Summary
Background
The occurrence rate and severity of gastroesophageal reflux disease with erosive esophagitis (EE) in patients after converting nasogastric tube (NGT) feeding to percutaneous endoscopic gastrostomy (PEG) are not well-known. The aim of this study was to determine the influence of PEG placement on the occurrence and severity of EE in patients with long-term PEG feeding.
Methods
This retrospective study included patients with NGT feeding who were converted to PEG feeding and received pre- and post-PEG endoscopy between January 2000 and June 2013. Factors predictive of the occurrence of EE after PEG were analyzed.
Results
One-hundred and twenty patients with NGT feeding were converted to PEG, and 47 patients were included. Before PEG, 21 (44.7%) NGT-feeding patients had EE. The mean follow-up time was 45.7 months (range, 6–147 months). Erosive esophagitis occurred in nine (19.1%) patients after PEG. The occurrence rate (p < 0.01) and severity (p < 0.05) of EE significantly improved after PEG, compared to before PEG. Hill’s classification of gastroesophageal valve was associated with the occurrence of EE after PEG (p < 0.01).
Conclusion
The occurrence and severity of esophagitis improved after converting the patient to PEG. Hill’s grading of gastroesophageal valve provides useful information for predicting the occurrence of EE after PEG.
Keywords
Esophagitis ; Gastroesophageal reflux disease ; Percutaneous endoscopic gastrostomy
Introduction
Percutaneous endoscopic gastrostomy (PEG) is increasingly used for long-term enteral nutrition in different chronic disorders. In the United State, the placement of a PEG tube increased from 2.71 to 3.75 placements per 1000 hospitalized patents in 1993 to 2003, respectively [1] . Percutaneous endoscopic gastrostomy is generally regarded as safer than the traditional surgical gastrostomy. However, several complications have been documented such as peristomal wound infection, pneumonia, gastroesophageal reflux disease (GERD), buried bumper syndrome, gastroparesis and bleeding [2] ; [3] ; [4] .
Gastroesophageal reflux disease is defined as “a disease comprising symptoms, end-organ effects and complications related to the reflux of gastric contents into the esophagus, oral cavity, and/or the lung” [5] . Several studies have provided conflicting evidences on the influence of PEG placement on GERD. In the absence of a gold standard method for diagnosing GERD, some investigators have only relied on clinical symptoms [6] . Other investigators have relied on the findings of pH metry [7] ; [8] ; [9] ; [10] ; [11] or scintigraphy [12] ; [13] , which cannot confirm the diagnosis of GERD. In addition, the follow-up period of these studies is short, ranging from 3 days to 9 weeks [8] ; [9] ; [10] ; [11] . The influence of PEG on GERD remains an incompletely answered question.
Endoscopy has excellent specificity for diagnosing GERD, especially when patients have GERD with erosive esophagitis (EE) [5] . In previous studies, patients have not received the same pre- and post-PEG endoscopic assessment for GERD [4] ; [8] . In addition, most studies only evaluate the incidence of gastroesophageal reflux episodes after PEG [10] ; [11] ; [12] . However, gastroesophageal reflux episodes do not necessarily indicate EE. Distinguishing gastroesophageal reflux episode from EE is particularly important to avoid an over- or underdiagnosis of the disease and its complications. To the best of our knowledge, no study using endoscopy directly compares the occurrence rate and severity of EE in patients before and after converting NGT feeding to PEG.
To elucidate the relation between PEG placement and EE, we performed a retrospective study with the data of pre- and post-PEG endoscopy to determine whether PEG placement would influence the occurrence and severity of EE in a long-term follow-up cohort.
Patients and methods
Patients
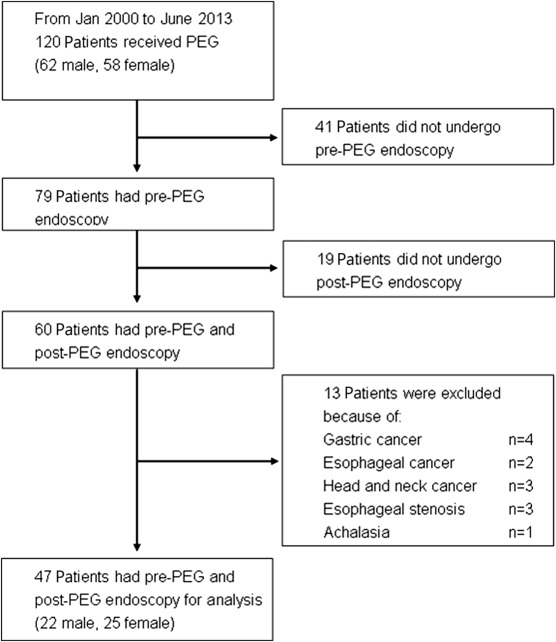
From January 1, 2000 to June 30, 2013, 120 patients with NGT feeding were converted to PEG feeding in Taipei Medical University Wan Fang Hospital (Taipei, Taiwan). Endoscopic images and reports of PEG procedure were documented in all patients. We recorded clinical history, primary diagnosis, complications, and morbidity during the follow-up period. Six months before PEG placement, 79 patients received endoscopy (i.e., pre-PEG endoscopy). However, 60 patients received endoscopy after PEG (i.e., post-PEG endoscopy). In this study, we included 47 patients with pre- and post-PEG endoscopy. Excluded were the remaining patients who had head and neck, esophageal, or gastric cancer; esophageal stenotic disorder, or achalasia (Figure 1 ). The Taipei Medical University–Joint Institutional Review Board approved the protocol of this study.
|
|
|
Figure 1. Flow chart representation of patient selection in the study of the occurrence of gastroesophageal reflux disease with erosive esophagitis before and after percutaneous endoscopic gastrostomy (PEG). |
Pre-PEG endoscopy
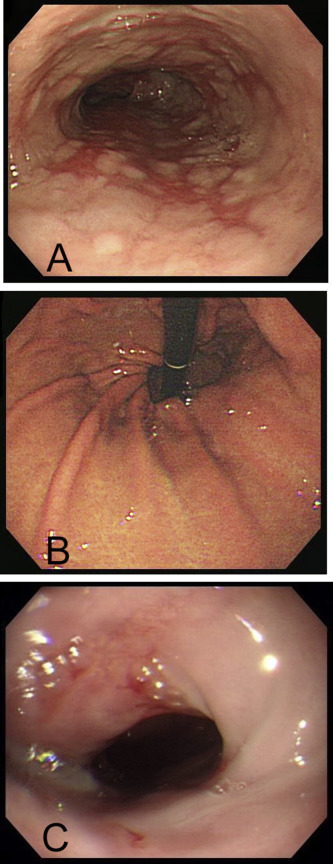
Six months before the PEG operation, all 47 patients had endoscopy for the evaluation of upper gastrointestinal complaints. If EE was diagnosed, the severity of EE was classified according to the Los Angeles (LA) classification [14] . Grading of the gastroesophageal valve was graded according to Hill’s classification [15] . Grade I is defined as a prominent fold of tissue along the lesser curvature that is closely opposed to the endoscope. In Grade II, a fold is present, but it is not as prominent and opens with respiration. In Grade III, a fold is not prominent and the hiatus is patulous. In Grade IV, there is a hiatal hernia without a fold, and the lumen of the esophagus is wide open (Figure 2 ). All reports and endoscopic images of the 47 patients were retrospectively reviewed by two authors (C.N.C. and F.M.S.). These two authors had performed > 3000 upper gastrointestinal endoscopies. The scores of the LA classification of GERD and Hill’s classification of gastroesophageal valve were evaluated, according to the defined criteria.
|
|
|
Figure 2. Endoscopic pictures of an 86 year-old woman. (A) Before percutaneous endoscopic gastrostomy, endoscopy shows Los Angeles (LA) Grade D erosive esophagitis; a mucosal break involves > 75% of the esophageal circumference. (B) Hills’ classification of Grade IV gastroesophageal valve presenting as a hiatal hernia without a fold; the lumen of the esophagus is wide open. (C) Forty-one months after percutaneous endoscopic gastrostomy, the follow-up endoscopy shows LA Grade B erosive esophagitis; one mucosal break is > 5 mm long. |
We treated all endoscopically diagnosed EE patients with a proton pump inhibitor (PPI), based on the indication of the Taiwan National Health Insurance Bureau reimbursement policy. A proton pump inhibitor was used for 4 months in LA Grade A and B patients and for 12 months in LA Grade C and D patients. After completing one treatment course and if another PPI treatment course was intended, the EE patients needed to receive a repeated endoscopy to confirm the continuous presence or occurrence of EE.
PEG procedure
The principle indication for replacing PEG for NGT feeding in patients with swallowing disorders is for the long-term maintenance of nutritional requirements. All patients received prophylactic antibiotics within 30 minutes before the procedure. We performed PEG with Ponsky and Gauderer’s [16] pull method. All PEGs were placed in the corpus of the stomach. On the 1st day, the PEG tube was flushed with sterile water, and feeding was started the next day.
Post-PEG endoscopy
At 6 months, the original PEG tube was replaced by a balloon tube under endoscopic guidance. A subsequent tube replacement was scheduled at the 6-month interval. At each time of tube replacement, we endoscopically observed the occurrence of EE and assessed the severity of EE, if it was present. If a patient received PPI treatment, the EE condition was assessed after discontinuing PPI treatment.
Statistical analysis
Descriptive statistics were used to describe the demographic characteristics of the patients. Univariate risk factor analysis was performed using the Chi square test and analysis of variance (ANOVA) test when appropriate. The severity of EE of pre- and post-PEG was analyzed with the McNemer–Bowker test. Weighted kappa statistic was used to evaluate the degree of agreement among observers. The range of possible values for κ is from −1 to +1. A κ < 0.00 indicates no agreement; κ = 0.00–0.20, slight agreement; κ = 0.21–0.40, fair agreement; κ = 0.41–0.60, moderate agreement; κ = 0.61–0.80, good agreement; and κ = 0.81–1.00, nearly perfect agreement.
We computed all study data with SPSS software, version 17 for Windows (SSPS, Inc., Chicago, IL, USA). The differences between groups were considered significant for p < 0.05.
Results
There was good agreement in the severity of GERD before PEG between reviewers (κ = 0.643; 95% confidence interval, 0294–0.992). The κ value was 0.694 (95 % confidence interval 0.547–0.841) for the severity of GERD after PEG. Agreement of the Hill’s classification of gastroesophageal valve had a κ value of 0.6 (95% confidence interval, 0.193–1.0).
Table 1 shows the demographic characteristics of 47 patients receiving PEG. Before PEG, 21 (44.7%) of 47 NGT-feeding patients had EE. The mean follow-up time was 45.7 months (range, 6–147 months). Erosive esophagitis occurred in nine (19.1%) patients after PEG. The discontinuation time of PPI before post-PEG evaluation of GERD condition ranged from 2 weeks to 2 months. Table 2 lists changes in the occurrence and severity of EE before and after PEG. The occurrence rate (p < 0.01) and severity (p < 0.05) of EE significantly improved after PEG, compared to before PEG. Table 3 describes the risk factors for the presence of EE after PEG. Only Hill’s classification of the gastroesophageal valve was associated with the occurrence of EE after the PEG operation (p < 0.01).
| Variables | N (%) |
|---|---|
| Age (y) | |
| Mean age (range) | 78 (30–96) |
| Gender | |
| M | 25 (53.2) |
| F | 22 (46.8) |
| Primary diagnosis | |
| Cerebrovascular accident | 29 (61.7) |
| Parkinsonism | 4 (8.5) |
| Head injury | 5 (10.6) |
| Dementia | 7 (14.9) |
| Nongastrointestinal malignant diseases | 2 (4.3) |
| Presence of EE (LA grade) | |
| 0 | 26 (55.3) |
| A | 2 (4.3) |
| B | 7 (14.9) |
| C | 7 (14.9) |
| D | 5 (10.6) |
| Hill’s classification | |
| I | 27 (57.4) |
| II | 2 (4.3) |
| III | 5 (10.6) |
| IV | 13 (27.7) |
0 = no EE; EE = erosive esophagitis; LA = Los Angeles classification, PEG = percutaneous endoscopic gastrostomy.
| Before PEG | After PEG * | |||||
|---|---|---|---|---|---|---|
| Patients with EE (LA grade) | 0 | A | B | C | D | |
| 0 | 26 | 26 | ||||
| A | 2 | 1 | 1 | |||
| B | 7 | 6 | 1 | |||
| C | 7 | 5 | 1 | 1 | ||
| D | 5 | 3 | 2 | |||
| n | 47 | 38 | 1 | 4 | 3 | 1 |
- p < 0.05.
0 = no erosive esophagitis; EE = erosive esophagitis; LA = Los Angeles classification; PEG = percutaneous endoscopic gastrostomy.
| Patients | Without EE (N = 38) | With EE (N = 9) | p |
|---|---|---|---|
| Age (y) | 0.147 | ||
| < 65 | 5 | 2 | |
| 65–74 | 2 | 1 | |
| 75–84 | 14 | 3 | |
| > 85 | 17 | 3 | |
| Gender | 0.515 | ||
| M | 19 | 6 | |
| F | 19 | 3 | |
| Underlying diagnosis | 0.702 | ||
| Cerebrovascular accident | 22 | 7 | |
| Parkinsonism | 4 | 0 | |
| Head injury | 4 | 1 | |
| Dementia | 6 | 1 | |
| Nongastrointestinal malignant diseases | 2 | 0 | |
| Hill’s classification * | 0.003 | ||
| I | 26 | 1 | |
| II | 1 | 1 | |
| III | 2 | 3 | |
| IV | 9 | 4 | |
| Follow-up interval (m)a | 46.6 ± 6.6 | 44.6 ± 6.5 | 0.862 |
- p < 0.01.
EE = erosive esophagitis; PEG = percutaneous endoscopic gastrostomy.
a. Follow-up interval is represented as month, mean ± standard error.
Discussion
We described the influence of PEG replacement on EE in patients with a swallowing disorder with endoscopic examination. In this study, 21 (44.7%) of 47 NGT-feeding patients had EE before they underwent the PEG operation (Table 1 ). Erosive esophagitis occurred only in nine (19.1%) patients after receiving PEG, and no patient without preexisting EE developed EE after receiving PEG (Table 2 ). Our study results imply that approximately one-half of NGT-feeding patients had EE before receiving PEG, and that PEG did not worsen or precipitate EE.
In our series, > 90% patients had neurological disorders (Table 1 ). The diagnosis of reflux esophagitis is more difficult in patients with neurologic disorders because the typical symptoms of esophagitis may be absent, and some subtle symptoms of esophagitis such as chest discomfort and heartburn may be difficult to detect in these disabled patients [10] ; [13] ; [17] . Even in the general population, clinical symptom is unreliable for predicting GERD. A systematic review has reported that the sensitivity of clinical symptoms for diagnosing EE is only 30–76% and its specificity is 62–96% [18] . Poor diagnostic agreement between pH metry and histological esophagitis has also been reported [19] . Salvatore et al [20] report no meaningful correlation between the results of the pH metry study and the findings of histological esophagitis. Endoscopy is an important investigation in diagnosing GERD and GERD-related complications such as Barrett’s esophagus. Therefore, we suggest performing endoscopy to evaluate the long-term evolution of the EE after PEG in this study.
Contradictory evidence exists in the literature on the relationship between PEG and GERD (Table 4 ). Some studies have shown that GERD does not precipitate after PEG [8] ; [9] ; [11] ; [12] , but these conclusions conflict with those of other studies [10] ; [21] .
| Source | Design | Type of patient | No. of patients | Pre-PEG assessments | Post-PEG assessments | Timescale of assessments | Conclusions |
|---|---|---|---|---|---|---|---|
| Douzinas et al 2009 [4] | Prospective | Ventilator support patients Age: 32–68 y | 29 | pH metry | pH metry endoscopy histology | 7 days | Grading of gastroesophageal flap valve and severity of reflux esophagitis predicted failure of PEG to reduce GER in mechanically ventilated patients |
| Samuel et al 2002 [8] | Prospective | NI children Age: 6.7 ± 4.2 y | 64 | N/A | 24-h pH monitoring | 9.4 ± 1.2 wk | PEG did not precipitate GER and an abnormal preoperation pH study predicted persistence or worsening reflux after PEG |
| Jung et al 2011 [9] | Prospective | Bed-ridden patients Age: 59.8 ± 14.1 y | 21 | 24-h pH monitoring | 24-h pH monitoring | 7.3 ± 2.2 d | PEG may prevent GER in patients receiving NGT feeding, especially in those patients with GER |
| Thomson et al 2011 [10] | Case series | NI children Age: 5.3 y | 10 | pH/MII | pH/MII | 12–384 d | PEG placement increases GER episodes in NI children |
| Razeghi et al 2002 [11] | Prospective | NI children Age: 5.2 ± 6.4 y | 68 | 24-h pH monitoring | 24-h pH monitoring | 3–1454 d | PEG did not provoke GER, and placement in the antrum may be unfavorable |
| Lee et al 2011 [12] | Prospective | NGT feeding patients > 6 months Age: 74.5 y | 15 | GER scintigraphy scan | GER Scintigraphy scan | 1 wk | Shifting from NGT to PEG feeding reduced GER |
| Nishiwaki et al 2006 [13] | Case-control | Dysphagia patients Age: 83 y | 178 | Endoscopy | GER Scintigraphy scan | 7–14 d | Hiatus hernia, severe reflux esophagitis, and a high GER index predicted aspiration or vomiting after PEG |
| Heine et al 1995 [21] | Case series | NI children Age: 6 y | 30 | pH Monitoring endoscopy | Questionnaire | 3–9 mo | PEG effectively provided nutrition, improved feed-related stresses, but may exacerbate GER |
GER = gastroesophageal reflux; N/A = not available; NI = neurologically impaired; NGT = Nasogastric tube; pH/MII = combined intraluminal pH and multiple intraluminal impedance; PEG = percutaneous endoscopic gastrostomy.
Several studies report potential risk factors that may contribute to the occurrence or worsening of GERD after replacing PEG. In scintigraphy study, a rapid gastrostomy bolus infusion may lead to a decrease in the lower esophageal pressure to an incompetent level and increase gastroesophageal reflux [22] . Razehgi et al [11] suggest that the location of the PEG tube may influence of the occurrence of gastroesophageal reflux, and that placing PEG in the antrum is associated with increased gastroesophageal reflux compared to placing PEG in the corpus [11] . In our study findings, all PEG procedures were performed in the corpus of stomach. As Table 3 shows, we found that Hill’s Grade IV gastroesophageal valve was significantly associated with the occurrence of EE after PEG (p < 0.01). The correlation between hiatal hernia and reflux esophagitis has been reported in general patients [23] . The presence of hiatal hernia may increase the frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients. Nishiwaki et al [13] also report that the presence of hiatal hernia is associated with gastroesophageal reflux after gastrostomy.
Our study has three major limitations. First, the sample size was limited in the number of study patients who had completed pre- and post-PEG endoscopic examinations. However, the long-term follow up of 47 patients who had the same endoscopic assessment of GERD condition before and after PEG operation represents one of the largest series in a single center. Most patients who received PEG were frail, and 31.5–61.9% patients died of their primary disease in the 1st year after PEG [2] ; [3] . Second, we did not perform pH measurements in the follow-up study patients; therefore, episodes of acidic reflux could not be evaluated in this study. Third, this was a retrospective study, and there is a patient selection bias in this study. Further prospective, randomized control studies are needed to provide stronger evidence for the association between PEG and GERD.
In conclusion, we reported that EE occurred in approximately one-half of patients on NGT feeding, and the occurrence and severity of EE were significantly improved after converting to PEG feeding. Endoscopic grading of the gastroesophageal valve provides useful information for predicting the occurrence of EE after PEG. We suggest that NGT-feeding patients should be evaluated for the presence of EE before and after PEG placement by endoscopy.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] P. Mendiratta, J.M. Tilford, P. Prodhan, K. Curseen, G. Azhar, J.Y. Wei; Trends in percutaneous endoscopic gastrostomy placement in the elderly from 1993 to 2003; Am J Alzheimers Dis Other Demen, 27 (2012), pp. 609–613
- [2] M.Y. Chen, Y.S. Cheng, G.S. Lien, C.N. Chen, F.M. Suk, M.S. Wu; Ten years’ experience of percutaneous endoscopic gastrostomy; Gastroenterol J Taiwan, 25 (2008), pp. 373–381
- [3] T. Tokunaga, T. Kubo, S. Ryan, M. Tomizawa, S. Yoshida, K. Takagi, et al.; Long-term outcome after placement of a percutaneous endoscopic gastrostomy tube; Geriatr Gerontol Int, 8 (2008), pp. 19–23
- [4] E.E. Douzinas, I. Andrianakis, O. Livaditi, D. Bakos, K. Flevari, N. Goutas, et al.; Reasons of PEG failure to eliminate gastroesophageal reflux in mechanically ventilated patients; World J Gastroenterol, 15 (2009), pp. 5455–5460
- [5] P.O. Katz, L.B. Gerson, M.F. Vela, M.F. Vela; Guidelines for the diagnosis and management of gastroesophageal reflux disease; Am J Gastroenterol, 108 (2013), pp. 308–328
- [6] J.A. Isch, F.J. Rescorla, L.R. Scherer, K.W. West, J.L. Grosfeld; The development of gastroesophageal reflux after percutaneous endoscopic gastrostomy; J Pediatr Surg, 32 (1997), pp. 321–322
- [7] L.J. Noble, A.M. Dalzell, W. El-Matary; The relationship between percutaneous endoscopic gastrostomy and gastro-oesophageal reflux disease in children: a systematic review; Surg Endosc, 26 (2012), pp. 2504–2512
- [8] M. Samuel, K. Holmes; Quantitative and qualitative analysis of gastroesophageal reflux after percutaneous endoscopic gastrostomy; J Pediatr Surg, 37 (2002), pp. 256–261
- [9] S.H. Jung, S.H. Dong, J.Y. Lee, N.H. Kim, J.Y. Jang, B.H. Kim, et al.; Percutaneous endoscopic gastrostomy prevents gastroesophageal reflux in patients with nasogastric tube feeding: a prospective study with 24-hour pH monitoring; Gut Liver, 5 (2011), pp. 288–292
- [10] M. Thomson, P. Rao, D. Rawat, T.G. Wenzl; Percutaneous endoscopic gastrostomy and gastro-oesophageal reflux in neurologically impaired children; World J Gastroenterol, 17 (2011), pp. 191–196
- [11] S. Razeghi, T. Lang, R. Behrens; Influence of percutaneous endoscopic gastrostomy on gastroesophageal reflux: a prospective study in 68 children; J Pediatr Gastroenterol Nutr, 35 (2002), pp. 27–30
- [12] T.H. Lee, Y.C. Shiun; Changes in gastroesophageal reflux in patients with nasogastric tube followed by percutaneous endoscopic gastrostomy; J Formos Med Assoc, 110 (2011), pp. 115–119
- [13] S. Nishiwaki, H. Araki, N. Goto, Y. Niwa, M. Kubota, M. Iwashita, et al.; Clinical analysis of gastroesophageal reflux after PEG; Gastrointest Endosc, 64 (2006), pp. 890–896
- [14] L.R. Lundell, J. Dent, J.R. Bennett, A.L. Blum, D. Armstrong, J.P. Galmiche, et al.; Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification; Gut, 45 (1999), pp. 172–180
- [15] L.D. Hill, R.A. Kozarek, S.J. Kraemer, R.W. Ave, C.D. Mercer, D.E. Low, et al.; The gastroesophageal flap valve: in vitro and in vivo observations; Gastrointest Endosc, 44 (1996), pp. 541–547
- [16] J.L. Ponsky, M.W.L. Gauderer; Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy; Gastrointestinal Endosc, 27 (1981), pp. 9–11
- [17] D.A. Johnson, M.B. Fennerty; Heartburn severity underestimates erosive esophagitis severity in elderly patients with gastroesophageal reflux disease; Gastroenterology, 126 (2004), pp. 660–664
- [18] P. Moayyedi, N.J. Talley, M.B. Fennerty, N. Vakil; Can the clinical history distinguish between organic and functional dyspepsia?; JAMA, 295 (2006), pp. 1566–1576
- [19] R.G. Heine, D.J. Cameron, C.W. Chow, D.J. Hill, A.G. Catto-Smith; Esophagitis in distressed infants: poor diagnostic agreement between esophageal pH monitoring and histopathologic findings; J Pediatr, 140 (2002), pp. 14–19
- [20] S. Salvatore, B. Hauser, K. Vandemaele, R. Novario, Y. Vandenplas; Gastroesophageal reflux disease in infants: how much is predictable with questionnaires, pH metry, endoscopy and histology?; J Pediatr Gastroenterol Nutr, 40 (2005), pp. 210–215
- [21] R.G. Heine, D.S. Reddihough, A.G. Catto-Smith; Gastro-oesophageal reflux and feeding problems after gastrostomy in children with severe neurological impairment; Dev Med Child Neurol, 37 (1995), pp. 320–329
- [22] R.M. Coben, A. Weintraub, A.J. DiMarino, S. Cohen; Gastroesopahageal reflux during gastrostomy feeding; Gastroenterology, 106 (1994), pp. 13–18
- [23] B. Kaul, H. Petersen, H.E. Myrvold, K. Grette, P. Roysland, T. Halvorsen; Hiatus hernia in gastroesophageal reflux disease; Scand J Gastroenterol, 21 (1986), pp. 31–34
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?