Summary
Background
The index of hemoglobin (IHb) has not been applied in colonoscopy to correlate the histological features of colon polyps. This study tested whether the net change of IHb values between polyp and normal mucosa correlates with the pathological features of colon polyps.
Patients and methods
This study consecutively enrolled patients who underwent colonoscopy during September 2011–August 2012 in a single tertiary referral colorectal unit. Endoscopic pictures and IHb values of each part of the colon were taken at the levels of cecum, ascending colon, transverse colon, sigmoid colon, and rectum. The net change of IHb values was calculated as follows: IHb value of colon polyp minus that of the surrounding mucosa.
Results
A total of 117 patients (32 with hyperplastic polyp, 5 with sessile serrated adenoma, 53 with tubular adenoma, 10 with villotubular adenoma, and 3 with adenocarcinoma) were recruited. The net change of IHb values increased in following order: hyperplastic polyp, tubular adenoma, sessile serrated adenoma, villotubular adenoma, and adenocarcinoma (−3.8 ± 6.3, −1.2 ± 1.7, −1.2 ± 5.7, 2.9 ± 8.1, and 12.7 ± 9.3, respectively; p < 0.001). Alcohol drinking and serum hemoglobin level were two independent factors related to the IHb values of non-polyp colon mucosa. Using a cutoff value of 2.4 for the net change of IHb values, selected based on the receiver operating characteristic curve analysis, the optimal sensitivity (52.9%) and specificity (75.6%) could be achieved for defining the polyp histology as an advanced colon lesion.
Conclusion
The net change of IHb values between colon polyp and nonpolyp mucosa can correlate with the pathological features of colon polyps. A positive net change of IHb values may indicate a more adverse histological pattern with a higher malignant potential.
Keywords
Advanced colon lesion ; Colonoscopy ; Colon polyp ; Index of hemoglobin
Introduction
Colorectal cancer is the third leading cause of cancer death in Taiwan [1] . An early screening with colonoscopy offers benefits such as interruption of the adenoma–carcinoma sequence of colorectal cancer [2] ; [3] . Endoscopic diagnosis of the colorectal disease is based on the observation of the color tone and the structure of lesion mucosa to predict the underlying pathological change. Despite the color change of the lesion being important for diagnosis, only subjective and descriptive terms can be provided in the endoscopic report. Thus, a more objective and quantitative measurement is really needed to define the exact degree of mucosal change or even to implicate the clinical–pathological significance.
The index of hemoglobin (IHb) can be used objectively to evaluate the hemoglobin (Hb) content in gastrointestinal mucosa instantly during endoscopy. It was designed based on the detection of the peak absorption of light by Hb, to objectively measure the mucosal redness [4] . The IHb assessed during gastrointestinal endoscopy can detect the gastric Helicobacter pylori infection [5] ; [6] , predict the treatment response to erosive reflux esophagitis [7] , and facilitate the detection of malignant gastric or colonic lesions [8] ; [9] . The study further determined whether the IHb modality in colonoscopy can be correlated with the adenoma carcinoma sequence and can predict the malignant risk of colon polyp instantly during colonoscopy.
Materials and methods
Patients and demographic background
The study consecutively enrolled patients who underwent colonoscopy during September 2011 to August 2012, in the Endoscopy Room of National Cheng Kung University Hospital, a tertiary referral medical center in southern Taiwan. All elective colonoscopies performed during the study period were screened for eligibility. Patients with poor bowel preparation, failure of cecal intubation, contraindication for biopsy or polypectomy, previous operation with altered vasculature at colorectal area, previous malignant diseases with a systemic chemotherapy history, and a history of inflammatory bowel disease, and those under 18 years of age were excluded. In addition, patients who declined to be a part of the study were also excluded. Prior to data collection, approval was obtained from the Institutional Review Board of National Cheng Kung University Hospital, Tainan, Taiwan.
Data on smoking, alcohol consumption, antihypertension medication, aspirin or clopidogrel use, body weight, body height, and body mass index were collected from the medical records. Laboratory data including those on blood Hb and creatinine levels were recorded.
Colonoscopy and IHb value acquisition
In this study, lower gastrointestinal electronic endoscopes (CF-Q260AL; Olympus Optical, Tokyo, Japan) were used as the standard equipment in all procedures. An endoscopic image database system (EVIS-NET) was used to take white light pictures and the IHb images on a personal computer equipped with a 24-bit color image capture board. During colonoscopy study, cecal intubation was achieved first and the adequacy of colon preparation was recorded. Endoscopic pictures were taken at normal mucosa of each part of the colon, including cecum, ascending colon, transverse colon, sigmoid colon, and rectum. IHb values were instantaneously measured by endoscopy image system on the frozen image and stored for further analysis. During measurement of IHb values of normal mucosa, endoscopists would make sure that there were no other lesions (such as arteriovenous malformation or diverticulum) in the field to avoid interference. Both white light pictures and IHb chart pictures with IHb values were recorded. The IHb value of each part of the colon is defined as the mean of two measurements on frozen endoscopic images when the endoscopic tip is placed at each level with 2 cm distance from normal mucosa.
For the area of detected polyps during screening colonoscopy, the white light images and the IHb chart with IHb values over the polyp and the adjacent normal mucosa were taken and recorded in pairs to obtain the net change of IHb values, defined as the difference between the IHb value of colon polyp and that of the adjacent nonpolyp mucosa. The size of each polyp was documented, and the final pathology would also be retrieved for analysis after complete removal of the polyp.
Statistical analysis
We first examined the clinical characteristics associated with IHb values of normal colon mucosa. Pearson’s correlation was used to determine the correlation between blood Hb and mucosal IHb value. Next, we calculated the net change of IHb values for each polyp. The continuous data were expressed as mean ± standard deviation. The net change of IHb values among different pathological types of polyps were compared with one-way analysis of variance with post hoc analysis by Tukey’s honest significant difference. Univariate linear regression analysis was carried out to identify the factors associated with IHb values in normal mucosa and the net change of IHb values in colon polyps. All variables with an effect (with p ≤ 0.15) were entered into multivariate linear regression models. The β coefficients are presented with 95% confidence intervals. A p value < 0.05 was considered statistically significant. PAWS version 17 (IBM, Chicago, IL, USA), R version 3.0.0 with packages “beanplot” and “car” (http://www.R-project.org ) were used for statistical analysis.
Results
A total of 117 patients who received colonoscopy were consecutively recruited. Analysis of Hb and IHb correlation was performed in 79 patients whose blood Hb was checked before colonoscopy. There were 32 patients with hyperplastic polyp, five with sessile serrated adenoma, 53 with tubular adenoma, 10 with villotubular adenoma, and three with adenocarcinoma. The colon polyps found in 103 patients were used to determine their net change of IHb values among the different pathological types of colon polyps.
Factors associated with IHb value of nonpolyp colon mucosa
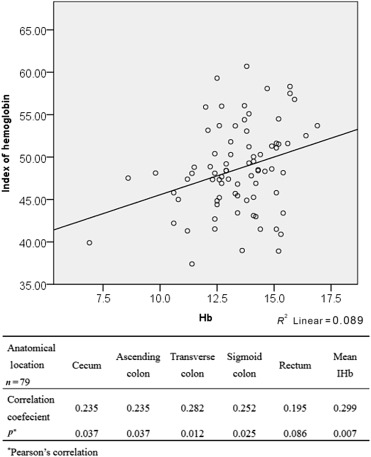
The demographic data and clinical characteristics of the patients are shown in Table 1 . The mean age of study cases was 57.2 years. In Fig. 1 , the serum Hb correlates with IHb in cecum, ascending colon, transverse colon, and sigmoid colon, and with the mean IHb of the five segments. In this study, thus, the mean IHb value of the five sessions was used as the nonpolyp colon mucosal IHb value in each case for univariate and multivariate analyses. Based on multivariate analysis, alcohol drinking and serum Hb level can be independent factors related to the value of IHb on nonpolyp colon mucosa (Table 2 ).
| All patients (n = 79) | |
|---|---|
| Age (y) | 57.2 ± 13.8 (23–87) |
| Male | 42 (53.2) |
| Smoking | 12 (15.2) |
| Hypertension | 26 (32.9) |
| Alcohol use | 8 (10.1) |
| Aspirin/clopidogrel use | 5 (6.3) |
| BMI | 23.9 ± 3.9 (15.2–36.7) |
| Indication | |
| FOBT positive | 29 (36.7) |
| LGI bleeding | 13 (16.5) |
| Previous colon polyp(s) for follow-up or removal | 12 (15.2) |
| Abdominal pain/tenesmus | 9 (11.4) |
| Bowel habit change | 5 (6.3) |
| Body weight loss | 2 (2.5) |
| Palpable rectal mass | 2 (2.5) |
| Other | 4 (5.1) |
Data are presented as n (%) or mean ± SD (range).
BMI = body mass index; FOBT = fecal occult blood test; LGI = lower gastrointestinal; SD = standard deviation.
|
|
|
Figure 1. Modest correlations of IHb values between normal mucosa at different segments of the colon and Hb. Hb = hemoglobin; IHB = index of hemoglobin. |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Age (per y) | 0.06 (−0.21 to 0.15) | 0.141 | 0.06 (−0.02 to 0.14) | 0.116 |
| Sex (male to female) | 1.78 (−0.50 to 4.06) | 0.124 | 0.48 (−1.83 to 2.77) | 0.684 |
| Smoking | 1.60 (−1.59 to 4.80) | 0.321 | ||
| Drinking* | 4.75 (1.07 to 8.42) | 0.012 | 3.94 (0.32 to 7.55) | 0.033 |
| Hypertension | 0.67 (−1.78 to 3.12) | 0.588 | ||
| Antiplatelet agent use | 0.37 (–4.37 to 5.11) | 0.877 | ||
| Hb (per g/dL increase)* | 0.89 (0.25 to 1.53) | 0.007 | 0.73 (0.06 to 1.41) | 0.033 |
| Renal insufficiency (Cr > 1.5 mg/dL) | −3.86 (−11.16 to 3.45) | 0.296 | ||
| Obesity (BMI > 27) | 0.004 (−3.33 to 3.34) | 0.998 | ||
BMI = body mass index; CI = confidence interval; Cr = creatinine; Hb = hemoglobin; IHb = index of hemoglobin.
- p < 0.05 by multivariate linear regression.
Net change of IHb values and its association with polyp pathology
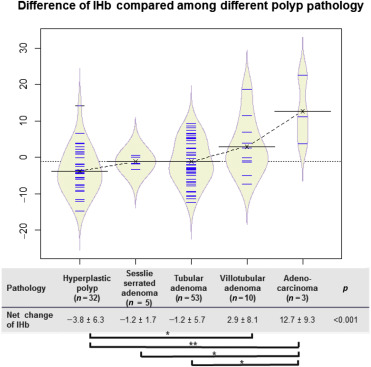
In cases with colon polyp detection during colonoscopy, the IHb values of each polyp and corresponding surrounding normal mucosa were recorded. The net change of IHb values in each polyp was calculated as follows: IHb value of the polyp (at 2 cm distance from the lesion) minus that of the adjacent nonpolyp mucosa. In Fig. 2 , the net change of IHb values increased in the following order: hyperplastic polyp, tubular adenoma, sessile serrated adenoma, villotubular adenoma, and adenocarcinoma (−3.8 ± 6.3, −1.2 ± 1.7, −1.2 ± 5.7, 2.9 ± 8.1, and 12.7 ± 9.3, respectively; p < 0.001). In Table 3 , hyperplastic polyp is associated with a negative net change of IHb value. In contrast, advanced colon lesions (defined as the detection of an adenocarcinoma, an adenoma > 1 cm in size, a villous component, or high-grade dysplasia) had a positive mean net change of IHb values. The serum Hb was not associated with the net change of IHb values in polyp.
|
|
|
Figure 2. Beanplot showing the trend of net changes of IHb values in polyps among different pathological types of colon polyps. The dotted line represents the trend of the net change of IHb values. The net change of IHb values between each polyp and corresponding normal mucosa was compared. Data are presented as mean ± standard deviation. *p < 0.05; one way ANOVA followed by Tukeys post hoc test. **p < 0.01; one way ANOVA followed by Tukey’s post hoc test. ANOVA = analysis of variance; IHB = index of hemoglobin. |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Age (per y) | 0.07 (−0.04 to 0.18) | 0.191 | ||
| Sex (male to female) | −0.54 (−3.30 to 2.22) | 0.699 | ||
| HP versus others | −3.76 (−6.53 to −0.99) | 0.008 | −2.45 (−5.28 to 0.37) | 0.088 |
| Advanced lesion versus othersa | 6.15 (2.79 to 9.52) | <0.001 | 4.98 (1.29 to 8.67) | 0.009 |
| Alcohol drinking | 3.40 (−0.42 to 7.21) | 0.080 | 0.79 (−3.11 to 4.69) | 0.689 |
| Hb (per g/dL increase)b | 0.100 (−0.80 to 1.00) | 0.825 | ||
CI = confidence interval; Hb = hemoglobin; HP = hyperplastic polyp; IHb = index of hemoglobin.
- p < 0.05 by multivariate linear regression.
a. Advanced lesion is defined as detection of an adenocarcinoma, or an adenoma with size > 1 cm, villous component, or high-grade dysplasia.
b. Analyzed in 72 cases with serum Hb available.
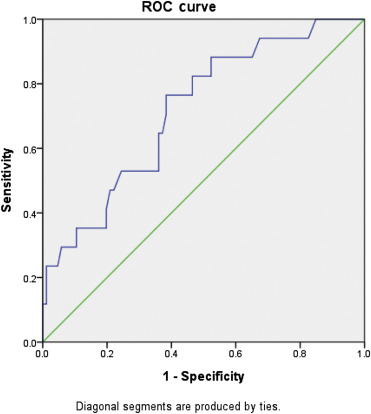
Based on the multivariate analysis model, only the advanced lesions could be the independent factors that are associated with a positive net change of IHb values. Based on the receiver operating characteristic curve analysis in Fig. 3 , the area under the receiver operating characteristic curve was 0.719 for a net change of IHb of ≥ 2.4 to achieve a sensitivity of 52.9% and specificity of 75.6%, to determine the polyp as an advanced colon adenoma lesion.
|
|
|
Figure 3. ROC curve revealing the net decrease of IHb values to determine a hyperplastic polyp. The area under the curve was 0.719. For a net IHb change, using ≥2.4, there were 52.9% sensitivity and 75.6% specificity for determining a polyp as an advanced lesion. ROC = receiver operating characteristic. |
Discussion
This study evaluated the clinical applicability of IHb in colonoscopy and whether the net change of IHb values correlated with different pathological types of colon polyps. We found that IHb values in normal colon mucosa are influenced by blood Hb and alcohol drinking status. Our study is original in illustrating that the net change of IHb values correlate with the pathology of colon polyps. This study suggests that the use of such net change of IHb values would be helpful in instantly identifying polyp lesions with risk as advanced lesions during colonoscopy.
We found that there was a weak but statistically significant correlation between blood Hb and colon mucosal IHb. The mean IHb of five different topographical segments of the colon had a positive correlation with blood Hb (Fig. 1 ). To our knowledge, this is the first study to demonstrate that IHb of nonpolyp colon mucosa would be influenced by blood Hb. In the multivariate analysis, increase of blood Hb and alcohol drinking status are associated with increased IHb values of colon mucosa (Table 2 ). To eliminate their influence, when assessing IHb values of colon polyps, we used the net change of IHb values since it was not confounded by blood Hb and alcohol drinking (Table 3 ).
Besides, our study disclosed that alcohol drinking would increase the IHb values of colon mucosa. It can, in part, be explained that alcohol consumption may impair the function of intestinal barrier by increasing endothelial permeability to macromolecules with chronic inflammation [10] .
We also found that the net change of IHb values increased in the following order: hyperplastic polyp, tubular adenoma/sessile serrated adenoma, villotubular adenoma, and adenocarcinoma. Angiogenesis is an important mechanism during colon tumorigenesis and induces aberrant vessel formation [11] . Thus, the IHb value of colon polyps should increase compared with that of the surrounding normal mucosa, except for hyperplastic polyps (Fig. 2 ). Such data seem to be compatible with the previous findings showing Paris classification 0-IIa or IIc colorectal lesions with higher IHb values [12] . Adaptive IHb color enhancement image can help endoscopists identify such flat or minute lesions during colonoscopy [13] . Our study is highly original in applying the model with the net change of IHb values between polyp and nonpolyp surrounding normal mucosa. Our data suggest that the positive value is associated with increased vasculature and Hb content, and thus can correlate with advanced pathology.
Our study has some limitations. First, our study did not cover all histological types of colon polyps; for example, traditional serrated adenoma and villous adenoma were lacking. Moreover, only three cases of adenocarcinoma (2 patients with T1N0M0 stage and 1 case with T2N0M0 stage) were included, and those with negative colonoscopy findings declined to be included in this study. The demographic data were collected from medical records, so details regarding alcohol consumption were lacking, including the amount, frequency, and duration of alcohol drinking. The sensitivity and specificity of determining the advanced colon pathology of polyps by applying a cutoff value of ≥ 2.4 of the net change of IHb values were relatively not so satisfactory. Accordingly, the current net change of IHb values can just serve as a supplementary tool to assist in the prepathologic differentiation instantly by endoscopists. It can tentatively serve as an instant supplementary marker during colonoscopy to judge the potential risk of colon adenoma. Finally, according to a previous study, image-enhanced endoscopy such as narrow band image could achieve 90% accuracy to differentiate colon neoplasms from non-neoplastic polyps [14] . Moreover, the accuracy of predicting invasion depth by chromoendoscopy with magnification could be as high as 98% [15] . However, the interobserver agreement varied [16] ; [17] . Our study with objective IHb values, which served as a different modality and needed less learning experience, had only 71% accuracy in the differentiation of advanced polyps. It would be interesting to test whether narrow band image plus IHb modality could improve both the accuracy and the interobserver agreement to predict malignant transformation and invasion depth of colon polyps in the future.
In conclusion, the net change of IHb values between colon polyp and nonpolyp mucosa may correlate with the pathological features of colon polyps. The positive net change of IHb values may indicate a more adverse histological pattern with a higher malignant potential.
Conflict of interest
The authors declare no financial relationship with any company involved in this study. There is no conflict of interest involved in this submission.
References
- [1] Department of statistics. Statistics on the causes of death, 2012. Taiwan: ministry of health and welfare, the Executive Yuan (2013) Available at: http://www.mohw.gov.tw/cht/DOS/Statistic.aspx?f_list_no=312&fod_list_no=2747 [Accessed 15 December 2013]
- [2] B. Levin, D.A. Lieberman, B. McFarland, R.A. Smith, D. Brooks, K.S. Andrews, et al.; Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology; CA Cancer J Clin, 58 (2008), pp. 130–160
- [3] D.A. Lieberman; Clinical practice. Screening for colorectal cancer; N Engl J Med, 361 (2009), pp. 1179–1187
- [4] S. Tsuji, N. Sato, S. Kawano, T. Kamada; Functional imaging for the analysis of the mucosal blood hemoglobin distribution using electronic endoscopy; Gastrointest Endosc, 34 (1988), pp. 332–336
- [5] G.H. Kim, K.B. Kim, E.K. Lim, S.H. Choi, T.O. Kim, J. Heo, et al.; Analysis of endoscopic electronic image of intramucosal gastric carcinoma using a software program for calculating hemoglobin index; J Korean Med Sci, 21 (2006), pp. 1041–1047
- [6] S. Nakagawa, M. Kato, Y. Shimizu, M. Nakagawa, J. Yamamoto, P.A. Luis, et al.; Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy; Gastrointest Endosc, 58 (2003), pp. 71–75
- [7] H. Cheng, Y.C. Tsai, W.Y. Chen, W.L. Chang, H.C. Cheng, B.S. Sheu; Supplementation of Los Angeles classification with esophageal mucosa index of hemoglobin can predict the treatment response of erosive reflux esophagitis; Surg Endosc, 25 (2011), pp. 2478–2486
- [8] K. Yao, M. Kato, J. Fujisaki; Techniques using the hemoglobin index of the gastric mucosa; Endoscopy, 37 (2005), pp. 479–486
- [9] K. Yao, T. Yao, T. Matsui, A. Iwashita, T. Oishi; Hemoglobin content in intramucosal gastric carcinoma as a marker of histologic differentiation: a clinical application of quantitative electronic endoscopy; Gastrointest Endosc, 52 (2000), pp. 241–245
- [10] A. Parlesak, C. Schafer, T. Schutz, J.C. Bode, C. Bode; Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease; J Hepatol, 32 (2000), pp. 742–747
- [11] T. Horimatsu, Y. Sano, K. Kaneko, H. Ikematsu, A. Katagiri, T. Yano, et al.; Relationship between MVD and meshed-capillaries using magnifying NBI colonoscopy in colorectal precursor lesions; Hepatogastroenterology, 56 (2009), pp. 372–377
- [12] Anon; The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002; Gastrointest Endosc, 58 (2003), pp. S3–S43
- [13] M. Igarashi, Y. Saitoh, T. Fujii; Adaptive index of hemoglobin color enhancement for the diagnosis of colorectal disease; Endoscopy, 37 (2005), pp. 386–388
- [14] M. Fujiya, Y. Kohgo; Image-enhanced endoscopy for the diagnosis of colon neoplasms; Gastrointest Endosc, 77 (2013), pp. 111–118
- [15] T. Matsuda, T. Fujii, Y. Saito, T. Nakajima, T. Uraoka, N. Kobayashi, et al.; Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms; Am J Gastroenterol, 103 (2008), pp. 2700–2706
- [16] A. Rastogi, K. Pondugula, A. Bansal, S. Wani, J. Keighley, J. Sugar, et al.; Recognition of surface mucosal and vascular patterns of colon polyps by using narrow-band imaging: interobserver and intraobserver agreement and prediction of polyp histology; Gastrointest Endosc, 69 (2009), pp. 716–722
- [17] H.J. Choi, B.I. Lee, H. Choi, K.Y. Choi, S.W. Kim, J.Y. Song, et al.; Diagnostic accuracy and interobserver agreement in predicting the submucosal invasion of colorectal tumors using gross findings, pit patterns, and microvasculatures; Clin Endosc, 46 (2013), pp. 168–171
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?