Summary
Objective
Liver transplantation (LT) is known to be a promising treatment for patients with liver cirrhosis associated with hepatocellular carcinoma (HCC). This study, however, found that HCC recurrence remains to be a concern.
Methods
A total of 126 HCC patients who had undergone LT between January 2000 and December 2009 were retrospectively reviewed. The clinicopathological features of the patients were analyzed by univariate and multivariate analyses to determine prognostic factors. Patients who had HCC recurrence were further analyzed in terms of recurrent pattern, management, and outcome.
Results
Seventeen patients (13.5%) exhibited HCC recurrence following LT. Univariate and multivariate analyses identified two prognostic factors: tumor number > three [hazard ratio (HR) = 3.249] and presence of microvascular invasion (HR = 4.336). Among patients with HCC recurrence, 15 out of 17 (88%) patients developed extrahepatic metastasis shortly after recurrence. The survival of patients after HCC recurrence was dismal with 18.3 months of median survival.
Conclusions
Multiple tumors (>three) are an important prognostic factor for HCC recurrence following LT, but an accurate assessment of tumor status by pretransplantation radiological examination is required. The outcome of patients with HCC recurrence after LT remains very poor because of a tendency of HCC to recur as extrahepatic metastasis.
Keywords
hepatocellular carcinoma;liver transplantation;outcome;prognostic factor;recurrence
1. Introduction
Hepatocellular carcinoma (HCC) is a common but lethal malignant disease with worldwide occurrence, and majority of the cases are associated with liver cirrhosis. Although hepatic resection is the preferred treatment that provides a favorable outcome for HCC patients, the proportion of suitable patients is limited by the underlying liver cirrhosis and poor liver function reserve. Therefore, liver transplantation (LT) was introduced as a promising treatment modality that simultaneously cures liver cirrhosis and HCC. Nonetheless, the initial results of LT were frustrating until the Milan criteria were proposed in 1996.1; 2 ; 3 The results of LT in HCC patients meeting the Milan criteria are excellent in terms of HCC recurrence and patients’ outcome; this fact has facilitated the worldwide use of these criteria for patient selection. Subsequently, expanded criteria, including the University of California, San Francisco (UCSF), and the up-to-seven criteria, were introduced to recruit more patients qualifying for LT, and this generated valuable results.4; 5 ; 6
However, HCC patients meeting these criteria are not completely free from the risk of tumor recurrence after LT. It is estimated that around 10–20% of the patients experienced HCC recurrence despite meeting these criteria.7; 8; 9 ; 10 Therefore, prediction of HCC recurrence following LT remains entirely elusive, and despite a growing experience and literature, it is still a hotly debated field. Therefore, in this study, we aimed to identify the factors associated with the prognosis of HCC patients undergoing LT, and to analyze the outcome of patients with HCC recurrence following LT.
2. Materials and methods
2.1. Patients
A total of 376 LTs were performed at the Organ Transplantation Institute of Chang Gung Memorial Hospital at Linkou, Taiwan, between January 2000 and December 2009. A retrospective review of the database showed that 137 patients had undergone LT because of HCC-associated liver cirrhosis. Apart from 11 (8.0%) hospital mortalities, the remaining 126 patients, including 97 men and 29 women with their ages ranging from 32 to 68 years [mean ± standard deviation (SD), 53.0 ± 7.3 years], were enrolled in the current study.
2.2. Diagnosis and treatment of HCC
The diagnosis of HCC was based on the findings of ultrasonography, computed tomography (CT), hepatic angiography, and/or magnetic resonance imaging (MRI), in accordance with the European Association for the Study of the Liver (EASL) guidelines and the American Association for the Study of Liver Diseases (AASLD) guidelines.11 ; 12 Ultrasound-guided biopsy was considered only in the case of equivocal imaging patterns and/or when clinically indicated; the concentration of the serological tumor marker, α-fetoprotein (AFP), was also assessed during diagnosis.
Primary HCC treatment consisted of a multimodal therapy, including hepatic resection, local ablation by radiofrequency ablation or percutaneous ethanol injection, transcatheter arterial chemoembolization (TACE), and an eventual referral for LT, or a combination of these treatments; the treatment was decided based on the consensus of the liver cancer committee composed of representatives from the liver surgery, oncology, hepatology, radiology, and interventional radiology departments. Treatment options mainly depend on tumor characteristics and the patient’s physical condition, and hepatic resection is always considered a preferred treatment. In particular, a patient with unresectable HCC and willing to undergo transplantation was evaluated for LT, but patients with imaging evidences of extrahepatic metastasis or vascular invasion were considered unsuitable for LT. HCC patients were listed in the waitlist only if they met the proposed Milan criteria1: single tumor ≤5 cm or up to three tumors, none >3 cm before 2002. They also needed to meet the expanded criteria from UCSF5: single tumor ≤6.5 cm, or ≤three tumors, none >3 cm with a total tumor diameter of ≤8 cm after 2002. TACE and local ablation were arranged to prevent tumor progression for patients who were potential transplantation candidates with HCC beyond the transplantation criteria; downstage patients awaiting a deceased donor LT; patients with different reasons for being unable to undergo LT within 3 months and for whom we anticipated a longer waiting time; etc.
The strategy for the treatment of recurrent HCC after LT was the same as that for primary HCC, depending on the consensus of the liver cancer committee.
2.3. Variables and clinicopathological features
Variables including demographics, laboratory data, transplantation features, and tumor characteristics were analyzed to determine the prognostic factors. Tumor characteristics were determined by the histopathological examination of the thin-sliced specimens (at 5 mm intervals) of the explanted liver.
2.4. Transplantation and follow-up
All LTs were performed using standard techniques without venovenous bypass. Postoperative immunosuppression consisted of two protocols: methylprednisolone plus cyclosporine for recipients who underwent transplantation before the introduction of tacrolimus, and a combination of three drugs including methylprednisolone, tacrolimus, and mycophenolate mofetil for patients who underwent transplantation after the introduction of tacrolimus. However, all immunosuppression procedures were switched to the latter protocol since 2001. Of these three drugs, the dose of methylprednisolone was tapered and discontinued by the third postoperative month in the majority of the recipients, while tacrolimus and mycophenolate mofetil were continued for the maintenance of immunosuppression. Serum levels of tacrolimus were kept at 5 ng/mL to 10 ng/mL as much as possible. Patients were aggressively followed up for tumor recurrence by AFP measurements and liver ultrasonography at monthly intervals in the initial 3 months and every 3 months thereafter. CT and/or MRI were performed annually or whenever HCC recurrence was suspected.
2.5. Statistical analysis
All the data were analyzed using the statistical software SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) and Prism version 5.0 (GraphPad Software, San Diego, CA, USA) for Windows. The outcome measurements included Recurrence-Free Survival (RFS) and overall survival. RFS was calculated from the date of LT to the date of HCC recurrence. Overall survival was measured from the date of LT to the date of death. Analyses of the variables were performed using the Cox proportional hazards regression model in order to identify the factors that influence RFS, and all the prognostic factors with a p value of <0.1 from univariate analysis were then selected for multivariate analysis in a forward stepwise manner. Cut-off values for the tumor factor in the statistical analysis were defined according to previous reports or transplantation criteria of Milan and UCSF. Differences in clinicopathological features among the groups were assessed using Chi-square or Fisher’s exact test. Survival rates were calculated by the Kaplan-Meier method, and the curves were compared by the log rank test. A p-value of <0.05 was defined as statistically significant.
3. Results
3.1. Clinicopathological characteristics affecting HCC recurrence
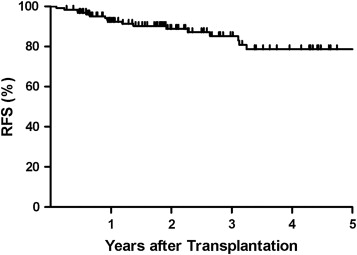
Overall, 17 of 126 patients (13.5%) had HCC recurrence following LT. The median time for HCC recurrence was 11.3 months, with a range of 1.1–38.9 months. RFS rates were 92.3%, 85.2%, and 78.6% at 1, 3, and 5 years, respectively (Fig. 1). For all the patients, the mean follow-up period was 35.2 months. The clinicopathological features of the patients with and without HCC recurrence after LT are listed and compared in Table 1. Meeting or not meeting the Milan and UCSF transplantation criteria were significant differences between the two groups.
|
|
|
Figure 1. Cumulative Recurrence-Free Survival (RFS) curve of HCC patients after LT. The 1-, 3-, and 5-year RFS rates were 92.3%, 85.2%, and 78.6%, respectively (n = 126). |
| Characteristics | HCC recurrence | p value | |
|---|---|---|---|
| Yes (n = 17) | No (n = 109) | ||
| Age (years), mean (range) | 48.9 (32–63) | 53.7 (33–68) | 0.029 |
| Sex (Male:Female) | 15:2 | 82:27 | 0.381 |
| Hepatitis status | |||
| Hepatitis B positive | 13 (76.5%) | 73 (67.0%) | 0.297 |
| Hepatitis C positive | 2 (11.7%) | 29 (26.6%) | |
| Hepatitis B and C positive | 1 (5.9%) | 5 (4.6%) | |
| None | 1 (5.9%) | 2 (1.8% ) | |
| Child-Pugh Class | |||
| A | 7 (41.2%) | 33 (30.3%) | 0.566 |
| B | 5 (29.4%) | 43 (39.4%) | |
| C | 5 (29.4%) | 33 (30.3%) | |
| MELD score, median (range) | 16 (8–41) | 15 (7–40) | 0.378 |
| AFP (ng/ml), median (range) | 54 (3–18250) | 14 (2–1360) | 0.081 |
| Liver transplantation type | |||
| DDLT | 3 (17.6%) | 33 (30.3%) | 0.433 |
| LDLT | 14 (82.4%) | 76 (69.7%) | |
| Meeting Milan criteria | 6 (35.3%) | 87 (79.8%) | <0.001 |
| Meeting UCSF criteria | 9 (52.9%) | 96 (88.1%) | 0.001 |
HCC: hepatocellular carcinoma; AFP: alpha-fetoprotein; MELD: model for end-stage liver disease; DDLT: deceased donor liver transplantation; LDLT: living donor liver transplantation; UCSF: University of California, San Francisco.
Univariate analysis identified the following 11 factors: age, AFP, preoperative locoregional therapy, tumor number, maximum tumor size, sum of tumor sizes, histological grade, presence of satellite nodules, presence of tumor capsules, presence of microvascular invasion, and mean tumor necrosis with a p value of <0.1. Subsequently, multivariate regression analysis of these 11 factors showed that tumor number > three [p = 0.024, hazard ratio (HR) = 3.249] and presence of microvascular invasion (p = 0.003, HR = 4.336) were independent prognostic factors of HCC recurrence ( Table 2).
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| p value | HR | 95% CI | p value (HR, 95% CI) | |
| Age (<55 versus ≥55 years) | 0.093 | 0.397 | 0.152–1.035 | NS |
| Sex (male versus female) | 0.558 | 0.649 | 0.183–2.301 | NS |
| Hepatitis B virus (positive versus negative) | 0.525 | 1.492 | 0.492–4.526 | NS |
| Hepatitis C virus (positive versus negative) | 0.358 | 0.562 | 0.194–1.629 | NS |
| Child Class (A, B, C) | 0.556 | — | — | NS |
| AFP (≥40 versus <40 ng/ml) | 0.021 | 2.904 | 1.006–8.379 | NS |
| MELD score (≥20 versus <20) | 0.569 | 1.351 | 0.440–4.141 | NS |
| Type of liver transplantation (DDLT versus LDLT) | 0.157 | 2.378 | 0.870–6.500 | NS |
| Preoperative locoregional therapy (>3 versus ≤3 months) | 0.062 | 0.417 | 0.143–1.216 | NS |
| GRWR (≥0.8% versus <0.8%) | 0.817 | 1.188 | 0.298–4.741 | NS |
| Tumor number (>3 versus ≤3) | 0.005 | 7.680 | 1.839–32.082 | 0.024 (3.249, 1.166-9.050) |
| Maximum tumor size (>3 versus ≤3 cm) | 0.077 | 2.328 | 0.754–7.193 | NS |
| Sum of tumor size (>8 versus ≤8 cm) | 0.059 | 2.807 | 0.570–13.812 | NS |
| Histology grade (I/II versus III/IV) | 0.026 | 0.284 | 0.093–0.865 | NS |
| Presence of satellite nodule (yes versus no) | 0.073 | 2.671 | 0.560–12.738 | NS |
| Presence of tumor capsule (yes versus no) | 0.098 | 0.416 | 0.147–1.179 | NS |
| Presence of microvascular invasion (yes versus no) | <0.001 | 4.767 | 1.293–17.600 | 0.003 (4.336, 1.649-11.403) |
| Mean tumor necrosis (≥60% versus <60%) | 0.007 | 0.171 | 0.065–0.445 | NS |
HCC: hepatocellular carcinoma; AFP: alpha-fetoprotein; DDLT: deceased donor liver transplantation; LDLT: living donor liver transplantation; MELD: model for end-stage liver disease; HR: hazard ratio; CI: confidence interval; GRWR; graft recipient weight ratio; NS: no significance.
3.2. Patients with HCC recurrence after liver transplantation
The clinical characteristics of 17 patients with HCC recurrence following LT are summarized in Table 3. There were 15 men and 2 women; their ages ranged from 32 to 63 years. Three patients received liver grafts from deceased donors; the others, from living donors. Two patients with intrahepatic recurrence were successfully treated by TACE, and no more recurrent HCCs were detected. Six patients who had intrahepatic recurrence followed by extrahepatic metastasis or synchronous intrahepatic and extrahepatic recurrence were managed by locoregional therapy for intrahepatic lesions along with systemic chemotherapy. The remaining nine patients were found to have extrahepatic metastasis at the time when HCC recurrence was detected, without intrahepatic HCC. Of these, four patients (patient number 6, 10, 12, and 16) had undergone surgical resection for isolated solitary extrahepatic metastases, and two of them (patient number 10 and 12) were alive and without any detectable recurrent tumor at the end of this study. Unfortunately, one patient (patient number 6) had systemic spreading including liver and bony metastasis 6 months after resection of intra-abdominal metastatic HCC. However, another patient (patient number 16) had undergone two surgical resections for solitary lung metastasis at intervals of 4.5 months, and no more recurrence was detectable at the last follow-up. In addition to surgical resection, there was no systemic adjuvant therapy added in these four patients. A total of 15 out of 17 (88%) patients exhibited extrahepatic metastasis during the follow-up examination.
| Patient No. | Age/sex | Tumor character | Recurrent location and management | LT to HCC recurrence (months) | Follow-up after HCC recurrence (months) | Outcome | |
|---|---|---|---|---|---|---|---|
| Number | Microvascular invasion | ||||||
| 1 | 48/M | 4 | (−) | Brain, Lung | 7.8 | 0.7 | Dead |
| 2 | 53/M | 2 | (+) | Liver (TACE), Lung (systemic C/T) | 31.7 | 27.2 | Alive |
| 3 | 63/M | 7 | (−) | Liver (TACE) | 37.3 | 18.7 | Alive |
| 4 | 38/M | 1 | (+) | Liver (TACE) | 27.3 | 16.5 | Alive |
| 5 | 32/M | 1 | (+) | Liver (TACE), Lung (systemic C/T) | 5.3 | 12.7 | Dead |
| 6 | 54/M | 9 | (−) | Intra-abdominal mass (resection), liver and bone. | 39 | 12.2 | Alive |
| 7 | 34/M | 3 | (+) | Liver (TACE), Bone | 1.2 | 21.1 | Dead |
| 8 | 48/M | 1 | (+) | Liver (RFA), Lung (systemic C/T) | 16.4 | 18.3 | Dead |
| 9 | 55/M | 1 | (−) | Bone and Lung (systemic C/T) | 5.4 | 3 | Dead |
| 10 | 51/M | 7 | (−) | Abdominal wall (resection) | 7.0 | 49 | Alive |
| 11 | 41/M | 7 | (+) | Intra-abdominal mass (resection) | 2.7 | 0.7 | Dead |
| 12 | 45/M | 5 | (−) | Lung (resection) | 11.3 | 40 | Alive |
| 13 | 53/M | 3 | (−) | Liver (TACE), Lung (systemic C/T) | 11.2 | 8.6 | Dead |
| 14 | 48/M | 1 | (−) | Bone (radiotherapy), Lung (systemic C/T) | 37.5 | 6.1 | Dead |
| 15 | 57/F | 2 | (−) | Carcinomatosis | 23.0 | 1.2 | Alive |
| 16 | 51/M | 3 | (+) | Lung (resection, twice) | 14.3 | 13.3 | Alive |
| 17 | 60/F | 1 | (+) | Liver (TACE), adrenal gland, lung | 10.9 | 3.7 | Dead |
LT: liver transplantation; TACE: transcatheter aterial chemoembolization; HCC: hepatocellular carcinoma; C/T: chemotherapy; RFA: radiofrequency ablation.
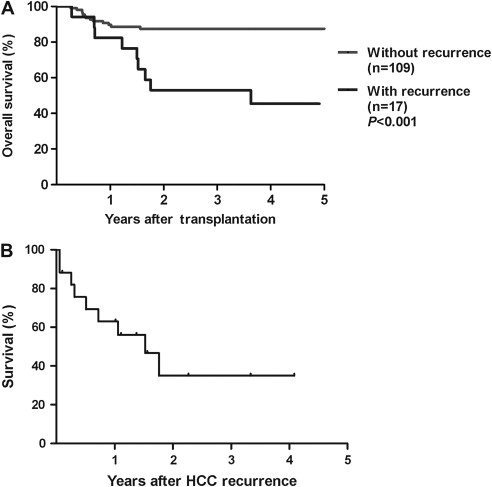
However, the prognosis of patients with HCC recurrence following LT remained very poor. The groups of patients with and without HCC recurrence were compared and it was found that HCC recurrence significantly affects the outcome of patients undergoing LT (Fig. 2A, p < 0.001). The analysis of survival curves showed that the overall survival rates at 3 and 5 years were 52.9% and 45.4% in the group of patients with HCC recurrence, while the corresponding values were 87.5% and 87.5% in the group of patients without HCC recurrence. After HCC recurrence, the median length of patient survival was 18.3 months, and the cumulative survival rates at 1 and 3 years were 63% and 35%, respectively ( Fig. 2B).
|
|
|
Figure 2. (A) Cumulative overall survival curves of patients according to the presence or absence of HCC recurrence, and patients with HCC recurrence after LT had significantly poor survival (p < 0.001). (B) The cumulative survival curve of patients after HCC recurrence. The median length of survival was 18.3 months. The 1- and 3-year survival rates were 63% and 35%, respectively (n = 17). |
4. Discussion
LT offers the best chance to restore impaired liver function and clear malignancy, and it has been considered an ideal treatment modality for patients with severe liver cirrhosis complicated by HCC.1; 4; 5 ; 13 However, like patients who have undergone surgical resection for malignancy, patients with HCC who undergo LT live with a fear of tumor recurrence. For decades, cancer research has continued to clarify prognostic factors and develop better treatment strategies for cancer patients. Although prevention of HCC recurrence through careful selection of patients is the best strategy to improve the outcome of HCC patients undergoing LT, it is also very important to treat patients with HCC recurrence following LT.
Many previous studies have reported several prognostic factors capable of predicting the outcome of HCC patients undergoing LT,7; 14; 15; 16 ; 17 and similar factors were also noted in our study. Although univariate analysis identified 11 factors for RFS, only tumor number and microvascular invasion were shown to be independent prognostic factors. However, the presence of microvascular invasion can only be accurately assessed in the explanted liver, and pretransplantation tumor biopsy is usually not suitable for all patients considering the patient’s clinical status and risk of tumor seeding. Albeit low, it is estimated that approximately 2% of patients experience tumor seeding following diagnostic biopsy,18 ; 19 and liver biopsy is usually more complicated in patients with severe liver cirrhosis. Additionally, multiple tumors have significant histological heterogeneity, and it is not practical to perform biopsy on each liver tumor even if liver biopsy is proposed for each of the suspected lesions.20 ; 21 However, numerous studies have reported that greater tumor burden and elevation of AFP level is associated with a greater chance of microvascular invasion,7; 22; 23 ; 24 indicating that tumor burden remains a key factor affecting the outcome of HCC patients undergoing LT.
According to our data, an accurate diagnosis of the tumor stage, in terms of number and size, at pretransplantation evaluation is very crucial and a better way to predict patient outcome. Nonetheless, patients with HCC are frequently accompanied by liver cirrhosis as well as associated with numerous regeneration nodules, making accurate diagnosis of HCC even more challenging, and understaging can be an issue encountered in patients previously treated with locoregional therapy. Additionally, all imaging modalities appear to be suboptimal for detecting small HCCs in a cirrhotic liver.25 Therefore, pretransplantation determination of tumor number by an imaging study, at times, does not correlate with the pathological tumor number of an explanted liver, indicating that a radiologically determined tumor number might be unable to reflect the real outcome in patients. In our patient group, pathological staging had shown that 21 out of 126 patients (16%) were transplanted beyond UCSF criteria. The discrepancy of clinical and pathologic stage might be related to the fact that most patients had received either pretransplantation bridging or downstaging therapy. Therefore, dedicated multimodality imaging examinations including MRI, dynamic liver CT scan, and hepatic angiography are helpful to evaluate viable tumor burden in such patients prior to LT.
As shown by our results and other reports,14 ; 26 HCC recurrence after LT seems to have a tendency to manifest as extrahepatic metastasis or rampantly cause a systemic spread after recurrence. With 88% of patients with recurrent HCC developing extrahepatic metastasis, the incidence is much higher than that estimated with up to 42% of patients experiencing extrahepatic metastatic HCC following liver resection.27 This incidence may be related to the patient’s immunosuppressed status after LT, in which the patient’s immunity is unable to stop tumor progression. Additionally, a study has also shown that overexposure to immunosuppressants increases the risk of HCC recurrence after LT,28 and recommended that tacrolimus should not exceed 10 ng/mL of serum level. Although the limited number of current study was unable to clarify the relationship of immunosuppressant and HCC recurrence, we usually kept the serum level of tacrolimus between 5 ng/mL and 10 ng/mL as much as possible. This level is not only for preventing HCC recurrence but also considering that the patient’s liver was relatively resistant to immunological rejection as well as the low amount of immunosuppressants required.
Similarly, early tumor recurrence occurred in patients undergoing LT for HCC as seen in liver resection for patients with HCC; there is currently no effective way to prevent this situation. It might be argued that inadequate pretransplantation assessment was performed, but it is possible that undetectable small tumor cells already systemically traveled before LT. Additionally, the procedure of total hepatectomy could also result in tumor dissemination during the course of liver mobilization. Importantly, unexplored factors other than pretransplantation evaluation and surgical factors may play a principal role leading to early HCC recurrence after LT, and further study in more patients may be able to clarify this issue in the future.
Interestingly, the locations of recurrent HCC were predominantly extrahepatic. A hypothetical explanation of this phenomenon could be that the immunity offered by the liver graft could work against the growth of host HCC within the liver, directing the HCC to spread systemically. This hypothesis, however, needs to be proven by a more detailed scientific study. Once the recurrent HCC becomes systemic, a poor prognosis is predictable, as shown by our study. However, the aggressive approach with multimodal treatment of the primary or recurrent HCC has greatly improved the overall outcome of patients with HCC.29 ; 30 The three patients who underwent surgical resection for extrahepatic metastatic HCC were alive and remained cancer-free until the end of this study, indicating that aggressive management can provide a good outcome for patients with HCC recurrence after LT.
In summary, we have identified two perioperative prognostic factors of tumor recurrence in HCC patients undergoing LT, and tumor number is the only factor that can be assessed preoperatively. Thus, accurate evaluation of tumor status before LT by radiological examination is essential. Although the outcome of patients with HCC recurrence after LT remains very poor because of a tendency of HCC to recur as extrahepatic metastasis, there is evidence proving that an aggressive procedure like surgical resection of extrahepatic metastasis can be beneficial in selected patients.
References
- 1 V. Mazzaferro, E. Regalia, R. Doci, et al.; Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis; N Engl J Med, 334 (1996), pp. 693–699
- 2 S. Iwatsuki, R.D. Gordon, B.W. Shaw Jr., T.E. Starzl; Role of liver transplantation in cancer therapy; Ann Surg, 202 (1985), pp. 401–407
- 3 R. Selby, Z. Kadry, B. Carr, A. Tzakis, J.R. Madariaga, S. Iwatsuki; Liver transplantation for hepatocellular carcinoma; World J Surg, 19 (1995), pp. 53–58
- 4 V. Mazzaferro, J.M. Llovet, R. Miceli, et al.; Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis; Lancet Oncol, 10 (2009), pp. 35–43
- 5 F.Y. Yao, L. Ferrell, N.M. Bass, et al.; Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival; Hepatology, 33 (2001), pp. 1394–1403
- 6 F. D’Amico, M. Schwartz, A. Vitale, et al.; Predicting recurrence after liver transplantation in patients with hepatocellular carcinoma exceeding the up-to-seven criteria; Liver Transpl, 15 (2009), pp. 1278–1287
- 7 S. Jonas, W.O. Bechstein, T. Steinmuller, et al.; Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis; Hepatology, 33 (2001), pp. 1080–1086
- 8 V. Mazzaferro, Y.S. Chun, R.T. Poon, et al.; Liver transplantation for hepatocellular carcinoma; Ann Surg Oncol, 15 (2008), pp. 1001–1007
- 9 M.E. Schwartz, F. D’Amico, A. Vitale, S. Emre, U. Cillo; Liver transplantation for hepatocellular carcinoma: are the Milan criteria still valid?; Eur J Surg Oncol, 34 (2008), pp. 256–262
- 10 M. Ravaioli, G. Ercolani, M. Cescon, et al.; Liver transplantation for hepatocellular carcinoma: further considerations on selection criteria; Liver Transpl, 10 (2004), pp. 1195–1202
- 11 J. Bruix, M. Sherman, J.M. Llovet, et al.; Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver; J Hepatol, 35 (2001), pp. 421–430
- 12 J. Bruix, M. Sherman; Management of hepatocellular carcinoma; Hepatology, 42 (2005), pp. 1208–1236
- 13 K.S. Mak, K.C. Tan; Liver transplantation for hepatocellular carcinoma: an Asian perspective; Asian J Surg, 25 (2002), pp. 271–276
- 14 S. Roayaie, J.D. Schwartz, M.W. Sung, et al.; Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis; Liver Transpl, 10 (2004), pp. 534–540
- 15 S. Tamura, T. Kato, M. Berho, et al.; Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation; Arch Surg, 136 (2001), pp. 25–30
- 16 R.A. Fisher, L.M. Kulik, C.E. Freise, et al.; Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation; Am J Transplant, 7 (2007), pp. 1601–1608
- 17 J.N. Vauthey, J.A. Ajani; Liver transplantation and hepatocellular carcinoma biology: beginning of the end of the era of educated guesses; J Clin Oncol, 21 (2003), pp. 4265–4267
- 18 S. Chang, S.H. Kim, H.K. Lim, W.J. Lee, D. Choi, J.H. Lim; Needle tract implantation after sonographically guided percutaneous biopsy of hepatocellular carcinoma: evaluation of doubling time, frequency, and features on CT; AJR Am J Roentgenol, 185 (2005), pp. 400–405
- 19 R. Stigliano, L. Marelli, D. Yu, N. Davies, D. Patch, A.K. Burroughs; Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC; Cancer Treat Rev, 33 (2007), pp. 437–447
- 20 J.W. Marsh, I. Dvorchik; Should we biopsy each liver mass suspicious for hepatocellular carcinoma before liver transplantation?–yes; J Hepatol, 43 (2005), pp. 558–562
- 21 F.Q. An, M. Matsuda, H. Fujii, et al.; Tumor heterogeneity in small hepatocellular carcinoma: analysis of tumor cell proliferation, expression and mutation of p53 AND beta-catenin; Int J Cancer, 93 (2001), pp. 468–474
- 22 E. Adachi, T. Maeda, K. Kajiyama, et al.; Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments; Cancer, 77 (1996), pp. 2022–2031
- 23 S. Sumie, R. Kuromatsu, K. Okuda, et al.; Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors; Ann Surg Oncol, 15 (2008), pp. 1375–1382
- 24 M. Ravaioli, G.L. Grazi, F. Piscaglia, et al.; Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria; Am J Transplant, 8 (2008), pp. 2547–2557
- 25 B. Taouli, G.A. Krinsky; Diagnostic imaging of hepatocellular carcinoma in patients with cirrhosis before liver transplantation; Liver Transpl, 12 (2006), pp. S1–S7
- 26 W.Y. Shin, K.S. Suh, H.W. Lee, et al.; Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma; Liver Transpl, 16 (2010), pp. 678–684
- 27 M.S. Si, F. Amersi, S.R. Golish, et al.; Prevalence of metastases in hepatocellular carcinoma: risk factors and impact on survival; Am Surg, 69 (2003), pp. 879–885
- 28 M. Vivarelli, A. Cucchetti, B.G. La, et al.; Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence; Ann Surg, 248 (2008), pp. 857–862
- 29 K.M. Chan, W.C. Lee, C.F. Hung, M.C. Yu, Y.Y. Jan, M.F. Chen; Aggressive multimodality treatment for intra-hepatic recurrence of hepatocellular carcinoma following hepatic resection; Chang Gung Med J, 28 (2005), pp. 543–550
- 30 K.M. Chan, M.C. Yu, T.J. Wu, et al.; Efficacy of surgical resection in management of isolated extrahepatic metastases of hepatocellular carcinoma; World J Gastroenterol, 15 (2009), pp. 5481–5488
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?