Summary
Objective
To compare the effectiveness of early termination (ET) and standard termination (ST) of radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) using internally cooled electrodes.
Methods
Eighty-three treatment naïve patients with HCC with 101 index tumor underwent RFA. ET was defined as termination of ablation if after 6 minutes the power emission was < 10 seconds for three times per minute under maximal power. Standard termination was defined as termination of ablation according to manufactures’ algorithms of 12 minutes’ procedure. Primary end point was complete ablation of index tumor after 4 weeks of RFA.
Results
Nineteen patients (22.9%) underwent ET and 64 patients (77.1%) underwent ST. The mean size of the index tumor was 2.2 + 0.9 cm. Patient had complete ablation of the index tumor at 92.0% for the ET and 88.2% for the ST (p = 0.593). Eight of 25 (32.0%) for the ET and 18 of 73 (24.7%) for the ST group had local tumor progression on last follow-up (p = 0.473). After a median follow-up of 23 weeks (range, 9–33 weeks), the 24 weeks cumulative probability of local tumor progression was not different between ET (46.2%) and ST (25.6%; p = 0.387). Complete ablation at 4 weeks was the only independent factor associated with local tumor progression (adjusted hazard ratio 0.04, 95% confidence interval 0.01–0.16, p < 0.001).
Conclusion
Using ET in RFA is as effective as the ST in achieving complete ablation and local tumor progression.
Keywords
Hepatocellular carcinoma ; Internally cooled electrode ; Local tumor progression ; Radiofrequency ablation ; Standard termination ; Termination of ablation
Introduction
Hepatic resection and transplantation are the potential curative therapy for hepatocellular carcinoma (HCC). Unfortunately, only 20–30% of those patients presenting with HCC are eligible candidates [1] ; [2] . With the introduction of radiofrequency ablation (RFA) in 1983, it has become one of the modalities that have been gaining acceptance in treating small inoperable HCC [3] ; [4] ; [5] ; [6] ; [7] ; [8] ; [9] ; [10] ; [11] ; [12] . RFA have been shown in several studies to have a high complete ablation rate of 85–96% in the management of HCC < 5 cm in diameter [4] ; [5] ; [6] ; [7] ; [8] ; [9] ; [10] ; [11] .
The mechanism behind RFA is based on adequate conversion of electrical energy to thermal energy to produce tissue heating. The heat has coagulation and direct cytotoxic effects on the tumor leading to tumor destruction [12] . Several electrodes are available commercially and the internally cooled electrode is among the commonly used electrode because of its ease of use and proven efficacy [3] ; [4] ; [5] ; [6] ; [7] ; [8] ; [9] ; [10] ; [11] ; [12] ; [13] ; [14] ; [15] ; [16] . Its tip is internally cooled to prevent tissue charring leading to a significant increase in energy delivery and lesion size [13] ; [14] ; [15] ; [16] . Previous studies showed that increasing the power or prolonged ablation procedure would produce larger coagulation area however a power > 130 watts would induce a frequent increase in impedance and multiple automatic reduction in current that might consequently diminish the total treated time [14] ; [15] ; [16] ; [17] ; [18] . A rising impedance may be due to irreversible dehydration and charring [19] ; [20] or due to reversible formation of electrically insulating gas between the electrode and the tissue due to boiling and evaporation of tissue fluid [21] .
Modification of treatment strategies such as performing percutaneous saline-enhanced impedance controlled RFA to minimize potential carbonization to produce a larger tumor response rate has been studied; though promising, it is not often convenient and applicable to all operators [21] ; [22] . Hence seeking for newer techniques, constant modification or refinement of treatment strategies is of value.
This study aimed to determine whether there is any difference in using the early termination technique than the standard termination in terms of complete ablation of the index tumor, local tumor progression, and complication rates.
Methods
Patients
Consecutively registered patients with HCC who underwent RFA using internally cooled electrode from May 2010 to April 2011 at Chang Gung Memorial Hospital (Linkou Branch), Taipei, Taiwan were screened. The study included patients with treatment-naïve HCC with less than three index tumors and < 4 cm in size. Excluded patients were those with Child-Pugh Class C cirrhosis, with macroscopic vascular invasion or extrahepatic metastases and those with malignancy other than HCC.
The diagnosis of HCC was through cytologic or histopathologic findings or the presence of a hypervascular liver mass in the arterial phase of a dynamic imaging study (computed tomography or magnetic resonance imaging) with contrast washout during the portal or delayed phase plus angiographic confirmation of a hypervascular mass or an α-fetoprotein concentration > 200 ng/mL. Patient demographics, hematology, biochemistry, size, and number of index tumor were recorded. Etiological factors of hepatitis B virus or hepatitis C virus were examined by serological assays. Patients included in the study gave informed written consent for RFA.
Radiofrequency ablation technique
This study was limited to patients treated with an internally cooled electrode with a 3-cm uninsulated tip (Cool-tip radiofrequency system, Valleylab Inc., Boulder, CO, USA) in all tumors. Cluster-type electrodes were not used in this study. All RFA were performed by three gastroenterologists with at least 10 years’ experience with sonographic-guided interventions. Multiple overlapping ablations were performed as needed to cover the whole tumor plus a 5- to 10-mm ablative margin around the tumor, when feasible [3] ; [12] ; [17] ; [18] . Electrode track thermocoagulation was routinely performed with a temperature over 70°C on withdrawal [3] ; [12] ; [17] ; [18] ; [23] .
A standard protocol for conscious sedation consisting of 30 mg meperidine and 3 mg midazolam were administered intravenously prior to ablation. These doses were then titrated to achieve an adequate comfort level throughout the procedure. Continuous monitoring of vital signs and oxygen saturation during the procedure was performed. Local anesthesia with 1% lidocaine hydrochloride was applied along the planned puncture site. The electrode was introduced percutaneously through the tumor for at least 0.5 cm beyond its deep margin under real-time sonographic guidance (Aplio SSA-700A, Toshiba, Tokyo, Japan). The radiofrequency generator contains an internal program that automatically runs a 12-min pulsed energy ablation cycle and adjusts the power output relative to tissue impedance to optimize the diameter of ablated tissue.
For the early termination group (ET) maximum power setting was done and if after 6 minutes the power emission was < 10 seconds for three times per minute the ablation was terminated after 9 minutes. As for the standard termination (ST) the modified automated algorithm as previously described was used [18] . Briefly, the ablation begins by activating the impedance control mode with a gradual increase in power output over the 1st minute to reach 120 W. The power is maintained at this level until tissue impedance rises to 20 Ω above the baseline impedance. The power output is automatically reduced to zero for 15 seconds once the 20 Ω of threshold is exceeded. The power is then returned to the initial peak power setting until the tissue impedance rises again. Successive cycles are continued for 12 minutes to complete a single ablation. The discretion to perform either technique was left to the decision of the operator.
Follow-up protocol
Four weeks after the ablation, a dynamic imaging study [computed tomography (CT) or magnetic resonance imaging (MRI)] was performed to assess the completeness of ablation. A completely ablated tumor was defined as an area of low attenuation on CT or low signal intensity on T2-weighted MRI that encompassed the area of the index tumor with no nodular peripheral enhancement on dynamic studies [23] . Any nodular enhancement seen in the treated tumor was considered to be a residual viable tumor. In cases of incomplete ablation, additional sessions using the same algorithm or percutaneous ethanol injection or transarterial chemoembolization (TACE) were performed. Treatment for each tumor is limited to three sessions within 3 months. An immediate follow-up dynamic study was carried out to confirm the absence of a residual tumor after the additional treatments. After complete ablation, monitoring for local recurrence with dynamic imaging studies, liver function tests, and α-fetoprotein measurement were done every 3–6 months.
Study endpoints
The primary endpoint of this study was complete ablation of the index tumor after one RFA session. Secondary end points were primary effectiveness defined as complete ablation of the index tumor after more than one RFA session and local tumor progression. Local tumor progression was defined as the appearance of nodular enhancement contiguous with the ablated tumor on dynamic study or an increase in the size of the ablated area on follow-up imaging of a tumor that was previously completely ablated [23] . Major complications were defined as those requiring therapy with hospitalization or involving permanent adverse sequelae. These endpoints were based on the guidelines set by the International Working Group on Image-Guided Tumor Ablation [23] .
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics for Windows version 19.0 (Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation or median (interquartile range), and compared using unpaired t test or Mann–Whitney U test as appropriate. Categorical variables were presented as frequency (percentage) and compared using the Chi-square test or Fisher’s exact test as appropriate. Cumulative probability local tumor progression was estimated with Kaplan–Meier curve. Multiple logistic regression analysis was used to determine independent predictors of complete ablation and Cox regression analysis with forward logistic regression was used to model independent predictors of local tumor progression. Covariates with p < 0.05 from univariate analysis were included in multivariate analysis. All statistical tests were two sided and a p < 0.05 was considered statistically significant. Survival analysis was not performed due to the short-term follow-up of the study population.
Results
Patients characteristics
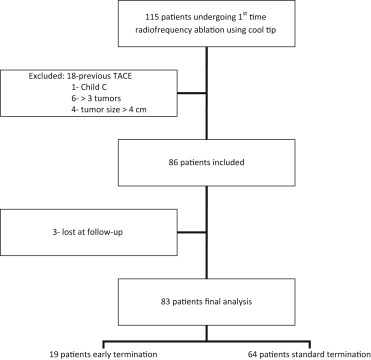
A total of 115 patients underwent first-time RFA using internally cooled electrode during the study period. Twenty-nine patients were excluded due to history of previous treatment (n = 18), Child-Pugh class C (n = 1), index tumor number of more than three (n = 6) and tumor size of more than four (n = 4). Eighty-three patients were eventually included in the final analysis, with 19 patients belonging to the ET group and 64 patients to the ST group ( Figure 1 ). The mean age of patients was 67.0 + 9.2 with a median follow-up of 23 weeks (range, 9–33 weeks). The mean size of the index tumor was 2.2 + 0.9 cm. More cirrhotic patients (89.1%) were included in the ST group than ET group (68.4%, p = 0.030; Table 1 ).
|
|
|
Figure 1. Flowchart of patient distribution. TACE = transarterial chemoembolization. |
| Characteristics | All (n = 83) | Early Termination (n = 19) | Standard Termination (n = 64) | p |
|---|---|---|---|---|
| Age (y) | 67.0 + 9.2 | 66.7 + 9.9 | 67.1 + 9.1 | 0.896 |
| Male (%) | 55 (66.3) | 15 (78.9) | 40 (62.5) | 0.183 |
| Etiologic factor | 0.560 | |||
| Hepatitis B virus | 25 (30.1) | 4 (21.1) | 21 (32.8) | |
| Hepatitis C virus | 40 (48.2) | 11 (57.8) | 29 (45.3) | |
| Othersa | 18 (21.7) | 4 (21.1) | 14 (21.9) | |
| Cirrhosis | 70 (84.3) | 13 (68.4) | 57(89.1) | 0.030 |
| Child-Pugh class (n = 70) | 0.627 | |||
| A | 50 (71.4) | 10 (76.9) | 40 (70.2) | |
| B | 20 (28.6) | 3 (23.1) | 17 (29.8) | |
| α-fetoprotein (ng/mL) | 9.7 (4.8–36.7) | 12.3 (5.7–39.6) | 8.1 (4.2–36.0) | 0.319 |
| Total bilirubin (mg/dL) | 0.9 + 0.6 | 1.0 + 0.6 | 0.9 + 0.6 | 0.428 |
| Albumin (g/dL) (n = 80) | 3.6 + 0.6 | 3.7 + 0.7 | 3.6 + 0.6 | 0.439 |
| Prothrombin time (international normalized ratio) | 1.2 + 0.1 | 1.2 + 0.2 | 1.2 + 0.1 | 0.342 |
| Platelet count (×109 /L) | 115.6 + 53.6 | 112.0 + 55.6 | 116.6 + 53.4 | 0.743 |
| Size of index tumor (cm) (n = 101) | 2.2 + 0.9 | 2.1 + 0.8 | 2.3 + 1.0 | 0.271 |
| Single tumor | 65 (78.3) | 13 (20) | 52 (80) | 0.216 |
| No. of index tumor | 1.2 + 0.4 | 1.3 + 0.5 | 1.2 + 0.4 | 0.239 |
| Patients with tumors in difficult-to-treat location | 24 (28.9) | 6 (31.5) | 18 (28.1) | 0.538 |
| Cool down temperature (°C) (n = 81) | 65.3 + 7.6 | 62.4 + 7.8 | 66.2 + 7.4 | 0.057 |
| Complete ablation at 4 wk (n = 101) | 90 (89.1) | 23/25 (92) | 67/76 (88.2) | 0.593 |
| Primary technique effectiveness (n = 101) | 98 (97) | 25/25 (100) | 73/76 (96.1) | 0.313 |
| Local progression (n = 98)b | 21 (21.4) | 6/25 (24) | 15/73 (20.5) | 0.717 |
| New HCC on follow-up (n = 98)b | 5 (5.1) | 2/25(8) | 3/73 (4.1) | 0.445 |
Data are presented as n (%), mean + standard deviation, or median (interquartile range) as appropriate.
a. Alcoholic liver disease (n = 6), idiopathic (n = 5), hepatitis B and C coinfection (n = 3), Wilsons disease (n = 1), primary biliary cirrhosis (n = 1), hepatitis B and alcoholic liver disease (n = 2).
b. Not included in analysis because dynamic images after complete ablation unavailable (n = 2) and complete ablation was not achieved (n = 1).
Complete ablation
A total of 101 index tumors were included in the analysis for complete ablation after 4 weeks of RFA with 25 tumors for the ET group and 76 tumors for the ST group. There was no significant difference between the 92% of the ET group and 88.2% of the ST group in achieving complete ablation after 4 weeks of RFA (p = 0.593). There was also no significant independent factor, including a tumor in a difficult-to-treat location, that would predict complete ablation after 4 weeks of RFA from our study ( Table 2 ).
| Variable | HR (95% CI) | p | |
|---|---|---|---|
| Cirrhosis | Yes (n = 70) | 0.41 (0.1–1.8) | 0.231 |
| No (n = 13) | 1.0 | ||
| Child-Pugh class | B (n = 20) | 0.38 (0.1–2.6) | 0.326 |
| A (n = 50) | 1.0 | ||
| Cool-down temperature (°C) | ≤ 70 (n = 8) | 0.98 (0.9–1.1) | 0.722 |
| > 70 (n = 75) | 1.0 | ||
| Early termination | No (n = 64) | 0.65 (0.1–3.2) | 0.595 |
| Yes (n = 19) | 1.0 | ||
| Number of needle passes | 2–3(n = 62) | 1.07 (0.6–1.8) | 0.803 |
| 1 (n = 21) | 1.0 | ||
| Size of index tumor | ≤ 2 cm (n = 78) | 1.00 (0.5–2.0) | 0.996 |
| > 2 cm (n = 23) | 1.0 | ||
| Patients with tumors in difficult-to-treat location | Yes (n = 24) | 1.56 (0.78–3.84) | 0.443 |
| No (n = 59) | 1.0 | ||
CI = confidence interval; HR = hazard ratio.
Primary technique effectiveness
Among the 11 tumors not completely ablated after one RFA session, eight were managed with further RFA session to achieve complete ablation. Of the remaining three tumors with incomplete ablation, two underwent transarterial chemoembolization and one underwent ethanol injection that was determined by patient’s preference. All patients in the ET group and 73 in the ST group (96.1%) achieved primary technique effectiveness (p = 0.313).
Local tumor progression
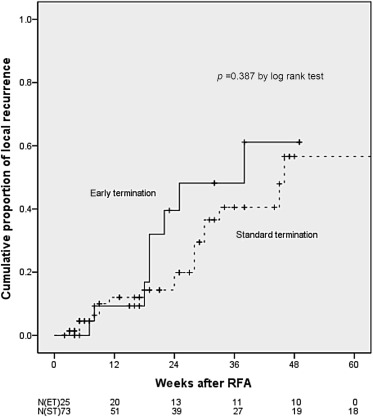
Out of the 101 tumors, only 98 tumors were included in the final analysis for local tumor progression because dynamic images after complete ablation were unavailable for two tumors and complete ablation was not achieved in one tumor. The follow up duration was 20.1 + 14.8 weeks (20.2 + 13.1 weeks for the ET group and 20.0 + 15.4 weeks for the ST group; p = 0.966). Twenty-one tumors had local recurrence and five tumors were new recurrences. Local tumor progression for the ET group and ST group were not significantly different (32% and 24.7% respectively, p = 0.473). Both cirrhosis and complete ablation after 4 weeks of RFA were significant in univariate analysis for predicting local tumor progression, however, on multivariate analysis only complete ablation after 4 weeks of RFA is the independent predictive factor for local tumor progression ( Table 3 ). The cumulative local tumor progression rate of patients in the ET group was not significantly different to those in the ST group (p = 0.387, Figure 2 ). The 24 weeks cumulative probability of local tumor progression was not different among those with ET group [46.2%; 95% confidence interval (CI) 0.191–0.733] and those with ST group (25.6%; 95% CI 0.119–0.393).
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Cirrhosis | Yes (n = 70) | 0.32 (0.13–0.77) | 0.011 | 0.56 (0.20–1.52) | 0.251 |
| No (n = 13) | 1.0 | 1.0 | |||
| Child-Pugh class | B (n = 20) | 0.4 6(0.17–1.29) | 0.142 | ||
| A (n = 50) | 1.0 | ||||
| Tumor size | ≤ 2 cm (n = 78) | 0.75 (0.44–1.28) | 0.285 | ||
| > 2 cm (n = 23) | 1.0 | ||||
| Patients with tumors in difficult-to-treat location | Yes (n = 24) | 1.89 (0.94–3.18) | 0.562 | ||
| No (n = 59) | 1.0 | ||||
| Early termination | No (n = 64) | 1.44 (0.62–3.32) | 0.394 | ||
| Yes (n = 19) | 1.0 | ||||
| Number of needle passes | 2–3 (n = 62) | 0.62 (0.36–1.09) | 0.096 | ||
| 1 (n = 21) | 1.0 | ||||
| Complete ablation of HCC after 4 w | Yes (n = 90) | 0.01 (0.01–0.11) | < 0.001 | 0.04 (0.01–0.16) | < 0.001 |
| No (n = 11) | 1.0 | 1.0 | |||
CI = confidence interval; HCC = hepatocellular carcinoma; HR = hazard ratio.
|
|
|
Figure 2. Cumulative proportion of local recurrence among patients with hepatocellular carcinoma treated with early termination (ET) radiofrequency ablation versus standard termination (ST). Cumulative local tumor progression rate of those patients with hepatocellular carcinoma treated with ET was not significantly different to those treated with ST of radiofrequency ablation (p = 0.387). n indicates the number of remaining patients who were not lost to follow-up and still had nonviable tumor. |
Complications
All patients tolerated the procedure well. No major complications occurred in both groups. Side effects seen were not significant in both groups (p = 0.612). One patient had post-ablation syndrome (fever, general malaise) and one patient had minimal asymptomatic perihepatic or renal fluid for the ET group. Although five patients of the ST group had asymptomatic pleural effusion, four had asymptomatic pleural effusion plus ascites, two had postablation syndrome, another two had minimal asymptomatic perihepatic or renal fluid, and one had minimal pain. During the follow-up period, no patients died but two patients were lost to follow-up.
Discussion
In this study, performing percutaneous RFA using the ET technique is not different from the ST technique in terms of achieving complete ablation. Local tumor progression and side effects were also comparable between the groups. To our knowledge, this study is the first of its kind in using such a technique in performing RFA.
RFA has gained important role in ablation of small HCC for its high rate of complete ablation and minimal invasiveness [4] ; [5] ; [6] ; [7] ; [8] ; [9] ; [10] ; [11] . The determinants of complete ablation of RFA include tumor factors including size, location, conspicuousness, and heat sink effect, and ablation procedure factors including technical success and complications [12] ; [13] ; [20] . The technical success was also dependent on the distribution of heat emission within the tumor [12] . To our knowledge, there is no universal ablation algorithm for RFA procedure [20] ; [23] . However, if the heat emission was interrupted by the abnormally high temperature related carbonization and charring tissue, the ablation effect and zone would be incomplete [20] ; [24] . A manufacture algorithm of radio-frequency was used an internal program that automatically runs a 12-min pulsed energy ablation cycle and adjusts the power output relative to tissue impedance to optimize the diameter of ablated tissue [3] ; [4] ; [12] ; [13] ; [16] ; [18] ; [20] ; [23] . Nevertheless, frequent rising of impedance between very short intervals was observed and it subsequently might hinder heat further emission due to more charring formation during frequent high impedance [17] ; [18] ; [24] ; [25] . Our finding in the current study showed a similar rate of complications and local tumor progression between standard and early termination of ablation procedures. The benefit of early termination may include shorter ablation time, less discomfort, and probably more useful for ablation of larger tumor that requires overlapping ablation.
Furthermore, to our knowledge, there is no definition of early termination procedure. However, based on the mechanism of radiofrequency emission, the power is maintained at the setting level until tissue impedance rises to 20 Ω above the baseline impedance [18] . The power output is automatically reduced to zero for 15 seconds (time of power temporary off) once the 20 Ω of threshold is exceeded. The power is then returned to the initial peak power setting until the tissue impedance rises again. Therefore, if the power emission is < 15 seconds of power temporary off, it may indicate that sufficient impedance will interrupt further power emission, particularly, as the ablation procedure is up to 9 minutes as compared with 12 minutes of standard ablation. Therefore, we defined the ET group if a maximum power setting was done and if after 6 minutes the power emission was < 10 seconds for three times per minute, the ablation was terminated after 9 minutes.
Although saline-linked RFA or using a novel needle perfusion technique currently can reduce the charring effects and enhance efficacy [15] ; [21] ; [22] ; [24] ; [25] , these devices are slightly cumbersome and not so popular in most institutes. Therefore, the current algorithm using ET might be an option to reduce charring effects and achieve comparable efficacy as the standard termination of RFA procedures.
The limitation of this study is its small sample size. Despite the small sample size, our study still showed that ET of the ablation procedure would achieve comparable complete ablation and local tumor progression compared with ST of the RF procedure. Further randomized study or a cohort study with larger sample size and longer follow-up may be needed to confirm our findings.
In conclusion, using ET in RFA is as effective as the ST in achieving complete ablation. It is also not associated with an increased risk of local tumor progression, and side effects between the two groups are comparable.
Conflicts of interest
All authors declared no conflicts of interest.
References
- [1] H. Bismuth, P.E. Majno, R. Adam; Liver transplantation for hepatocellular carcinoma; Semin Liver Dis, 19 (1999), pp. 311–322
- [2] R.T. Poon, S.T. Fan, C.M. Lo, I.O. Ng, C.L. Liu, C.M. Lam, et al.; Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years; Ann Surg, 234 (2001), pp. 63–70
- [3] S.M. Lin; Recent advances in radiofrequency ablation in the treatment of hepatocellular carcinoma and metastatic liver cancers; Chang Gung Med J, 32 (2009), pp. 22–31
- [4] R. Tateishi, S. Shiina, T. Teratani, S. Obi, S. Sato, Y. Koike, et al.; Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases; Cancer, 103 (2005), pp. 1201–1209
- [5] S.M. Lin, C.J. Lin, C.C. Lin, C.W. Hsu, Y.C. Chen; Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm; Gastroenterology, 127 (2004), pp. 1714–1723
- [6] C. Camma, V. Di Marco, A. Orlando, L. Sandonato, A. Casaril, P. Parisi, et al.; Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study; J Hepatol, 42 (2005), pp. 535–540
- [7] R.A. Lencioni, H.P. Allgaier, D. Cioni, M. Olschewski, P. Deibert, L. Crocetti, et al.; Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection; Radiology, 228 (2003), pp. 235–240
- [8] S. Rossi, E. Buscarini, F. Garbagnati, M. Di Stasi, P. Quaretti, M. Rago, et al.; Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode; AJR Am J Roentgenol, 170 (1998), pp. 1015–1022
- [9] T. Livraghi, S.N. Goldberg, S. Lazzaroni, F. Meloni, T. Ierace, L. Solbiati, et al.; Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions; Radiology, 214 (2000), pp. 761–768
- [10] S.M. Lin, C.H. Shen, D.Y. Lin, S.H. Kuo, C.J. Lin, C.W. Hsu, et al.; Cytologic changes in small hepatocellular carcinomas after radiofrequency ablation; Acta Cytol, 46 (2002), pp. 490–494
- [11] L. Buscarini, E. Buscarini, M. Di Stasi, D. Vallisa, P. Quaretti, A. Rocca; Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results; Eur Radiol, 11 (2001), pp. 914–921
- [12] S.M. Lin; Local ablation for hepatocellular carcinoma in Taiwan; Liver Cancer, 2 (2013), pp. 73–83
- [13] T. Lorentzen; A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design; Acad Radiol, 3 (1996), pp. 556–563
- [14] T. de Baere, A. Denys, B.J. Wood, N. Lassau, M. Kardache, V. Vilgrain, et al.; Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems; AJR Am J Roentgenol, 176 (2001), pp. 187–192
- [15] P.L. Pereira, J. Trubenbach, M. Schenk, J. Subke, S. Kroeber, I. Schaefer, et al.; Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers; Radiology, 232 (2004), pp. 482–490
- [16] T. Shibata, Y. Maetani, H. Isoda, M. Hiraoka; Radiofrequency ablation for small hepatocellular carcinoma: prospective comparison of internally cooled electrode and expandable electrode; Radiology, 238 (2006), pp. 346–353
- [17] T.C. Macatula, C.C. Lin, C.J. Lin, W.T. Chen, S.M. Lin; Radiofrequency ablation for hepatocellular carcinoma: use of low versus maximal radiofrequency power; Br J Radiol, 85 (2012), pp. e102–92011
- [18] I.H. Cua, C.C. Lin, C.J. Lin, W.T. Chen, C.W. Hsu, Y.C. Chen, et al.; Treatment of hepatocellular carcinoma using internally cooled electrodes. A prospective comparison of modified automated vs. manual pulsed radiofrequency algorithms; Oncology, 72 (2007), pp. 76–82
- [19] S.N. Goldberg, G.S. Gazelle, P.R. Mueller; Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance; AJR Am J Roentgenol, 174 (2000), pp. 323–331
- [20] S.N. Goldberg, G.S. Gazelle, E.F. Halpern, W.J. Rittman, P.R. Mueller, D.I. Rosenthal; Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size; Acad Radiol, 3 (1996), pp. 212–218
- [21] T. Livraghi, S.N. Goldberg, F. Monti, A. Bizzini, S. Lazzaroni, F. Meloni, et al.; Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases; Radiology, 202 (1997), pp. 205–210
- [22] J. Kettenbach, W. Kostler, E. Rucklinger, B. Gustorff, M. Hupfl, F. Wolf, et al.; Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients; AJR Am J Roentgenol, 180 (2003), pp. 1537–1545
- [23] S.N. Goldberg, C.J. Grassi, J.F. Cardella, J.W. Charboneau, G.D. Dodd 3rd, D.E. Dupuy, et al.; Image-guided tumor ablation: standardization of terminology and reporting criteria; J Vasc Interv Radiol, 20 (2009), pp. S377–S390
- [24] S.A. Topp, M. McClurken, D. Lipson, G.A. Upadhya, J.H. Ritter, D. Linehan, et al.; Saline-linked radiofrequency ablation: factors affecting steam popping and depth of injury in the pig liver; Ann Surg, 239 (2004), pp. 518–527
- [25] P. Abitabile, C.A. Maurer; Radiofrequency ablation of liver tumors: a novel needle perfusion technique enhances efficacy; J Surg Res, 159 (2010), pp. 532–537
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?