Abstract
Background
Advanced age is associated with left ventricular (LV) remodeling and impaired diastole. The association among aging, mitral leaflet closure (EF slope), cardiac structures, and diastolic indices in an asymptomatic Taiwanese population is largely unknown.
Methods
We studied 8103 asymptomatic participants (49.5 ± 11.6 years, 38.2% women) from a health evaluation cohort (2004–2012) in a tertiary center in Taiwan. Echo-derived LV structure/function, and M-mode based EF slope (mm/s) and serum NT-proBNP level were obtained. The association between EF slope and the other clinical or echo-based parameters was investigated.
Results
Average values for EF slope among various age groups in the Taiwanese population were determined for both genders. Advanced age was associated with reductions in EF slope (adjusted estimate: − 0.35/per decade). Reduced EF slope was associated with older age, higher blood pressure and greater body mass index in multivariate models (all p < 0.05). Reduced EF slope was correlated with greater cardiac concentricity, abnormal E′ and E/E′ (AUROC: 0.74 and 0.77, respectively, both p < 0.05) and elevated NT-proBNP (Coef: 5.98 pg/mL, per − 10 mm/s EF slope, 95% CI: 7.82 to 4.17, p < 0.001). EF-slope also clearly discriminated individuals with abnormal estimated LV filling (E/E′ categorized by < 8, ≥ 8 & < 15, ≥ 15, ANOVA p < 0.001).
Conclusions
EF-slope reduction in the asymptomatic Taiwanese population was correlated with age, several unfavorable LV remodeling, and impaired diastolic function parameters, and EF-slope can be an effective clinical diagnostic tool for identifying poor E′ and elevated LV filling pressure. In addition, our data provided reference values for EF-slope in various age groups.
Keywords
Echo;Mitral EF slope;NT-proBNP;Diastology;Age;Gender
1. Introduction
Increasing age has long been shown to be associated with a higher incidence of cardiovascular disease. [1] Cardiac remodeling with aging, an adaptation tightly linked to clinical heart failure, had been reported to be produced by numerous physiological and pathological mechanisms [2] ; [3]. Currently, echocardiographic measurement of M-mode based and 2-dimensional (2D)-defined left ventricular (LV) systolic function, especially left ventricular ejection fraction (LVEF), is load dependent, and these measurements are regarded as having low sensitivity to the earlier stages of myocardial dysfunction [4] ; [5]. Instead, the cardiac remodeling process with aging can be detected by worsened functional presentation in several parameters, for example, diastolic dysfunction.
Of the several diastolic indices that have been investigated for use in the past decades, the EF slope measurement from conventional M-mode echocardiography, a measurement reflecting the rate of early diastolic mitral leaflet closing [6], has long been proposed as a measurement for diastolic function [7]. As a variety of new diastolic parameters including Tissue Doppler Imaging (TDI) have been proposed in recent years, the clinical utilization of EF slope has been largely replaced by those diastolic indices that are less influenced by load status [8] ; [9]. However, the clinical use of these parameters, especially the Doppler-based techniques, is highly dependent on technique, may heavily rely on the operators experience, and produces limited information on regional rather than global cardiac function [9] ; [10].
There are few current data scarce regarding how EF slope correlates with age-related cardiac remodeling or related elevated load status in the Taiwanese population. In addition, sex differences in such associations remain relatively unexplored. Understanding this information may provide insight as to how EF slope measurement may be clinically useful when performed in daily practice. Based on these considerations, we aimed to investigate the relationship between EF slope and cardiac remodeling using an asymptomatic population that had undergone a cardiovascular survey.
2. Methods
2.1. Study subjects
This study utilized the dataset from subjects who underwent a cardiovascular survey from Mackay Memorial Hospital, a tertiary medical center at Northern Taipei, that was conducted during the period from July 2003 to December 2012. A total of 11,376 persons underwent anthropometric measurements, body fat composition assessment, comprehensive echocardiography studies and 12-lead resting electrocardiography and had blood drawn for biochemical information and circulating biomarkers. Structured questionnaires were obtained from all participants. Of the total number of subjects, 9058 had baseline visits that included non-repeated echocardiography data. We excluded subjects with missing baseline variables, known implanted pacemaker, severe pulmonary hypertension (defined as peak systolic pulmonary artery pressure ≥ 60 mm Hg), hypertrophic cardiomyopathy, atrial fibrillation, primary significant valvular heart disease (aortic or mitral valve), or prevalent symptoms of heart failure (n = 1045). Some participants underwent repeated visits, and for these patients, we used information from the first visit as data for this study.
2.2. Echocardiography
All study subjects undergoing transthoracic echocardiography were positioned in the left decubitus position after an adequate resting time. A Philips (Hewlett-Packard) Sonos 5500 ultrasound was initially used for conventional echocardiography scans, with M-mode, two-dimensional (2D) and hemodynamic Doppler images acquired per standardized protocol using a 2–4-MHz adult cardiac transducer (S4, phased array transducer). Pulsed wave TDI was available after July 2007, and enabled myocardial and mitral annular sampling (1.5 mm) and contraction/relaxation velocity (S′/E′, unit in cm/s) to be available and feasible with the highest frame rate to be performed, thus facilitating diastolic function grading. After January 2009, echocardiography was uniformly performed using a GE system (Vividi, Vingmed, Horten, Norway) equipped with the 2–4-MHz transducer. For both systems, the standard echocardiography imaging protocol included M-mode based measurement of LA diameter, LV end-diastolic/-systolic diameter (LVIDd and LVIDs), wall thickness, and LV mass calculation (American Society of Echocardiography criteria) [11] with LV volumes obtained by the modified biplane Simpson method by 2D. All M-mode images were acquired and recorded at a speed of 60–100 mm/s with the transducer placed at the third to the fifth inter-costal space.
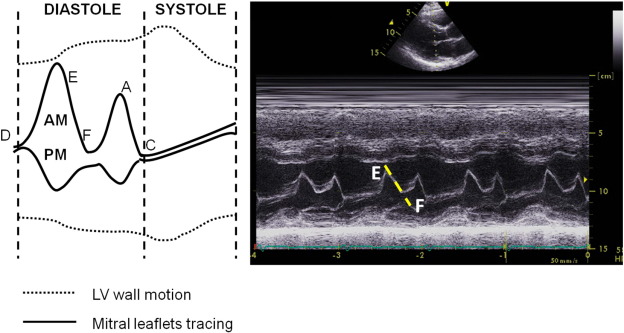
For EF slope measure, recordings were made using leading-edge methods according to the standards suggested by the American Society of Echocardiography [12]. The manual tracing of anterior mitral leaflet closure after rapid early filling was displayed by M-mode imaging, and was determined by drawing a line from the E to the F point (Fig. 1) by a single experienced technician. A steeper EF slope (unit: mm/s) represented rapid and normal filling of LV during the early diastolic phase while a more damped and slowed slope pattern was observed in subjects with impaired LV diastolic function [13]. The intra- and inter-observer variability (coefficient of variance) for LV wall thickness, diastolic diameter and M-mode based EF slope measurement from a random set of 30 subjects in our laboratory was 7.2%, 6.1% and 5.8% and 7.4%, 6.8% and 6.2%, respectively.
|
|
|
Fig. 1. Illustration of individual landmarks (A, C, D, E and F points) of a transthoracic parasternal long-axis M-mode tracing (left panel) and a representative EF slope from a manual tracing (right panel). |
LV hemodynamic diastolic assessment was determined by using pulsed-wave transmitral inflow Doppler imaging of early (E) and late diastolic (A) LV filling velocities measured at the tip of the mitral leaflets from an apical 4-chamber view, with high frame rates. LV filling pressure was estimated by the mitral E/E′ ratio. Impaired mitral annulus diastolic relaxation velocities (E′ < 8 cm/s, E′ as mean for both medial and lateral mitral annular sampling) was used to make the diagnosis of diastolic dysfunction [14], with an E/E′ (same averaged E′ from both medial and lateral annulus) ratio greater than the proposed threshold of 15 as the diagnostic criteria for an elevated end-diastolic pressure or filling, as previously described [8] ; [9].
2.3. Serum NT-proBNP test and renal functional assessment
NT-proBNP (pg/mL) was available for 5207 study participants (65%) using an electrochemiluminescence immunoassay (Roche E170, Roche Diagnostics). Major baseline demographic information including age, sex distribution, body size, blood pressure and medical history did not differ significantly between study participants with or without NT-proBNP data. Renal function was assessed using the Modification of Diet in Renal Disease (MDRD) formula.
2.4. Statistical analysis
Continuous data were shown as mean and standard deviation and compared using the t test. Categorical data were expressed as frequency and proportion of occurrence in all subjects and were compared using the chi-square test. The associations among EF slope, age, NT-proBNP and various cardiac structural/functional indices were analyzed using univariate regression models. We also used a multivariate model to determine the significance of the relationship between EF slope and age adjusted with clinical covariates such as fasting glucose, cholesterol, HDL, eGFR, and medical history of hypertension, diabetes, and hyperlipidemia. Receiving operative characteristic (ROC) curves were used to test the hypothesis that EF slope provides value in the detection of abnormal diastolic dysfunction, including impaired myocardial relaxation and impaired LV filling capacity. p value was set at two-tailed probability, and a p value less than 0.05 was considered statistically significant. Software packages IBM SPSS version 22.0 (SPSS, Chicago, IL, USA) and STATA 8.2 (StataCorp, College Station, TX, USA) were used to conduct the statistical analyses.
3. Results
3.1. Baseline characteristics
In Table 1, we show the baseline characteristics of the study participants (n = 8013) by genders. In general, females were older, and had lower body length and body weight, smaller waist circumference, and smaller body size (all p < 0.001). Men showed greater blood pressure profiles (all p < 0.001) and had a higher fasting glucose level, lower HDL and lower eGFR compared to women (all p < 0.001).
| Women (N = 3060) | Men (N = 4953) | p value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, y | 51.1 ± 12.0 | 49.5 ± 11.3 | < 0.0001 |
| Height, cm | 157 ± 5.7 | 170 ± 6.2 | < 0.0001 |
| Weight, kg | 56.8 ± 9.3 | 72.0 ± 11.1 | < 0.0001 |
| Body mass index | 23.2 ± 3.7 | 25.0 ± 3.4 | < 0.0001 |
| SBP, mm Hg | 120 ± 19.1 | 124.8 ± 16.3 | < 0.0001 |
| DBP, mm Hg | 72.5 ± 10.6 | 77.7 ± 10.6 | < 0.0001 |
| MBP, mm Hg | 88.4 ± 12.5 | 93.4 ± 11.6 | < 0.0001 |
| Heart rate, 1/min | 74.1 ± 9.6 | 74.3 ± 10.6 | 0.5222 |
| Waist circumference, cm | 77.0 ± 9.8 | 86.4 ± 8.9 | < 0.0001 |
| Biochemical data | |||
| Fasting glucose, mg/dL | 98.3 ± 23.8 | 102.7 ± 24.6 | < 0.0001 |
| Cholesterol, mg/dL | 200.7 ± 38 | 198.9 ± 36 | 0.0375 |
| HDL, mg/dL | 62 ± 15.7 | 48.8 ± 12.1 | < 0.0001 |
| eGFR, mg/dL | 92.6 ± 20.1 | 85.7 ± 15.7 | < 0.0001 |
| Medical history, % | |||
| Hypertension | 586 (19.2%) | 956 (19.3%) | 0.891 |
| Hyperlipidemia | 75 (2.5%) | 125 (2.5%) | 0.897 |
| Diabetes mellitus | 206 (6.7%) | 322 (6.5%) | 0.72 |
| Cardiovascular disease | 282 (9.2%) | 369 (7.5%) | 0.006 |
DBP: diastolic blood pressure, eGFR: estimated glomerular filtration rate, HDL: high density lipoprotein, MBP: mean blood pressure and SBP: systolic blood pressure.
Table 2 shows age-related changes in EF slope stratified by gender for all participants (n = 8013) (Table 2, Fig. 2A), and for those in the healthy population (n = 5839) without histories of hypertension, diabetes, use of medication for hyperlipidemia, and known cardiovascular diseases. The mean EF slope in all study participants and in the healthy population was 89.7 ± 27.7 and 93.6 ± 26.8 mm/s, respectively. Both populations showed a similar and significant trend toward a more reduced EF slope with older age groups (both trends p < 0.001), with a nearly 30–40% reduction from the < 30 to the > 70 year age group in men and women. In general, men showed a greater EF slope compared to women.
| Age groups | All (n = 8013) | Men | Women | p value | Healthy (n = 5893) | Men | Women | p value |
|---|---|---|---|---|---|---|---|---|
| ≤ 30 (years) | Mean ± SD | 111 ± 26.6 | 101 ± 23.9 | < 0.001 | Mean ± SD | 111 ± 27 | 100.5 ± 24.4 | < 0.001 |
| N | 247 | 133 | N | 223 | 120 | |||
| 31–40 (years) | Mean ± SD | 103.7 ± 25.6 | 99.5 ± 23.9 | 0.004 | Mean ± SD | 104.9 ± 25 | 99.5 ± 23.6 | 0.003 |
| N | 984 | 436 | N | 867 | 394 | |||
| 41–50 (years) | Mean ± SD | 96.1 ± 25.9 | 93.2 ± 23.5 | 0.006 | Mean ± SD | 97.4 ± 25.9 | 93.2 ± 23.4 | 0.02 |
| N | 1677 | 892 | N | 1320 | 757 | |||
| 51–60 (years) | Mean ± SD | 85.1 ± 27.1 | 83.2 ± 22.8 | 0.0771 | Mean ± SD | 87.2 ± 27.2 | 83.2 ± 22.3 | 0.203 |
| N | 1377 | 948 | N | 904 | 650 | |||
| 61–70 (years) | Mean ± SD | 73.7 ± 25.8 | 71.3 ± 25.1 | 0.155 | Mean ± SD | 75.6 ± 24.9 | 71.3 ± 24.9 | 0.895 |
| N | 478 | 469 | N | 231 | 235 | |||
| ≥ 71 (years) | Mean ± SD | 64.8 ± 23 | 59 ± 22.4 | 0.0147 | Mean ± SD | 71 ± 24 | 59 ± 20.2 | 0.003 |
| N | 190 | 182 | N | 73 | 65 | |||
| p for trend (across age groups) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| All subjects | Mean ± SD | 91.9 ± 28.4 | 86 ± 26.1 | Mean ± SD | 95.6 ± 27.7 | 90.3 ± 25 | < 0.001 | |
| N | 4953 | 3060 | n | 3618 | 2221 |
SD: standard deviation.
|
|
|
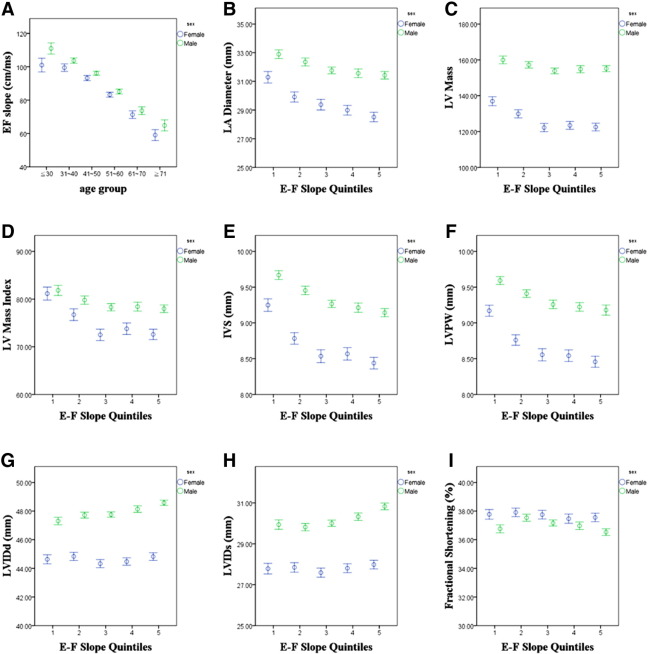
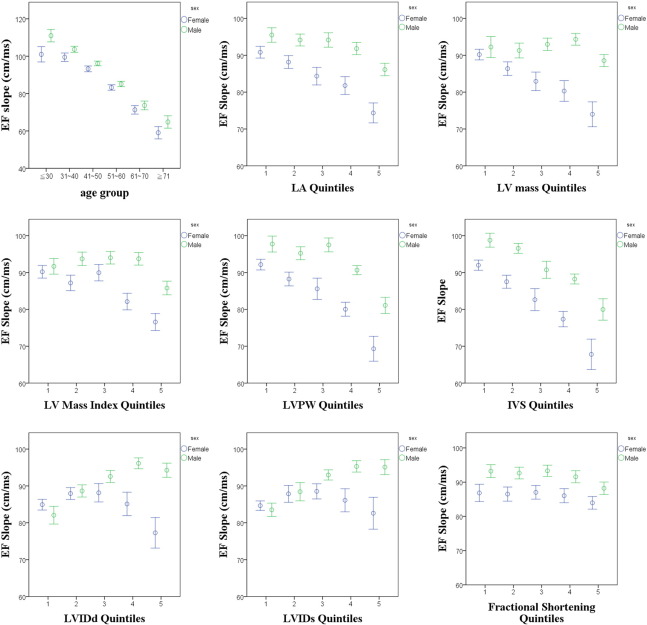
Fig. 2. Association between age, EF slope, and several cardiac structural parameters. Unfavorable cardiac remodeling, including greater wall thickness and larger mass, are all associated with reduced EF slope, with men demonstrating a positive linear trend between higher EF slope and greater LV internal diameter. |
Table 3 also shows the association between age and reduction of EF slope in univariate and multivariate models. For all study participants (n = 8013, Table 3A) and healthy group participants (n = 5839, Table 3B), an increase in age (per decade) is associated with a significant decline in the EF slope for both genders even after adjustment for clinical covariates (Fig. 2A). In addition, higher blood pressure and greater body size are both independently related to EF slope reduction (all p < 0.05) in multivariate models from both genders.
| EF slope (mm/s) | ||||||
|---|---|---|---|---|---|---|
| Female (n = 3060) | Male (n = 4953) | All study participants (n = 8013) | ||||
| Beta | p | Beta | p | Beta | p | |
| Univariate | ||||||
| Age (per decade) | − 9.63 | < 0.001 | − 10.19 | < 0.001 | − 10.11 | < 0.001 |
| Multivariate | ||||||
| Model 1 | ||||||
| Age (per decade) | − 8.19 | < 0.001 | − 9.54 | < 0.001 | − 9.32 | < 0.001 |
| SBP (per 10 mm Hg) | − 1.91 | < 0.001 | − 2.04 | < 0.001 | − 1.69 | < 0.001 |
| Model 2 | ||||||
| Age (per decade) | − 8.03 | < 0.001 | − 9.68 | < 0.001 | − 9.37 | < 0.001 |
| SBP (per 10 mm Hg) | − 1.6 | < 0.001 | − 1.71 | < 0.001 | − 1.42 | < 0.001 |
| BMI (per 5 kg/m2) | − 2.56 | < 0.001 | − 3.25 | < 0.001 | − 2.01 | < 0.001 |
| Model 3a | ||||||
| Age (per decade) | − 7.51 | < 0.001 | − 8.95 | < 0.001 | − 8.9 | < 0.001 |
| SBP (per 10 mm Hg) | − 0.98 | 0.001 | − 1.45 | < 0.001 | − 1.04 | < 0.001 |
| BMI (per 5 kg/m2) | − 1.45 | 0.036 | − 2.36 | < 0.001 | − 1.57 | 0.001 |
| Female (n = 2221) | Male (n = 3618) | Healthy participants (n = 5893) | ||||
| Beta | p | Beta | p | Beta | p | |
| Univariate | ||||||
| Age (per decade) | − 8.4 | < 0.001 | − 9.47 | < 0.001 | − 9.17 | < 0.001 |
| Female (n = 3060) | Male (n = 4953) | Healthy participants (n = 5893) | ||||
| Beta | p | Beta | p | Beta | p | |
| Multivariate | ||||||
| Model 1 | ||||||
| Age (per decade) | − 7.66 | < 0.001 | − 9.13 | < 0.001 | − 8.82 | < 0.001 |
| SBP (per 10 mm Hg) | − 1.33 | < 0.001 | − 1.59 | < 0.001 | − 1.1 | < 0.001 |
| Model 2 | ||||||
| Age (per decade) | − 7.39 | < 0.001 | − 9.26 | < 0.001 | − 8.86 | < 0.001 |
| SBP (per 10 mm Hg) | − 0.9 | 0.009 | − 1.28 | < 0.001 | − 0.81 | < 0.001 |
| BMI (per 5 kg/m2) | − 3.65 | < 0.001 | − 2.89 | < 0.001 | − 2.08 | < 0.001 |
| Model 3b | ||||||
| Age (per decade) | − 6.99 | < 0.001 | − 8.68 | < 0.001 | − 8.65 | < 0.001 |
| SBP (per 10 mm Hg) | − 0.89 | 0.016 | − 1.46 | < 0.001 | − 0.91 | < 0.001 |
| BMI (per 5 kg/m2) | − 2.94 | 0.001 | − 2.28 | 0.002 | − 2.03 | < 0.001 |
Beta: beta co-efficiency, BMI: body mass index, SBP: systolic blood pressure.
a. Model 3: CV indicates clinical variables including fasting glucose, cholesterol, HDL, eGFR, medical history of hypertension, diabetes, CVD, and hyperlipidemia.
b. Model 3: CV indicates clinical variables including fasting glucose, cholesterol, HDL, and eGFR.
3.3. Association between EF slope and cardiac structure by conventional measures
Table 4 and Fig. 2B–I show the relationship between EF slope and several cardiac structure and functional indices in all participants (n = 8013) and in healthy participants without hypertension, diabetes, hyperlipidemia and prior cardiovascular diseases (n = 5893). For all study participants, greater LV wall thickness (both IVS and LVPW) is associated with lower EF slope (r = − 0.17 & − 0.14, both p < 0.001, Table 4, Fig. 2E and F). In contrast, greater LV internal chamber diameter is associated with greater EF slope (all p < 0.001, Table 4, Fig. 2G and H). A modest inverse relationship was observed between EF slope and fractional shortening (r = − 0.05, p < 0.001, Fig. 2I), with greater LV mass (with or without index, Fig. 2C and D) related to more reduced EF slope (r = − 0.11 & -0.04, respectively, both p < 0.001). The association between EF slope and LV internal diameter (both systolic and diastolic) is more pronounced in men than that in women (p for interaction: < 0.05). Similar results are shown for healthy participants. Finally, the relationships between various cardiac structural quintiles and EF slope measurement from all study participants (n = 8013) are shown in Supplemental Fig. 1.
| (Per 1 SU increase) | All study participants (n = 8013) | (Per 1 SU increase) | Healthy participants (n = 5893) | ||||
|---|---|---|---|---|---|---|---|
| Beta | S.E. | p value | Beta | S.E. | p value | ||
| IVS | − 0.17 | 0.29 | < 0.001 | IVS | − 0.11 | 0.34 | 0.29 |
| LVPW | − 0.14 | 0.29 | < 0.001 | LVPW | − 0.08 | 0.35 | 0.29 |
| LVIDd | 0.14 | 0.09 | < 0.001 | LVIDd | 0.18 | 0.1 | 0.09 |
| LVIDs | 0.11 | 0.1 | < 0.001 | LVIDs | 0.16 | 0.11 | 0.1 |
| FS | − 0.05 | 0.08 | < 0.001 | FS | − 0.07 | 0.1 | 0.08 |
| LV mass | − 0.04 | 0.01 | < 0.001 | LV mass | 0.02 | 0.01 | 0.111 |
| LV mass index | − 0.11 | 0.02 | < 0.001 | LV mass index | − 0.03 | 0.03 | 0.011 |
| LA diameter | − 0.12 | 0.07 | < 0.001 | LA diameter | − 0.05 | 0.08 | 0.001 |
| DT | − 0.29 | 0.01 | < 0.001 | DT | − 0.15 | 0.01 | < 0.001 |
| IVRT | − 0.2 | 0.03 | < 0.001 | IVRT | − 0.16 | 0.03 | < 0.001 |
| E | 0.12 | 0.03 | < 0.001 | E | 0.11 | 0.03 | < 0.001 |
| A | − 0.42 | 0.02 | < 0.001 | A | − 0.37 | 0.02 | < 0.001 |
| E/A | 0.41 | 0.9 | < 0.001 | E/A | 0.36 | 1.05 | < 0.001 |
| TDI E′ | 0.46 | 0.15 | < 0.001 | TDI E′ | 0.4 | 0.17 | < 0.001 |
| E/E′ | − 0.32 | 0.16 | < 0.001 | E/E′ | − 0.25 | 0.22 | < 0.001 |
A: late mitral inflow velocity, DT: mitral inflow deceleration time, E: early mitral inflow velocity, E/A: mitral inflow E/A ratio, E/E: early mitral inflow E to mitral annulus relaxation velocity E′ ratio, FS: fractional shortening, IVRT: iso-volumic relaxation time, IVS: inter-ventricular septal wall thickness, LA: left atrial/atrium, LV: left ventricular/ventricle, LVIDd/LVIDs: diastolic/systolic left ventricular internal diameter, LVPW: left ventricular posterior wall, SU: standardized unit and TDI E′: mitral annulus relaxation velocity E′.
3.4. Association between EF slope, diastolic indices, and NT-proBNP
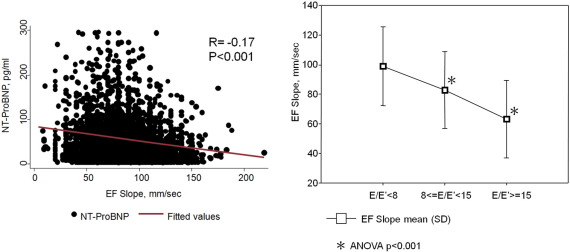
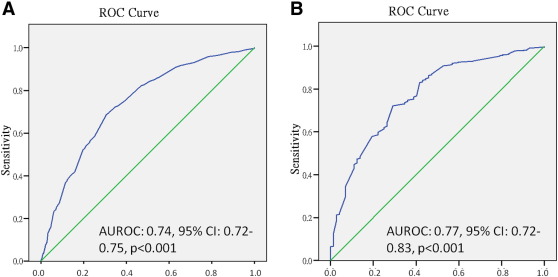
Table 4 shows the association between EF slope and clinically defined impaired diastolic indices. An inverse association between lower EF slope value and greater LA size (Table 4, Fig. 2B) and more prolonged DT/IVRT is observed, with higher E, lower A and higher E/A related to greater EF slope (all p < 0.001). Higher mitral annular relaxation velocity E′ and lower E/E′ are both associated with higher EF slope (both p < 0.001), with lower EF slope related to higher serum NT-proBNP levels (r = − 0.17, p < 0.001) (Fig. 3, left panel). A significant graded reduction in EF slope was observed over categorized E/E′ (98.9 ± 26.6 vs 82.8 ± 26 vs 63.2 ± 26.2 mm/s for E/E′ categorized as < 8, 8–15, ≥ 15, respectively) (Fig. 3, right panel, ANOVA p < 0.001). Lower EF slope is associated with increased risk of having diastolic dysfunction in terms of impaired myocardial relaxation (defined by E′ < 8 cm/s) (OR: 2.64, 95% confidence interval: 2.43–2.87, p < 0.001) or impaired LV filling capacity or compliance (defined by E/E′ ≥ 15) (OR: 3.01, 95% confidence interval: 2.24–4.05, p < 0.001). For every 10 mm/s reduction in EF slope, there was a significantly higher NT-proBNP concentration (Coef: 5.98 pg/mL, per − 10 mm/s EF slope, 95% CI: 7.82 to 4.17, p < 0.001). The area under the ROC curve indicates a value of 0.74 (95% confidence interval: 0.72–0.75) and 0.77 (95% confidence interval: 0.72–0.83) for identifying significantly worsened E′ (< 8 cm/s) and abnormally high E/E′ (Fig. 4).
|
|
|
Fig. 3. Linear correlation between EF slope and NT-proBNP level (left panel). Significant graded reduction of EF slope based on categorized LV filling pressure defined by E/E′ (98.9 ± 26.6 vs 82.8 ± 26 vs 63.2 ± 26.2 mm/s, respectively). |
|
|
|
Fig. 4. Panels A and B show AUROC for predicting abnormally low E′ (< 8 cm/s) and high E/E′ (> 15) by EF slope. The cut-off values for EF slope in identifying abnormal E′ and E/E′ are 87.5 and 79.5 cm/s2, with sensitivity/specificity 70%/67.9% and 72.2%/70.8%, respectively. |
4. Discussion
In our current work, we demonstrated that advanced age is associated with worsening EF slope in a large asymptomatic population, and lower EF slope is associated with unfavorable LV remodeling, including greater LV wall thickness (both IVS and LVPW), and greater LV mass. In addition, we further observed that lower EF slope may parallel several diastolic functional changes and elevated NT-proBNP level. These features, when taken together, have long been regarded as clinical hallmarks of cardiac aging. The clinical significance of our current work can be two-folds. Firstly, EF slope measure is tightly associated with unfavorable geometries together with aging, with lower EF slope reflecting greater cardiac concentricity. The second, we show in our current data that daily conventional echocardiography measures based on M-mode EF slope, except for the Doppler-based method, is capable of providing a clinical alternative marker for identifying subjects present with key features of diastolic dysfunction. To this end, we further provided the reference ranges based on sex and various age groups in relative large Taiwanese population.
4.1. The association between advanced age and EF slope reduction
With aging, both the arterial system and the ventricles may undergo a degree of remodeling, or stiffening, due to a number of biological/pathological changes that lead to excessive extracellular or interstitial deposition of matrix proteins and collagen fibers, impaired cardiac diastolic filling, worsened compliance and functional uncoupling [15]; [16]; [17] ; [18]. At the same time, changes in contractile mechanics, such as LV twist and torsion, may occur that compensate for age-related functional loss at early stages to preserve global LV systolic function in terms of ejection fraction [19] ; [20]. Measures capable of evaluating distensibility or the ability of the ventricle to relax may be able to detect cardiac change at earlier times than detection of systolic functional decline or clinical heart failure [21].
EF slope represents mechanical motion in response to early diastolic closure of the anterior mitral leaflet [22], and reflects the mechanical motion that is driven by decelerating transmitral inflow hemodynamics of the global LV [6] ; [23]. Therefore, factors related to diminished ventricular diastolic compliance, such as cardiac remodeling in relation to the aging process or even mildly elevated blood pressure [24], may lead to a decrease in transmitral flow and a lower EF slope value. Although previous research has indicated better sensitivity for early diastolic filling as a structural/functional indicator of LV function, our current findings on detecting abnormal key diastolic surrogates (defined as abnormal E′ or E/E′) by EF slope based on M-mode show that this method could be an important clinical alternative measure to supplement the current Doppler-based diastolic parameters used for this purpose [25].
4.2. Association between reduced EF slope and impaired conventional diastolic indices
More recently, data from several non-invasive, echocardiographic measures have shown that simultaneous and combined evaluation of transmitral flow velocity (hydrostatic driving force) and annular velocity (active myocardial relaxation), such as E/E′ ratio by TDI, may be most comparable or close to real LV filling pressure when evaluated by invasive catheterization [8] ; [9]. Though EF slope as a measure or LV diastolic function has been proposed to be highly correspondent to early diastolic mitral inflow deceleration velocity and been shown to be a function of the amount and velocity of blood passing through the mitral leaflet [26] ; [27], its specificity and the exact correlation between these two measures based on larger sample sizes has not been thoroughly explored. Nevertheless, factors postulated to be associated with the decrease of EF slope by M-mode in the context of elevated LV stiffness, altered afterload status or worsened LV compliance may also explain the more prolonged early diastolic mitral inflow deceleration time (DT) [15] ; [28], as observed in our data.
4.3. The association between reduced EF slope and impaired myocardial relaxation and filling
While it has been proposed in recent years that diastolic filling energy may start from the isovolumic phase and facilitate ventricular suction, mechanistic insight into the understanding of diastolic rheology is still a challenge, despite the more novel echo-based techniques that are still evolving [6]; [14]; [29] ; [30]. Interestingly, we observed a good correlation between myocardial/mitral annulus relaxation velocity E′, and also a good prediction ability of reduced EF slope in identifying worse E′ and elevated LV filling pressure (AUROC: 0.74 & 0.77, respectively, both p < 0.001). The most likely cause for such findings may be that myocardial relaxation velocity could be the major mechanical driving force for rapid early mitral filling, causing a faster and synergistic suction flow toward the LV and a steeper descent of the EF slope [8] ; [9]. These associations were further confirmed by the inverse relationship observed between EF slope and serum NT-proBNP level, an observation that further strengthened our hypothetical link between impeded diastolic filling as the main reason for worsened mitral leaflet closure because NT-proBNP is an effective indicator of heart diastolic function [31]. Though we acknowledge that our current work may be limited by the lack of a causal-relationship between older age or more elevated afterload on the effect of reduction in the M-mode based EF slope measurement, we have shown in our data that in such evaluation, a conventional measurement with high availability in daily practice, may be clinically feasible and useful in identifying subjects with suspected impaired diastolic function. Therefore, we propose EF slope to be a clinical alternative marker for diastolic functional assessment in asymptomatic subjects. In addition, whether such data may be extended to a population presenting with clinical heart failure as a continuum of more advanced diastolic dysfunction of myocardial asynergy, will need further investigation. To our best knowledge, this is the first work comparing and exploring the correlations between mitral leaflet EF slope and various new diastolic parameters with a relatively large sample size in an asymptomatic population.
5. Conclusions
Though clinical practice and the body of knowledge on diastolic function continues to evolve, we have shown in our work that EF slope measurement by M-mode echocardiography, a potential marker relevant to the rate of early mitral leaflet closure, is tightly associated with age and load status. Further, this simple measurement may be related to several advanced diastolic indices and have the highest correlation with mitral annulus relaxation velocity, and be regarded as the most sensitive diastolic parameter in daily use. The limitation of this study is that it is a single center study. Therefore, it may not represent general community data, and studies in different populations are needed. However, we have also provided a normal reference range for EF slope measurement in different age groups for the Taiwanese population that may serve as a useful guide in evaluating subjects with suspected diastolic dysfunction without other feasible markers in clinical practice.
The following are the supplementary data related to this article.
|
|
|
Supplemental Fig. 1. |
Acknowledgement
This work was in part supported in part by grants from the National Science Council (NSC 103-2314-B-010-005-MY3, 103-2314-B-195-001-MY3, 101-2314-B-195-020-MY1, MOST 103-2314-B-195-006-MY3), and Mackay Memorial Hospital (10271, 10248, 10220, 10253, 10375, 10358, E-102003).
References
- [1] J.Y. Wei; Age and the cardiovascular system; N. Engl. J. Med., 327 (1992), pp. 1735–1739
- [2] J.N. Cohn, R. Ferrari, N. Sharpe; Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling; J. Am. Coll. Cardiol., 35 (2000), pp. 569–582 T
- [3] D.L. Mann; Mechanisms and models in heart failure: a combinatorial approach; Circulation, 100 (1999), pp. 999–1008
- [4] T. Vartdal, H. Brunvand, E. Pettersen, H.J. Smith, E. Lyseggen, T. Helle-Valle, et al.; Early prediction of infarct size by strain Doppler echocardiography after coronary reperfusion; J. Am. Coll. Cardiol., 49 (2007), pp. 1715–1721
- [5] M.S. Maurer, D.L. King, L. El-Khoury Rumbarger, M. Packer, D. Burkhoff; Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms; J. Card. Fail., 11 (2005), pp. 177–187
- [6] A.N. DeMaria, R.R. Miller, E.A. Amsterdam, W. Markson, D.T. Mason; Mitral valve early diastolic closing velocity in the echocardiogram: relation to sequential diastolic flow and ventricular compliance; Am. J. Cardiol., 37 (1976), pp. 693–700
- [7] H. Kim, H.J. Yoon, H.S. Park, Y.K. Cho, C.W. Nam, S.H. Hur, et al.; Usefulness of tissue Doppler imaging–myocardial performance index in the evaluation of diastolic dysfunction and heart failure with preserved ejection fraction; Clin. Cardiol., 34 (2011), pp. 494–499
- [8] S.R. Ommen, R.A. Nishimura, C.P. Appleton, F.A. Miller, J.K. Oh, M.M. Redfield, et al.; Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures; Circulation, 102 (2000), pp. 1288–1294
- [9] S.F. Nagueh, R.J. Middleton, H.A. Kopelen, W.A. Zoghbi, M.A. Quin˜ones; Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures; J. Am. Coll. Cardiol., 30 (1997), pp. 1527–1533
- [10] G. Thomas; Tissue Doppler echocardiography — a case of right tool, wrong use; Cardiovasc. Ultrasound, 2 (2004), pp. 12–18
- [11] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, et al.; Chamber Quantification Writing Group; American Society of Echocardiographys Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiographys Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology; J. Am. Soc. Echocardiogr., 18 (2005), pp. 1440–1463
- [12] D.J. Sahn, A. DeMaria, J. Kisslo, A. Weyman; Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements; Circulation, 58 (1978), pp. 1072–1083
- [13] J.M. Gardin, J.I. Drayer, M. Weber, M.K. Rohan, M. Knoll, V.W. Shu, et al.; Doppler echocardiographic assessment of left ventricular systolic and diastolic function in mild hypertension; Hypertension, 9 (1987), pp. II90–II96
- [14] V.K. Munagala, S.J. Jacobsen, D.W. Mahoney, et al.; Association of newer diastolic; Echocardiography, 16 (2003), pp. 1049–1056
- [15] J.M. Gardin, M.K. Rohan, D.M. Davidson, et al.; Doppler transmitral flow velocity parameters: relationship between age, body surface area, blood pressure and gender in normal subjects; Am. J. Noninvasive Cardiol., 1 (1987), pp. 3–10
- [16] M.L. Muiesan, M. Salvetti, C. Monteduro, B. Bonzi, A. Paini, S. Viola, et al.; Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients; Hypertension, 43 (2004), pp. 731–738
- [17] W.H. Gaasch, M.R. Zile; Left ventricular diastolic dysfunction and diastolic heart failure; Annu. Rev. Med., 55 (2004), pp. 373–394
- [18] M.M. Redfield, S.J. Jacobsen, B.A. Borlaug, R.J. Rodeheffer, D.A. Kass; Age and gender-related ventricular–vascular stiffening: a community-based study; Circulation, 112 (2005), pp. 2254–2262
- [19] J. Wang, D.S. Khoury, Y. Yue, G. Torre-Amione, S.F. Nagueh; Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure; Eur. Heart J., 29 (2008), pp. 1283–1289
- [20] K. Yoneyama, O. Gjesdal, E.Y. Choi, C.O. Wu, W.G. Hundley, A.S. Gomes, et al.; Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: the multi-ethnic study of atherosclerosis; Circulation, 126 (2012), pp. 2481–2490
- [21] M. Takeuchi, W.B. Borden, H. Nakai, T. Nishikage, M. Kokumai, T. Nagakura, et al.; Reduced and delayed untwisting of the left ventricle in patients with hypertension and left ventricular hypertrophy: a study using two-dimensional speckle tracking imaging; Eur. Heart J., 28 (2007), pp. 2756–2762
- [22] A. Zaky, W.K. Nasser, H. Feigenbaum; A study of mitral valve action recorded by reflected ultrasound and its application in the diagnosis of mitral stenosis; Circulation, 37 (1968), pp. 789–796
- [23] M.A. Quinones, W.H. Gaasch, E. Waisser, J.K. Alexander; Reduction in the rate of diastolic descent of the mitral valve echogram in patients with altered left ventricular diastolic pressure–volume relations; Circulation, 49 (1974), pp. 246–254
- [24] L.M. Gardin, M.K. Rohan, O.M. Davidson, et al.; Doppler transmitral flow velocity parameters: effects of age, body surface area, blood pressure and gender in normal subjects; Am. J. Noninvasive Cardiol., 1 (1987), pp. 3–10
- [25] J.M. Gardin, J.I.M. Drayer, M. Weber, et al.; Doppler echocardiographic assessment of left ventricular systolic and diastolic function in mild hypertension; Hypertension, 9 (suppl II) (1987), pp. 90–96 II
- [26] P.A. Vignola, H.J. Walker, G.M. Pohost, L.M. Zir; Relation between phasic mitral flow and the echocardiogram of the mitral valve in man; Br. Heart J., 39 (1977), pp. 1292–1298
- [27] H. Feigenbaum; Echocardiography; (3rd ed.)Lea & Febiger, Philadephia (1981), pp. 189–190
- [28] M.A. Quinones, W.H. Gaasch, E. Waisser, J.K. Alexander; Reduction in the rate of diastolic descent of the mitral valve echogram in patients with altered left ventricular diastolic pressure–volume relations; Circulation, 49 (1974), pp. 246–254
- [29] M.S. Firstenberg, N.L. Greenberg, M.L. Main, et al.; Determinants of diastolic myocardial tissue Doppler velocities: influences of relaxation and preload; J. Appl. Physiol., 90 (2001), pp. 299–307
- [30] P.P. Sengupta, B.K. Khandheria, J. Narula; Twist and untwist mechanics of the left ventricle; Heart Fail. Clin., 4 (2008), pp. 315–324
- [31] A. Barragán, J. Lacalzada, A. de la Rosa, A.M. de Vera, A. Duque, C. Perera, et al.; Relationship between slightly elevated NT-proBNP and alterations in diastolic function detected by echocardiography in patients without structural heart disease; Int. J. Cardiol., 129 (2008), pp. 430–432
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?