 |
| Infarct (n = 221) | 1.6 (0.8) | 1.6 (0.8)a& | 1.2(0.6)b& | 0.15 | 0.011 |
| No infarct (n = 773) | 1.1 (1.1) | 1.2 (0.8)a§ | 0.6 (0.9)b§ | 0.218 | < 0.0001 |
Independent t-test to compare gender and average extent and vessel score.
Values are Mean (SD); r = correlation coefficient adjusted for age.
Extent score: ⁎Male infarct vs non-infarct, P = 0.02; #female infarct vs non-infarct P < 0.0001; %male vs female infarct, P = 0.179; $male vs female non-infarct, P < 0.0001.
Vessel score: aMale infarct vs non-infarct, P < 0.0001; bfemale infarct vs non-infarct P < 0.0001.
&Male vs female infarct, P = 0.005; §male vs female non-infarct, P < 0.0001.
The predictors of extent score in men were age, systemic hypertension, body mass index, diabetes and evidence of recent myocardial infarction (Table 5). However in women, the predictors for extent score in order of significance were age, smoking, myocardial infarction and hypercholesterolemia.
| Variable | Male (χ2) | P | Female (χ2) | P |
|---|---|---|---|---|
| Age | 50.6 | < 0.0001 | 21.5 | 0.011 |
| Systemic hypertension | 9.3 | 0.026 | 0.3 | 0.97 |
| Body mass index | 9.4 | 0.024 | 1.3 | 0.73 |
| Diabetes | 27.4 | < 0.0001 | 5.1 | 0.16 |
| Cigarette smoker | 6.9 | 0.077 | 15.3 | 0.002 |
| Hypercholesterolemia | 5.4 | 0.144 | 4.8 | 0.028 |
| Infarction | 9.6 | 0.022 | 8.6 | 0.035 |
Chi-squared (χ2) test for predictors of extent score.
P-values significant at P < 0.05.
4. Discussion
We have shown a significant difference in CAD severity and extent between men and women presenting with suspected ischemic chest pain. These findings are concordant with studies showing that compared to men, age-matched women have a much lower burden of obstructive and non-obstructive CAD [3]; [15]; [16] ; [17]. We also found that women with suspected ischemic chest pain undergoing invasive coronary angiography had less extensive epicardial atheroma. There is a paucity in the literature of data showing gender differences using the extent scoring system to grade CAD.
The pattern of CAD in women was found to be substantially different to that in men [2]. Pressure wire studies as well as nuclear and magnetic resonance imaging have shown that women can display evidence of myocardial ischemia and microvascular dysfunction in the absence of obstructive coronary disease, often unrelated to the presence of Framingham risk factors [8]; [9] ; [10]. Further, women without significant luminal obstruction have a higher prevalence of atherosclerosis with positive remodeling and preserved lumen size as demonstrated by intravascular ultrasound (IVUS) [9] ; [10]. A clear pathophysiologic explanation for the observed sex-specific differences, however, is lacking.
Our study uses the Extent (Sullivan) score to determine the severity and burden of coronary atherosclerosis in a large population presenting for the evaluation of suspected angina [14]. We used the extent score as an alternative method of analyzing CAD on angiography as it provides anatomical perspective on atherosclerotic burden and has been shown previously to be a potential marker of adverse outcome [18]. Bigi and colleagues [18] studied 228 patients with low risk, stable CAD and found the extent score determines the risk of death and myocardial infarction. A recent study by Neeland et al. [19] showed that even in the absence of significant luminal stenosis, angiographic CAD scoring systems are correlated with each other and with plaque burden as determined by IVUS. A further IVUS study by Khuddus et al. [10] was performed in a small population of women with suspected ischemia and non-obstructive CAD. They reported that up to 80% of women without significant luminal narrowing, had a high prevalence of mild to moderate atherosclerosis by IVUS. This suggests that traditional methods of grading angiograms using stenosis scoring are suboptimal in the female population and that an anatomical approach of examining the burden and extent of atherosclerosis is perhaps a more useful tool.
In our population, being male led to a much greater risk of significant CAD. Men showed a three-fold higher risk than women of having a positive extent score when presenting with chest pain. We also showed that for each ascending age-group, the mean adjusted score increased substantially. Importantly, the proportion of men with normal coronary arteries fell dramatically after age 30–44 years, so that only approximately 16% of men aged 45–59 years and 12% of men aged 60–74 years had normal coronary arteries. Compared to the youngest group, being male aged over 45 years dramatically increased the odds of having a positive extent score, compared to that in women, with the odds increasing with ascending age.
This pattern was not seen in women. Women presenting with chest pain had a lower CAD burden, with up to 50% having normal coronary arteries in the 30–44 year age group and 40% in the 45–59 year age group. Compared to men, women with chest pain were more likely to have normal coronary arteries for each age band. Significant CAD appears to occur at a later age in women than men. Although there was a trend of increasing risk of a positive score with age, it was only of borderline significance among women aged over 75 years. This finding contrasts to that of men where the risk increased with age and with extent score. This may suggest that the extent of atheroma is not as predictive of the severity of CAD in women as it is for men.
Several previous studies have examined the age-related prevalence of obstructive CAD in women presenting with angina. The Womens Ischemia Syndrome Evaluation (WISE) study, found that the prevalence of significant CAD for females was < 5% in women aged under 35 years [2]. This changed dramatically from age 50 years but was highest from age 65 years. A study by Lerner et al. [20] found that the rate of CAD was low in women until age 75 years.
Enbergs et al. [21] studied 331 patients of which 39 men and 31 women had chest pain [21]. They reported higher extent scores in men than women and that asymptomatic men had a lower score than symptomatic men. In their study, women did not display differences in their extent score by presentation. Our findings conflict to some degree with this study, albeit that our population was symptomatic. Interestingly, women who presented with myocardial infarction had lower extent scores than men, although this difference did not reach statistical significance. However, women without infarct had a much lower score than those with evidence of recent infarction. These findings suggest two interpretations: first, the pathophysiology of myocardial infarction may be similar for both genders and second, the extent score may provide different information on the burden of coronary artery atherosclerosis than traditional scoring systems utilizing stenosis severity. This also suggests that CAD is more likely to be diffuse in women as the number of significant stenoses was found to be less in women than in men.
Several studies have demonstrated gender differences in angiographic findings among men and women presenting with myocardial infarction. Milcent et al. [22] studied a large series of patients who presented with acute myocardial infarction and found that women had a higher mortality [22]. Further, it has been previously described that the mortality in both ST elevation and non-ST elevation acute coronary syndrome was higher for women despite a lower incidence of obstructive CAD [3] ; [4].
The ability of myocardial infarction to predict the extent score was demonstrated by the multivariate analysis showing a moderately strong positive association for men and women. Our study found that predictors of extent score in men were older age, systemic hypertension, body mass index, diabetes and myocardial infarction. By contrast, the predictors in women were older age, smoking, hyperlipidemia and recent myocardial infarction. Horimoto et al. found similar predictors, though in contrast, diabetes was a predictor among women and smoking among men in their study of 437 patients with suspected CAD [23]. We found that traditional risk factors were less predictive of the extent score in women than in men, except smoking, which is a powerful predictor. As such, despite risk factors being more predictive of future cardiovascular events in women [24] ; [25], there is a disconnect in predicting the overall burden of coronary atheroma. We should therefore be cautious when reassuring women in the presence of normal arteries or minor CAD.
The difference in the burden of CAD between men and women presenting with chest pain has potential clinical implications. Our study suggests that traditional invasive coronary angiography may not always assist in establishing the cause of symptoms in women presenting with suspected ischemic chest pain. Using the Extent score may be a useful adjunct to traditional angiogram analyses to further risk stratify women. Angiography provides falsely reassuring results in women when there is minimal obstructive disease as they continue to have recurrent hospital admissions, repeated investigations for persistent or progressive symptoms and higher cardiovascular event rates [26]; [27]; [28] ; [29]. We need to investigate the utility of non-invasive methods as well as examine the role of non-traditional risk markers in the diagnosis of angina in the female population. From there, an optimal approach to treatment and prevention can be made.
4.1. Limitations
Although this is a large study from a single center examining patients from a broad demographic, all our patients were symptomatic and being investigated for suspected ischemia and therefore there was no predefined or standardized patient population. This limits the generalizability of our results to similar care settings and the general population. Further, the low proportion of women recruited in the study may have influenced the outcomes. This does highlight the lower number of women presenting for coronary angiography as well as the perceived lower risk of ischemic heart disease. Selection bias cannot be completely excluded even after adjusting for a wide range of patient characteristics. Last, we did not collect information on non-invasive investigations (such as stress testing, myocardial perfusion imaging) performed preceeding angiography as these were not available. Further, we were unable to perform novel ancillary investigations such as coronary flow reserve, PET scan or cardiac MRI which would be useful to correlate the findings.
5. Conclusions
We found a significant contrast in CAD severity and burden between women and men presenting for the investigation of suspected angina. Even in the presence of traditional risk factors, women were more likely to have normal coronary arteries or less severe disease than men. The extent score may be a useful tool to determine the diffusion of atherosclerosis seen on invasive coronary angiography and may help risk stratify CAD in women. Future studies should correlate the extent score with pressure wire studies, coronary artery calcification, myocardial perfusion imaging and/or PET scanning to determine if higher scores infer a predictive value for ischemia or microvascular dysfunction.
Disclosures and competing interests
None.
Acknowledgments
The authors would like to gratefully acknowledge the funding provided by the Australian National Health and Medical Research Council (Grant No. 571012); and the Westmead Millennium Institute, University of Sydney.
References
- [1] V. Vaccarino, L. Parsons, N.R. Every, H.V. Barron, H.M. Krumholz; Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants; N. Engl. J. Med., 341 (4) (1999), pp. 217–225 https://doi.org/10.1056/NEJM199907223410401
- [2] C.N. Bairey Merz, L.J. Shaw, S.E. Reis, et al.; Insights from the NHLBI-Sponsored Womens Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease; J. Am. Coll. Cardiol., 47 (3 Suppl.) (2006), pp. S21–S29 https://doi.org/10.1016/j.jacc.2004.12.084
- [3] E.R. Gehrie, H.R. Reynolds, A.Y. Chen, et al.; Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) Quality Improvement Initiative; Am. Heart J., 158 (4) (2009), pp. 688–694 https://doi.org/10.1016/j.ahj.2009.08.004
- [4] J.S. Berger, L. Elliott, D. Gallup, et al.; Sex differences in mortality following acute coronary syndromes; JAMA, 302 (8) (2009), pp. 874–882 https://doi.org/10.1001/jama.2009.1227
- [5] L. Jespersen, A. Hvelplund, S.Z. Abildstrom, et al.; Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events; Eur. Heart J., 33 (6) (2012), pp. 734–744 https://doi.org/10.1093/eurheartj/ehr331
- [6] E. Jones, W. Eteiba, N.B. Merz; Cardiac syndrome X and microvascular coronary dysfunction; Trends Cardiovasc. Med., 22 (6) (2012), pp. 161–168 https://doi.org/10.1016/j.tcm.2012.07.014
- [7] C.J. Pepine, R.D. Anderson, B.L. Sharaf, et al.; Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Womens Ischemia Syndrome Evaluation) study; J. Am. Coll. Cardiol., 55 (25) (2010), pp. 2825–2832 https://doi.org/10.1016/j.jacc.2010.01.054
- [8] C.N. Bairey Merz, C.J. Pepine; Syndrome X and microvascular coronary dysfunction; Circulation, 124 (13) (2011), pp. 1477–1480 https://doi.org/10.1161/circulationaha.110.974212
- [9] H.R. Reynolds, M.B. Srichai, S.N. Iqbal, et al.; Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease; Circulation, 124 (13) (2011), pp. 1414–1425 https://doi.org/10.1161/circulationaha.111.026542
- [10] M.A. Khuddus, C.J. Pepine, E.M. Handberg, et al.; An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Womens Ischemia Syndrome Evaluation (WISE); J. Interv. Cardiol., 23 (6) (2010), pp. 511–519 https://doi.org/10.1111/j.1540–8183.2010.00598.x
- [11] D.R. Sullivan, T.H. Marwick, S.B. Freedman; A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors; Am. Heart J., 119 (6) (1990), pp. 1262–1267
- [12] W.S. Weintraub, R.P. Karlsberg, J.E. Tcheng, et al.; ACCF/AHA 2011 key data elements and definitions of a base cardiovascular vocabulary for electronic health records: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards; J. Am. Coll. Cardiol., 58 (2) (2011), pp. 202–222 https://doi.org/10.1016/j.jacc.2011.05.001
- [13] T. Norgaz, G. Hobikoglu, H. Aksu, et al.; Retinopathy is related to the angiographically detected severity and extent of coronary artery disease in patients with type 2 diabetes mellitus; Int. Heart J., 46 (4) (2005), pp. 639–646
- [14] J.M. Bland, D.G. Altman; Statistical methods for assessing agreement between two methods of clinical measurement; Lancet., 1 (8476) (1986), pp. 307–310
- [15] C.N. Merz; The Yentl syndrome is alive and well; Eur. Heart J., 32 (11) (2011), pp. 1313–1315 https://doi.org/10.1093/eurheartj/ehr083
- [16] R. Bugiardini; Normal coronary arteries: clinical implications and further classification; Herz, 30 (1) (2005), pp. 3–7 https://doi.org/10.1007/s00059-005–2659–8
- [17] R. Ferrari, H. Abergel, I. Ford, et al.; Gender- and age-related differences in clinical presentation and management of outpatients with stable coronary artery disease; Int. J. Cardiol., 167 (6) (2013), pp. 2938–2943 https://doi.org/10.1016/j.ijcard.2012.08.013
- [18] R. Bigi, L. Cortigiani, P. Colombo, A. Desideri, J.J. Bax, O. Parodi; Prognostic and clinical correlates of angiographically diffuse non-obstructive coronary lesions; Heart, 89 (9) (2003), pp. 1009–1013
- [19] I.J. Neeland, R.S. Patel, P. Eshtehardi, et al.; Coronary angiographic scoring systems: an evaluation of their equivalence and validity; Am. Heart J., 164 (4) (2012), pp. 547 e1–552 e1 https://doi.org/10.1016/j.ahj.2012.07.007
- [20] D.J. Lerner, W.B. Kannel; Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population; Am. Heart J., 111 (2) (1986), pp. 383–390
- [21] A. Enbergs, R. Burger, H. Reinecke, M. Borggrefe, G. Breithardt, S. Kerber; Prevalence of coronary artery disease in a general population without suspicion of coronary artery disease: angiographic analysis of subjects aged 40 to 70 years referred for catheter ablation therapy; Eur. Heart J., 21 (1) (2000), pp. 45–52 https://doi.org/10.1053/euhj.1999.1763
- [22] C. Milcent, B. Dormont, I. Durand-Zaleski, P.G. Steg; Gender differences in hospital mortality and use of percutaneous coronary intervention in acute myocardial infarction: microsimulation analysis of the 1999 nationwide French hospitals database; Circulation, 115 (7) (2007), pp. 833–839 https://doi.org/10.1161/CIRCULATIONAHA.106.664979
- [23] M. Horimoto, A. Hasegawa, T. Ozaki, T. Takenaka, K. Igarashi, H. Inoue; Independent predictors of the severity of angiographic coronary atherosclerosis: the lack of association between impaired glucose tolerance and stenosis severity; Atherosclerosis, 182 (1) (2005), pp. 113–119
- [24] W.C. Willett, A. Green, M.J. Stampfer, et al.; Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes; N. Engl. J. Med., 317 (21) (1987), pp. 1303–1309 https://doi.org/10.1056/NEJM198711193172102
- [25] R. Huxley, F. Barzi, M. Woodward; Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies; BMJ, 332 (7533) (2006), pp. 73–78 https://doi.org/10.1136/bmj.38678.389583.7C
- [26] P. Lamendola, G.A. Lanza, A. Spinelli, et al.; Long-term prognosis of patients with cardiac syndrome X; Int. J. Cardiol., 140 (2) (2010), pp. 197–199 https://doi.org/10.1016/j.ijcard.2008.11.026
- [27] R.D. Anderson, C.J. Pepine; Gender differences in the treatment for acute myocardial infarction: bias or biology?; Circulation, 115 (7) (2007), pp. 823–826 https://doi.org/10.1161/CIRCULATIONAHA.106.685859
- [28] L.J. Shaw, R.E. Shaw, C.N. Merz, et al.; Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry; Circulation, 117 (14) (2008), pp. 1787–1801 https://doi.org/10.1161/CIRCULATIONAHA.107.726562
- [29] M. Gulati, R.M. Cooper-DeHoff, C. McClure, et al.; Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Womens Ischemia Syndrome Evaluation Study and the St. James Women Take Heart Project; Arch. Intern. Med., 169 (9) (2009), pp. 843–850 https://doi.org/10.1001/archinternmed.2009.50
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?