Summary
Objective
To observe the effects of intermittent one-lung ventilation (OLV) before and after surgery on the inflammatory cytokines and biomarkers of oxidative stress in serum of lung cancer patients undergoing open thoracotomy.
Methods
Between June 2011 to March 2012, 80 patients undergoing lobectomy were classified into four groups nonrandomly: Group A, control group; B, OLV preconditioning group; C, OLV postconditioning group; D, OLV preconditioning-combined-with-postconditioning group. Neutrophil granulocyte (PMN), interleukin 6 (IL-6), superoxide dismutase (SOD), and malondialdehyde (MDA) were assayed in plasma samples taken preoperatively (T1), intraoperatively (T2), and 2 hours postoperatively (T3).
Results
Comparison of T1 with T2 and T3 documented significant increase in MDA, PMN, and IL-6 levels and decrease in SOD in the control group (p < 0.01). Compared with the control group, the levels of IL-6 and MDA decreased and SOD increased significantly at T2 in the OLV preconditioning group, at T3 in the OLV preconditioning combined postconditioning group (p < 0.05).
Conclusion
Preconditioning of intermittent OLV before thoracotomy combined with postconditioning of intermittent returning two-lung ventilation after surgery maybe alleviate systematic inflammatory response and oxidative stress for lung cancer patients.
Keywords
acute lung injury;one-lung ventilation;oxidative stress;preconditioning;postconditioning
1. Introduction
One-lung ventilation (OLV) is used widely in thoracic surgeries. Lots of studies implied that OLV can cause lung injury.1; 2 ; 3 Animal study indicated OLV for 2 hours could provoke inhomogeneous lung injury that the injury in the nondependent lung was more severe than the other one.1 Besides, OLV is an important induced factor of acute lung injury (ALI), which is a potential complication after thoracic surgery. At present, ALI has become one of the major causes of death after thoracic surgery and the mortality is stable in 2–5%.4 The hypothesis of ventilator-induced lung injury during OLV has been further supported by the association between tidal volume exceeding 7–8 mL/kg predicted body weight and the release of systemic and pulmonary inflammatory mediators.5 The clinical benefits of lung-protective strategies using lower tidal volume combined with positive end-expiratory pressure (PEEP) have been supported by some studies.6 ; 7
Oxidative stress is an important factor of ALI during OLV, and it is found that oxidative stress plays an important part in the structure, function, and inflammatory response of the ALI model.8 Severe oxidative stress is caused by oxygen free radicals induced during the process of OLV and re-oxygenation,9 ; 10 and the degree of the amount of generated oxygen free radicals is associated with the duration of OLV, especially for patients with lung cancer.10 However, Cheng YJ et al11 stated that resuming two-lung ventilation (TLV) induces a massive superoxide production, but there is no significant decrease in the total antioxidant status, which indicates adequate antioxidant capacity to counteract it, and they thought that severe oxidative injuries after OLV/TLV should be considered in patients without adequate antioxidative capacity, such as those with cancer and trauma. Therefore, the new question is how to reduce the oxidative stress injury for patient with lung cancer during the surgery.
As we all know, the protective effect of ischemic preconditioning and postconditioning on the heart has been confirmed. Here, we wish to investigate whether preconditioning and postconditioning can be used in OLV. The aim of the study is to evaluate the effect of OLV preconditioning before surgery and OLV postconditioning after surgery on inflammatory cytokines and oxidative stress, and to seek a new protective strategy for ALI.
2. Materials and methods
The nonrandomly controlled study protocol was approved by the ethical committee at Xiangya Hospital of Central South University, Changsha, China.
2.1. Study subjects
Collecting the patients with lung cancer admitted to the Department of Cardiothoracic Surgery in Xiangya Hospital from June 2011 to March 2012.
2.1.1. Patients' eligibility
(1) Age < 70 years; (2) no pulmonary infection; (3) forced expiratory volume in 1 second (FEV1) ≥ 1.5 L; (4) lung cancer patients confirmed before or during the operation; (5) no metastasis of liver, gallbladder, kidney, spleen, adrenal gland, brain, and bone by computed tomography (CT) and type-B ultrasound; (6) single lobectomy combined with lymph node dissection; (7) Grade I–III of American Society of Anesthesiologists (ASA) level.
2.1.2. Patients rejected
(1) Patients with pneumonectomy and sub-lobectomy; (2) patients who underwent a peri-operative blood transfusion; (3) duration of OLV more than 2 hours; (4) The tidal volume (VT) is more than 8 or less than 6 mL/kg or respiratory frequency is more than 20 or less than 12 breaths per minute during the process of OLV and TLV alteration; (5) patients with severe pleural adhesion.
2.2. Grouping and management
The work of collecting, grouping, and management of subjects was done simultaneously. All eligible patients were divided into four groups nonrandomly with 20 in each group.
Group A is the control group. Patients accepted the conventional anesthesia ventilation scheme, which is TLV before opening the pleural cavity and then OLV during the operation, and then back to TLV after pulmonary lobectomy and lymph nodes dissection.
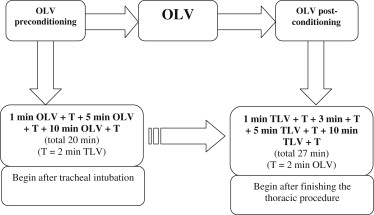
Group B is the OLV preconditioning group. Intermittent OLV for 20–30 minutes before opening the pleural cavity is done after the successful tracheal intubation. The specific program is 1 min + T + 5 min + T + 10 min + T, and it means 1-minute OLV and T-minute TLV and 5-minute OLV and T-minute TLV and 10-minute OLV and T-min TLV and then real OLV (T = 2).
Group C is the OLV postconditioning group. The program of OLV postconditioning is intermittent back to TLV after the lobectomy and lymph node dissection. The specific program is 1 min + T + 3 min + T + 5 min + T + 10 min + T, which means 1-minute TLV and T-minute OLV and 3-minute TLV and T-minute OLV and 5-minute TLV and T-minute OLV and 10-minute TLV and T-min OLV and then the real TLV after surgery (T = 2).
Group D is preconditioning-combined-with-postconditioning group. The program is a combination of those in Group B and Group C.
Double lumen tracheal cannula and intermittent positive pressure ventilation (IPPV) are used in the anesthesia. Regarding ventilator parameters, VT is controlled between 6 and 8 mL/kg and the PEEP is 0 cmH2O. Besides, the inspired oxygen concentration is set 100% and the allowed respiratory frequency is 12–20 breaths per minute. During the operation, VT and respiratory frequency are permitted to be regulated slightly to maintain the oxygen saturation and airway pressure less than 30 mmHg. In order to reduce the influence of human factors, the same operator and anesthesiologist are employed for the entire clinical study (Fig. 1).
|
|
|
Figure 1. The model of OLV preconditioning combined with OLV post-conditioning. OLV = one-lung ventilation; TLV = two-lung ventilation. |
2.3. Biomarker assays in plasma
The levels of the plasma markers were determined by ELISA following the manufacturers' instructions, including interleukin 6 (IL-6), and the polymorphnuclear neutrophils (PMN), superoxide dismutase (SOD), and malondialdehyde (MDA) (The kits were purchased from the American company R&D Systems).
Three milliliters of the arterial blood sample of patients were taken at three different time points during the operation, which were at the time of tracheal intubation (T1), before the lobectomy during the operation (T2), and 2 hours after operation (T3). Out of these, 1 mL of the sample was used to test the blood routine and the rest of the sample was centrifuged and used to test the inflammatory cytokines IL-6 and oxidation stress indices, SOD and MDA.
2.4. Statistical analysis
The data were recorded as mean ± standard deviation (x̄ ± s), and the independent sample t test and paired t test were used, respectively, between and within the groups. In addition, the Chi-square test was used to analyze the sex ratio of patients in each group and analysis of variance (ANOVA) was used to analyze the age of patients and the OLV time. All the experimental results were analyzed using the SPSS17.0 software, and p values < 0.05 indicated statistically significant difference.
3. Results
Eighty lung cancer patients were enrolled in our research. Their clinical characteristics are compared in Table 1, and there was no significant difference between the groups.
| Group | A | B | C | D | p |
|---|---|---|---|---|---|
| Number of patients | 20 | 20 | 20 | 20 | >0.99 |
| Sex | 0.545 | ||||
| Male | 16 | 12 | 15 | 14 | — |
| Female | 4 | 8 | 5 | 6 | — |
| Age (y) | 56 ± 9.8 | 55 ± 9.1 | 61 ± 9.0 | 58 ± 7.8 | 0.161 |
| Weight (kg) | 58 ± 7.3 | 61 ± 6.9 | 57 ± 7.6 | 60 ± 7.3 | 0.226 |
| Side of OLV | 0.282 | ||||
| Right | 14 | 8 | 11 | 10 | — |
| Left | 6 | 12 | 9 | 10 | — |
| Lobe of lobectomy | 0.585∗ | ||||
| LU | 10 | 5 | 4 | 6 | — |
| LL | 4 | 3 | 7 | 4 | — |
| RU | 5 | 7 | 5 | 7 | — |
| RM | 0 | 0 | 0 | 0 | — |
| RL | 1 | 4 | 4 | 3 | — |
| RM + RL | 0 | 1 | 0 | 0 | — |
| Operative time (h) | 127 ± 20 | 138 ± 18 | 124 ± 15 | 119 ± 14 | 0.058 |
| Time of OLV (h) | 75 ± 19 | 81 ± 17 | 70 ± 15 | 67 ± 16 | 0.064 |
| Input fluid volume (mL) | 1640 ± 315 | 1825 ± 327 | 1580 ± 371 | 1710 ± 302 | 0.116 |
| ASA score | 0.353 | ||||
| 1 | 0 | 0 | 0 | 0 | — |
| 2 | 15 | 17 | 12 | 14 | — |
| 3 | 5 | 3 | 8 | 6 | — |
| 4 | 0 | 0 | 0 | 0 | — |
∗p value is calculated by Fishers exact test.
Group A is the control group.
Group B is the OLV preconditioning group.
Group C is the OLV postconditioning group.
Group D is the preconditioning-combined-with-postconditioning group.
Input fluid volume is the mean of input fluid volume of patient during the operation.
ASA = American Society of Anesthesiologists; LL = left lower; LU = left upper; OLV = one-lung ventilation; RL = right lower; RM = right middle; RU = right upper.
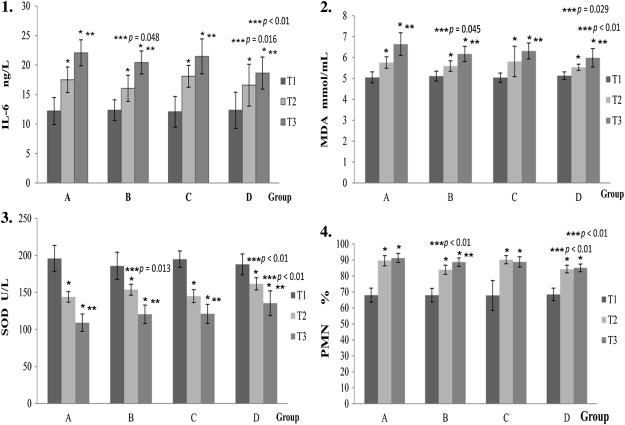
The levels of IL-6, MDA, SOD in serum, and PMN are given in Table 2. The results of statistical analysis are presented in Fig. 2, from which we can see that comparison of T1 with T2 and T3 shows a significant increase in MDA, PMN, and IL-6 levels and a decrease in SOD in the control group. Compared with the control group, there was a decrease in the levels of IL-6, MDA, and PMN, and SOD increased significantly at T2 in the OLV preconditioning group and at T3 in the OLV preconditioning-combined-with-postconditioning group.
| Group | No. | T1 | T2 | T3 | |
|---|---|---|---|---|---|
| IL-6 (ng/L) | A | 20 | 12.19 ± 2.30 | 17.49 ± 2.17 | 22.05 ± 2.20 |
| B | 20 | 12.33 ± 1.76 | 16.02 ± 2.21 | 20.42 ± 1.95 | |
| C | 20 | 12.06 ± 2.61 | 18.07 ± 1.84 | 21.45 ± 2.96 | |
| D | 20 | 12.32 ± 3.06 | 16.58 ± 3.54 | 18.63 ± 2.72 | |
| MDA (mmol/mL) | A | 20 | 5.05 ± 0.26 | 5.76 ± 0.27 | 6.64 ± 0.54 |
| B | 20 | 5.11 ± 0.24 | 5.59 ± 0.25 | 6.17 ± 0.36 | |
| C | 20 | 5.04 ± 0.22 | 5.81 ± 0.73 | 6.31 ± 0.38 | |
| D | 20 | 5.13 ± 0.18 | 5.53 ± 0.15 | 5.99 ± 0.44 | |
| SOD (U/L) | A | 20 | 195.87 ± 17.71 | 143.94 ± 7.08 | 109.16 ± 11.73 |
| B | 20 | 185.91 ± 18.29 | 153.70 ± 7.45 | 120.51 ± 12.42 | |
| C | 20 | 194.95 ± 11.08 | 145.05 ± 8.8 | 121.07 ± 12.91 | |
| D | 20 | 187.94 ± 14.02 | 161.69 ± 8.22 | 135.50 ± 16.79 | |
| PMN (%) | A | 20 | 67.99 ± 4.34 | 89.52 ± 3.20∗ | 91.20 ± 2.82 |
| B | 20 | 67.97 ± 4.21 | 83.88 ± 2.84 | 88.62 ± 2.67 | |
| C | 20 | 67.76 ± 9.34 | 90.13 ± 2.57 | 88.78 ± 3.21 | |
| D | 20 | 68.42 ± 3.95 | 84.27 ± 2.39 | 85.04 ± 2.37 |
Group A is the control group; Group B is the OLV preconditioning group; Group C is the OLV postconditioning group; Group D is the preconditioning-combined-with-postconditioning group. T1 is at the time of tracheal intubation (preoperatively); T2 is before the lobotomy during the operation (intraoperatively); T3 is 2 hours after operation (postoperatively). IL-6 = interleukin 6; MDA = malondialdehyde; OLV = one-lung ventilation; PMN = neutrophil granulocyte; SOD = superoxide dismutase.
|
|
|
Figure 2. Histograms of IL-6, MDA, SOD, and PMN in each group at different times: T1, T2, and T3. Group A is the control group; Group B is the OLV preconditioning group; Group C is the OLV post-conditioning group; Group D is the preconditioning-combined-with-postconditioning group. T1 is at the time of tracheal intubation (preoperatively); T2 is before the lobotomy during the operation (intraoperatively); T3 is 2 hours after operation (postoperatively). ∗p < 0.01 versus T1. ∗∗p < 0.01 versus T2. ∗∗∗p < 0.05 versus Group A. IL-6 = interleukin 6; MDA = malondialdehyde; OLV = one-lung ventilation; PMN = neutrophil granulocyte; SOD = superoxide dismutase. |
4. Discussion
4.1. Analysis of results and mechanisms
In thoracic surgery, ALI occurs due to many reasons, including OLV, operative procedure, fluid overload, and the patients basic condition. The results of the control group show that inflammatory indexes, IL-6 and PMN, in the serum of lung cancer patients undergoing pulmonary lobecetomy, increase gradually from the beginning of surgery to 2 hours after surgery, and so does MDA, while the level of SOD decreases gradually. It reveals that different degrees of lung injury and oxidative stress exist during the process.
The results show that intermittent prolonged OLV preconditioning before thoracic surgery can significantly reduce the concentration of the IL-6 and MDA and increase the level of SOD in the operation. Moreover, OLV preconditioning combined with OLV postconditioning of intermittent return to TLV reduced the level of the IL-6 and MDA, but increased SOD significantly at 2 hours after surgery. The results also suggests that OLV is one of the important factors that induces lung injury in thoracic surgery. In our study, OLV postconditioning reduced the level of IL-6 and MDA and increased the level of SOD in serum nonsignificantly postoperatively. It may be associated with too small a sample size and the short duration of postconditioning. Perhaps there is no apparent lung injury during the process of reoxygenation from OLV to TLV after operation. In this regard, further study needs to be done. From the results, it is further confirmed that OLV induced lung injury is associated with oxidative stress10 and OLV preconditioning improves the antioxidant capacity of lung tissue by enhancing activity and concentration of SOD.12
The mechanism of OLV-induced lung injury is not clear, but some factors should not be disregard, such as hypoxemia, hyper tidal volume of ventilated lung, and oxidative stress. It has a 5–10% incidence of hypoxemia during the OLV,13 which can cause lung injury by inducing many active oxygen free radicals or releasing some active factors. For ventilated lung, hyper tidal volume causes hyper mechanical stretch. High tidal volume is an independent risk factor,14 and it can increase the incidence of respiratory failure after pneumonectomy.15 It is supported that oxidative stress exits during the OLV and reoxygenation,9; 10 ; 11 which can lead to lung injury by various mechanisms, including: (1) direct damage to DNA; (2) lipid peroxidation; (3) oxidation of proteins; and (4) alteration of transcription.16
We cannot conclude about the mechanisms of OLV preconditioning/postconditioning for protection of lung injury in our study, but we can infer that it maybe associated with alleviation of inflammatory and oxidative stress according to our results. In fact, OLV preconditioning/postconditioning provides a beforehand adaptation of hyper tidal volume of ventilated lung and lung re-expansion during the surgery, and we think its protection maybe the same with ischemic preconditioning/postconditioning by priming the endogenous protective mechanism.
4.2. Analysis of influencing factors
In this study, the operative procedure can influence the results, including the duration of operation, operative extrusion, and operative extent. Besides, anesthesia is another important factor, including anesthetic method, the type of anesthetic machine, and ventilation parameters. In addition, intubations also cause the damage of trachea, and each patients ability of hypoxic tolerance is different. In our study, we tried our best to control and reduce the bias. First, we employed a strict screening procedure for the patients before selection or rejection. Second, the same operator, anesthetist, and anesthetic machine were employed in the study. Third, the operations were all open lobectomy combined with lymph nodes dissection. Finally, we made a comparison between the groups about age, sex, weight, time and side of OLV, ASA, and lobe of lobectomy, and the results were not significant (as shown Table 1). However, we have to admit that the study is not a random one and the sample capacity is limited; therefore, some differences do not show up as significant.
4.3. Analysis of preconditioning and postconditioning
The clinical study is based on the actual clinical operation and anesthesia, and the program are built on the condition that OLV preconditioning and postconditioning do not prolong the operative duration and increase the operative difficulty, but it does not imply that the program in the research is the best program for clinical application.
There are the following characteristics about the clinical OLV preconditioning and postconditioning program. The first characteristic is operability, and it implies that the program is easy to build and accomplish in a clinical setting. It just needs a big curved forceps with a rubber hose to change OLV and TLV. The second one is practicality that the set times of 20 minutes preconditioning and about 30 minutes postconditioning are based on lots of clinical observation in order not to prolong the operative duration. The third one is innovation, as there is no report about combination between OLV and preconditioning/postconditioning until now.
Up to February 11, 2012, we could find 26 relevant documents when we entered the keywords acute lung injury and one-lung ventilation in a PubMed search, eight relevant documents when the keywords were one-lung ventilation and oxidative stress, 401 relevant documents for acute lung injury and oxidative stress, and 24 documents with the keywords hypoxic preconditioning and lung. Therefore, there are very few current studies regarding OLV-induced lung injury. Further studies and discussion need to be done so that we can continue to investigate the protective effect of OLV preconditioning and postconditioning for ALI by changing the clinical programs. Besides, since the study supports that repetitive OLV preconditioning in vivo for 20 minutes before the thoracic surgery combined with repetitive OLV postconditioning in vivo for 30 minutes after OLV can alleviate the inflammatory reaction and oxidative stress significantly, what effect the clinical program will bring for recovery of patients and incidence rate of postoperative complications? It still requires further research.
5. Summary
In summary, ALI is a potential postoperative complication and should be paid more attention in clinical settings. Compared with treating lung injury, it is of greater benefit for patients to prevent the lung injury and reduce the incidence of ALI, by optimizing the medical process. The study is an exploratory trial of a combination preconditioning/postconditioning with OLV in an actual clinical setting. The programs of OLV preconditioning and postconditioning have an endogenous protective effect on the body; therefore it will have a broad prospect of clinical application in the OLV of thoracic surgery. Although it is still in the process of exploration and clinical study, we believe that a set of actual operative programs about OLV preconditioning and postconditioning will be realised with an increasing number of animal experiments and clinical studies.
Acknowledgments
This work was supported by grants from the National Natural Scientific Foundation of China (Nos. 81171841 and 81372515), the Province Natural Scientific Foundation of Hunan (No. 09JJ5024) and Scientific technological Foundation of Hunan (Nos. 2009RS3034 and 2010FJ3134).
References
- 1 Z.J. You, S.L. Yao, Y. Yuan; Comparison of lung injury degrees after one-lung ventilation; Central China Med J, 31 (2007), pp. 75–79
- 2 Y.T. Gao, S. Cao, F. Jiang, et al.; Effects of one-lung ventilation on systemic inflammatory responses in patients undergoing thoracic surgery; Jiang su Med J, 36 (2010), pp. 169–171
- 3 A.J. Bastin, H. Sato, S.J. Davidson, et al.; Biomarkers of lung injury after one-lung ventilation for lung resection; Respirology, 1 (2011), pp. 138–145
- 4 M. Licker, P. Fauconnet, Y. Villiger, J.M. Tschopp; Acute lung injury and outcomes after thoracic surgery; Curr Opin Anaesthesiol, 22 (2009), pp. 61–67
- 5 M.J. Schultz; Lung-protective mechanical ventilation with lower tidal volumes in patients not suffering from acute lung injury: A review of clinical studies; Med Sci Monit, 14 (2008), pp. RA22–RA26
- 6 M. Licker, J. Diaper, Y. Villiger, et al.; Impact of intra-operative lung-protective interventions in patients undergoing lung cancer surgery; Crit Care, 13 (2009), p. R41
- 7 W.Q. Lin, X.Y. Lu, L.H. Cao, et al.; Effects of the lung protective ventilatory strategy on proinflammatory cytokine release during one-lung ventilation; Ai Zheng, 27 (2008), pp. 870–873
- 8 E.T. Lima Trajano, C. Sternberg, M. Caetano, et al.; Endotoxin-induced acute lung injury is dependent upon oxidative response; Inhal Toxicol, 23 (2011), pp. 918–926
- 9 M. Iqbal, A.S. Multz, L.J. Rossoff, et al.; Re-expansion pulmonary edema after VATS successfully treated with continuous positive airway pressure; J Ann Thorac Surg, 70 (2000), pp. 669–671
- 10 P. Misthos, S. Katsaragakis, N. Milingos, et al.; Postresectional pulmonary oxidative stress in lung cancer patients. The role of one-lung ventilation; Eur J Cardiothorac Surg, 27 (2005), pp. 379–382
- 11 Y.J. Cheng, K.C. Chan, C.T. Chien, et al.; Oxidative stress during 1-lung ventilation; J Thorac Cardiovasc Surg, 132 (2006), pp. 513–518
- 12 T.F. Chou, M.C. Ma, C.P. Tsai, C.F. Chen; Enhancement of superoxide dismutase activity in rat lungs after hypoxic preconditioning; Chin J Physiol, 52 (5 suppl) (2009), pp. 376–383
- 13 W. Karzai, K. Schwarzkopf; Hypoxemia during one-lung ventilation: prediction, prevention, and treatment; Anesthesiology, 110 (2009), pp. 1402–1411
- 14 L. Mascia, E. Zavala, K. Bosma, et al.; High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study; Crit Care Med, 35 (2007), pp. 1815–1820
- 15 E.R. Fernandez-Perez, M.T. Keegan, D.R. Brown, et al.; Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy; Anesthesiology, 105 (2006), pp. 14–18
- 16 C.W. Chow, M.T. Herrera Abreu, T. Suzuki, et al.; Oxidative stress and acute lung injury; Am J Respir Cell Mol Biol, 29 (2003), pp. 427–431
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?