Summary
Choledochoduodenal fistula (CDF) complicated by peptic diseases or following surgical or endoscopic approaches of the common bile duct is not uncommon. However, it usually occurs without significant symptoms and can be well controlled with conservative treatment in normal immunized patients. Here we report a case involving a 58-year-old male patient with diabetic nephropathy, who received a choledocholithotomy for choledocholithiasis in November 2007 and renal transplantation in March 2008. The patient had recurring cholangitis during the 5 months following his renal transplantation. Cholangiography and liver biopsy revealed sclerosing cholangitis. The patient underwent liver transplantation (LT) in May 2009 because radiological and endoscopic procedures failed to control his jaundice. A proximal CDF was found during the LT procedures. We considered that the patients advanced secondary sclerosing cholangitis was induced by this fistula. At the 16 months' follow-up, the patient was surviving well and the graft remained intact. To our knowledge, this is the first report of a renal transplantation recipient receiving LT because of uncontrolled cholangitis caused by a CDF.
Keywords
choledochoduodenal fistula;liver transplantation;renal transplantation;secondary sclerosing cholangitis
1. Introduction
Secondary sclerosing cholangitis (SSC), which is morphologically and clinically similar to primary sclerosing cholangitis (PSC), usually originates from known pathological processes, including choledochoduodenal fistula (CDF).1 ; 2
Clinically, CDF is fairly common, and in patients in large endoscopic retrograde cholangiopancreatography studies, it has been reported to have an incidence ranging from 2.53% to5.3%.3 ; 4 However, surgery for this complication, especially liver transplantation (LT), is rarely necessary.1 ; 2
Here we report a case involving a uremic patient requiring LT as a result of advanced SSC 19 months after a choledocholithotomy and 10 months after renal transplantation. During the LT, we found a CDF. To our knowledge, this represents the first report of a renal transplantation recipient requiring LT due to advanced SSC caused by a CDF.
2. Case report
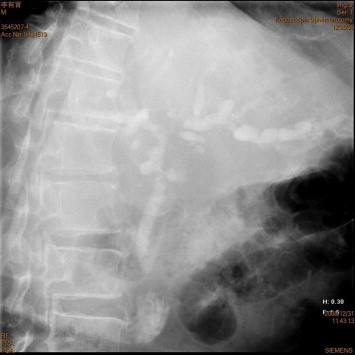
A 58-year-old male uremic patient received open cholecystectomy and choledocholithotomy for gall bladder stones and choledocholithiasis in November 2007. The T-tube was removed January 2008. The cholangiography showed no biliary-enteric fistula (Fig. 1). The patient also received living donor renal transplantation in March 2008 for diabetic nephropathy. The allograft was donated by his wife and this graft was implanted in the left iliac fossa. The maintenance immunosuppressive agents consisted of a triple-drug regimen included tacrolimus, mycophenolate sodium, and prednisolone. This patient did not suffer from any clinically evident rejection episode and the serum levels of blood sugar were acceptable after renal transplantation.
|
|
|
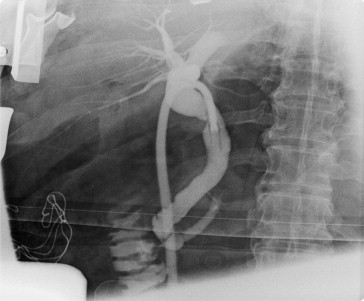
Figure 1. The cholangiography showed normal configuration of the biliary tract. There was no residual choledocholithiasis, no leakage of contrast, and no biliary-enteric fistula found. |
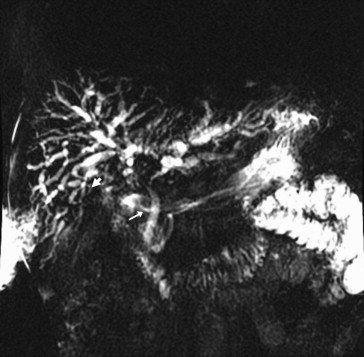
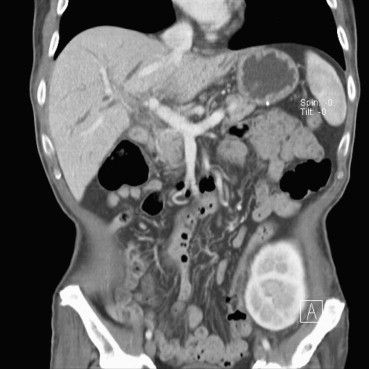
Five months after renal transplantation, he started having recurring episodes of intractable suppurative cholangitis. Magnetic resonance cholangiography in March 2009 revealed rapid progressive dilatation of both extra- and intrahepatic ducts and multifocal stricture and beading throughout the bile ducts (Fig. 2). During cholangiography, we found a proximal CDF (see Fig. 2, arrow), not previously shown by endoscopic or radiological images. Computed tomography (CT) showed normal perfusion of the extra- and intrahepatic artery (Fig. 3).
|
|
|
Figure 2. Multifocal stricture and beading throughout the extra- and intrahepatic ducts was found by magnetic resonance cholangiography in March 2009. The arrow shows the proximal choledochoduodenal fistula. |
|
|
|
Figure 3. The normal distribution of the extra- and intrahepatic artery and the patency of the portal vein were shown by computed tomography. The renal graft was implanted in the left iliac fossa. |
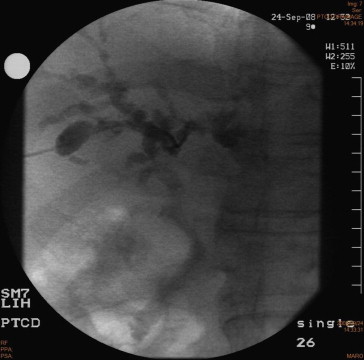
Before liver transplantation, the patients liver enzymes were:total bilirubin 12.43 mg/dL (0.1–1.6), aspartate aminotransferase 76 IU/L (1–40), alanine aminotransferase 91 IU/L (1–40), and alkaline phosphatase 565 IU/L (30–115), gamma glutamyl transpeptide 472 IU/L (8–35). Serological tests for viral hepatitis B or C, antimitochondria antibody, antinuclear antibody, and human immunodeficiency virus were all negative. Neither cryptosporidium nor cytomegalovirus was found in the bile or blood samples of this patient. Colonoscopy showed no inflammatory bowel disease. Liver and bile duct biopsy findings were compatible with sclerosing cholangitis and without evidence of cholangiocarcinoma. LT was indicated because biliary cirrhosis and hepatic failure were unresponsive to conservative treatment, including percutaneous transhepatic biliary drainage and endoscopic sphincterostomy (Figs. 4 and 5).
|
|
|
Figure 4. The cholangiography of initial cholangitis (September 2008) showed there was dilated intrahepatic biliary tract and no choledochoduodenal fistula visible when percutaneous transhepatic biliary drainage was undergone. |
|
|
|
Figure 5. The cholangiography of endoscopic sphincterostomy (December 2008) showed progressive dilation of both the intra- and extrahepatic biliary tract. There was no choledochoduodenal fistula detected during the period of treatments. |
The patient received deceased donor LT in May 2009. Intraoperatively, he was confirmed to have diffuse sclerosing cholangiopathy and a proximal type CDF, 1 X 0.7 X 0.2 cm, over the middle third common bile duct. Pathological analysis of the native explanted liver found periductal fibrosis and ductular proliferation, plus centrilobular cholestasis and necrosis. The CDF showed inflammatory granulation tissue. This fistula was thought to have caused the sclerosing cholangitis. The patients bile duct was reconstructed with Roux-en-Y hepaticojejunostomy and, 16 months later, he is surviving well and the liver graft has good function. No biliary complications, such as bile leakage or stenosis of the anastomatosis, have been found.
3. Discussion
Compared with PSC, the natural history and prognosis of SSC maybe more favorable if recognition is prompt and appropriate therapy timely.1 ; 2 Conservative treatment often fails in patients with advanced SSC, generally because of ischemic changes in the biliary tract of patients receiving surgical trauma or patients who are critically ill.2 Gossard et al5 reported that patients with advanced SSC who do not undergo LT may have significantly lower survival rates than patients with PSC.
In this case, the hepatic artery was intact (Fig. 3). Thus, instead of diffuse ischemia, his sclerosing cholangitis was probably caused by repeated cholangitis induced by proximal CDF. Because cholangiopathy was diffuse and liver parenchyma had irreversible sclerosing change, it would not be appropriate to perform a simple fistula closure or bypass.
Experience of using LT to treat SSC is limited. In Gossards report,5 29% (9/31) of the patients with advanced SSC needed LT, and four of those nine died, including one who was waiting to receive the procedure. Mohsine,6 however, reported that five SSC patients survived well after LT and all patients had no recurrence of cholangitis after a median follow-up of 39 months.
Compression of the duodenal bulb by T-tube may have contributed to the formation of the CDF in this patient. Its small caliber made it difficult to recognize during previous endoscopic and radiological procedures (Figs. 4 and 5). Thus, no operation was performed to salvage the liver before LT.
Tacrolimus and cyclosporine have cholestatic and lithogeniceffects.7 ; 8 Abdo et al9 reported the recovery of a renal transplant patient suffering from reversible SSC caused by cryptosporidiosis infection following a reduction in tacrolimus dosage. Therefore, these calcineurin inhibitors perhaps exacerbate the injury caused by toxin-producing bacteria, viruses, or other opportunistic pathogens to the biliary epithelium during the recurring episodes of cholangitis.8 ; 9 The dosage of tacrolimus prescribed for this patient was adjusted to maintain serum trough levels of 5–10 ng/mL for the first postoperative month and 5–7 ng/mL thereafter. These levels were lower than the usually recommended protocol. Furthermore, SSC is not more frequently encountered in LT patients who receive a hepaticojejunostomy or choledochoduodenostomy and take calcinerin inhibitors.10 Thus, the relationship between calcineurin inhibitors and SSC in this case needs further investigation.
In conclusion, the severe sequel of SSC due to CDF should not be neglected in such immunocompromised patients, as recipients of renal transplantations. LT can be used to treat these patients with advanced SSC.
References
- 1 R. Abdalian, E.J. Heathcote; Sclerosing cholangitis: a focus on secondary causes; Hepatology, 44 (2006), pp. 1063–1074
- 2 P. Ruemmele, F. Hofstaedter, C.M. Gelbmann; Secondary sclerosing cholangitis; Nat Rev Gastroenterol Hepatol, 6 (2009), pp. 287–295
- 3 M. Tanaka, S. Ikeda; Parapapillary choledochoduodenal fistula: an analysis of 83 consecutive patients diagnosed at ERCP; Gastrointest Endosc, 29 (1983), pp. 89–93
- 4 B.S. Sheu, J.S. Shin, X.Z. Lin, et al.; Clinical analysis of choledochoduodenal fistula with cholelithiasis in Taiwan: assessment by endoscopic retrograde cholangiopancreatography; Am J Gastroenterol, 91 (1996), pp. 122–126
- 5 A.A. Gossard, P. Angulo, K.D. Lindor; Secondary sclerosing cholangitis: a comparison to primary sclerosing cholangitis; Am J Gastroenterol, 100 (2005), pp. 1330–1333
- 6 R. Mohsine, M.C. Blanchet, Z. El Rassi, T. Ponchon, O. Boillot; [Liver transplantation for secondary sclerosing cholangitis following biliary surgery]; Gastroenterol Clin Biol, 28 (2004), pp. 181–184
- 7 S. Sanchez-Campos, R. Lopez-Acebo, P. Gonzalez, J.M. Culebras, M.J. Tuñon, J. Gonzalez-Gallego; Cholestasis and alterations of glutathione metabolism induced by tacrolimus (FK506) in the rat; Transplantation, 66 (1998), pp. 84–88
- 8 M. Kadmon, C. Klunemann, M. Bohme, et al.; Inhibition by cyclosporin A of adenosine triphosphate-dependent transport from the hepatocyte into bile; Gastroenterology, 104 (1993), pp. 1507–1514
- 9 A. Abdo; Reversible sclerosing cholangitis secondary to cryptosporiodiosis in a renal transplant patient; J Hepatol, 38 (2003), pp. 688–691
- 10 W. Bennet, M.A. Zimmerman, J. Campsen, et al.; Choledochoduodenostomy is a safe alternative to Roux-en-Y choledochojejunostomy for biliary reconstruction in liver transplantation; World J Surg, 33 (2009), pp. 1022–1025
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?