Abstract
Millions of people are exposed to arsenic through their drinking water and food, but the mechanisms by which it impacts embryonic development are not well understood. Arsenic exposure during embryogenesis is associated with neurodevelopmental effects, reduced weight gain, and altered locomotor activity, and in vitro data indicates that arsenic exposure inhibits stem cell differentiation. This study investigated whether arsenic disrupted the Wnt3a signaling pathway, critical in the formation of myotubes and neurons, during the differentiation in P19 mouse embryonic stem cells. Cells were exposed to 0, 0.1, or 0.5 μM arsenite, with or without exogenous Wnt3a, for up to 9 days of differentiation. Arsenic exposure alone inhibits the differentiation of stem cells into neurons and skeletal myotubes, and reduces the expression of both β-catenin and GSK3β mRNA to ∼55% of control levels. Co-culture of the arsenic-exposed cells with exogenous Wnt3a rescues the morphological phenotype, but does not alter transcript, protein, or phosphorylation status of GSK3β or β-catenin. However, arsenic exposure maintains high levels of Hes5 and decreases the expression of MASH1 by 2.2-fold, which are anti- and pro-myogenic and neurogenic genes, respectively, in the Notch signaling pathway. While rescue with exogenous Wnt3a reduced Hes5 levels, MASH1 levels stay repressed. Thus, while Wnt3a can partially rescue the inhibition of differentiation from arsenic, it does so by also modulating Notch target genes rather than only working through the canonical Wnt signaling pathway. These results indicate that arsenic alters the interplay between multiple signaling pathways, leading to reduced stem cell differentiation.
Keywords
Arsenic ; Stem cell ; Differentiation ; P19 ; Sensory neuron ; Skeletal myotube ; Wnt3a ; MASH1
1. Introduction
Arsenic is a contaminant in water systems around the world [37] and [58] . Although the U.S. EPA and the W.H.O. have set a limit of 10 ppb arsenic, millions of people drink water with higher arsenic levels. In addition, arsenic has also been found at high levels in some foods, such as rice [11] , [28] and [41] . Arsenic readily crosses the placental barrier [10] , [24] and [72] , and in utero exposure to arsenic has been linked to an increased incidence of stillbirths, preterm births, and miscarriages starting at drinking water concentrations of ∼40 ppb arsenic [7] , [19] , [35] , [51] and [65] .
Human epidemiological studies show that newborn weight and weight gain in early childhood is reduced after arsenic exposure. For example, drinking water containing 40ppb arsenic has been associated with an average 57 g reduction in birth weight [19] , while maternal blood levels >5.3 ppb arsenic are associated with a 220 g reduction in birth weight [16] . The mechanisms behind the loss of weight are not fully known. But, studies indicate that arsenic can reduce myoblast differentiation into myotubes by decreasing myogenin, the transcription factor important in muscle cell differentiation [59] and [73] . Embryonic arsenite exposure can also alter muscle fiber subtype [15] .
Additionally, in utero and in vitro arsenic exposure is linked to impacts on the developing nervous system. For example, arsenic exposure is correlated with reductions in neuronal cell migration and maturation at concentrations of 1–4 μM in embryonic primary rat neuroepithelial cells [55] , at concentrations of 5–10 μM arsenic in PC12 and Neuro2a cells [14] and [68] , and in postnatal day 11 pups whose mothers were injected twice with 1 or 2 mg/kg arsenite during pregnancy [12] . Arsenic exposure is also correlated with reduced intellectual function in children at mean drinking water levels ranging between 50 and 185 ppb [52] , [63] , [66] , [69] and [70] . These studies collectively suggest that arsenic can disrupt muscle and neuronal development. Indeed, in embryonic stem cells, 0.5 μM arsenite reduces the expression and localization of transcription factors such as Myf5, MyoD, myogenin, NeuroD, and neurogenins, all of which are needed to differentiate stem cells into skeletal myotubes and sensory neurons [18] . Therefore, signaling pathways upstream of neurons and skeletal myocytes are potential targets of arsenic.
The canonical Wnt/β-catenin signaling pathway is one such pathway that is important during embryogenesis. In the absence of Wnt ligand signaling, the co-transcription factor β-catenin is ubiquitinated and degraded in the proteasome after phosphorylation by casein kinase 1 (CK1α) and glycogen synthase kinase 3 β (GSK3β). These proteins are held together by the scaffolding protein Axin and the adenomatous polyposis coli (APC) protein. When a Wnt ligand is present and bound to the cell surface proteins Frizzled (Fz) and low-density lipoprotein receptor related protein 6 (LRP6), Axin is recruited to the plasma membrane, which inhibits β-catenin’s phosphorylation, and leads to its nuclear translocation. β-catenin binds with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors to activate downstream target genes (reviewed in Ref. [31] ).
Wnt signaling molecules, such as Wnt1, Wnt3, Wnt3a, and Wnt5 [23] , [26] and [33] , play a critical role in the development of muscle and neurons. Wnt3a is specifically important for both paraxial mesoderm and neural tube development [23] , [48] , [60] and [74] . For example, the Wnt/β-catenin pathway induces early mesodermal markers such as Brachyury and FoxA2 [62] and activates Myf5 in somites, which is one of the early transcription factors needed for skeletal muscle development [1] and [5] . In human embryonic stem cells, Wnt3a promotes the commitment to myogenic cells [22] . Wnt3a is also needed for neurogenesis, specifically in the growth of the neural tube [40] and in neural crest cell specification [13] , which are needed for sensory neuron formation. In vitro , the addition of Wnt3a-conditioned medium to P19 mouse embryonic stem cells increased the expression of βIII-tubulin, a specific marker of neuronal cells [36] .
Our previous study demonstrated that arsenic exposure inhibited the ability of P19 cells to differentiate into skeletal myotubes and sensory neurons. We hypothesized that a potential mechanism for the reduced differentiation was via the Wnt/β-catenin pathway, since β-catenin protein expression was decreased [18] . Indeed others have shown that arsenic can alter proteins within the Wnt signaling pathway. For example, after an in utero exposure to arsenic, the lungs of embryonic day 18 rats had reductions in both β-catenin and GSK3β transcripts [49] , while arsenic exposure to SH-SY5Y neuroblastoma cells decreases GSK3β activity [71] .
The current study shows that exogenous Wnt3a can effectively rescue the arsenic-mediated inhibition of P19 stem cell differentiation into skeletal myotubes, but not sensory neurons. Although arsenic exposure reduces the expression of β-catenin and GSK3β transcripts, these are not rescued by exogenous Wnt3a. Arsenic exposure reduces levels of the pro-neurogenic transcript Mash1 while maintaining high levels of Hes5. However, the addition of Wnt3a reduces Hes5 levels, and helps to rescue the inhibition of cellular differentiation caused by arsenic. This study underscores the complexity of arsenic-induced changes in cellular differentiation and highlights the cross-talk between the Wnt and Notch signaling pathways.
2. Materials and methods
2.1. P19 cell culture and differentiation
P19 mouse embryonic stem cells (ATCC, Manasass, VA) were cultured in α-MEM containing 7.5% bovine calf serum (Hyclone, Logan, UT), 2.5% fetal bovine serum (Mediatech, Manassas, VA), and 1% l -glutamine (Hyclone) in an incubator at 37 °C with 5% CO2 . Cells were subcultured every 2 days. For differentiation experiments, 1% DMSO was added to the medium, which is a typical compound used with these cells to form skeletal muscle cells [57] . Cells were aggregated for 2 days in hanging drops (500 cells/20 μL drop) [67] with 0, 0.1, or 0.5 μM arsenic as sodium arsenite (Sigma, St. Louis, MO). We have previously shown that these arsenic concentrations do not affect cell viability, but do reduce their ability to differentiate into sensory neurons and skeletal myotubes [18] .
After 2 days, each drop was transferred to an individual well in a 96-well ultralow attachment plate for an additional 3 days (day 5) to form a mature embryoid body. To further differentiate the cells, they were transferred to 48 well plates coated with 0.1% gelatin for up to 4 additional days (day 9). The medium was renewed every 2 days until the cells were harvested, and the arsenic exposures continued throughout the differentiation process. When day 5 and 9 cells were harvested, all the wells from each 96-well plate were combined into one replicate (n = 3 plates per arsenic concentration). Cells were harvested in TRI Reagent (Sigma, St. Louis, MO) and stored at −80 °C.
2.2. Exosome isolation
Cell culture medium was also collected from day 5 embryoid bodies to collect exosomes. Briefly, medium was passed through a 0.2 μm filter and incubated with ExoQuick-TC isolation reagent overnight at 4 °C (Systems Biosciences, Mountain View, CA). Exosomes were isolated by centrifugation per manufactuer’s instructions and lysed in RIPA buffer with protease and phosphtase inhibitors. Protein concentrations were quantified using Bio-Rad’s DC protein assay kit.
2.3. L-cell and wnt3a conditioned medium collection
L-Wnt-3A cells and L-cells (ATCC) were cultured in DMEM containing 10% FBS and 1% l -glutamine. To produce conditioned medium, cells were split 1:10 and cultured for 4 days. The medium was removed and another 10 mL fresh culture medium added for an additional 3 days. The two sets of conditioned medium were combined, filtered, and stored at 4 °C. Once enough medium was obtained, it was filtered through Amicon Pro 30 kD Ultra filtration system (EMD Millipore, Billerica, MA) to concentrate the proteins. The conditioned concentrate was resuspended to its original volume in P19 cell differentiation medium and stored at −20 °C until use.
2.4. Exposure of P19 cells to L- or Wnt3a-conditioned medium
P19 cells were aggregated into embryoid bodies and differentiated into sensory neurons and skeletal myotubes as described above in either L-cell or Wnt3a conditioned medium. Concomitantly, they were exposed to sodium arsenite at 0, 0.1, or 0.5 μM. Embryoid bodies or differentiated cells were collected after 5 or 9 days in either TRI Reagent (Sigma) for qPCR experiments or in RIPA buffer (Pierce Biotechnology, Rockford, IL) containing both phosphatase and protease inhibitors (Pierce) for immunoblotting and immunoprecipitation experiments.
2.5. qPCR
Total RNA was extracted using TRI Reagent, quantitated by spectrophotometry, and cDNA (2 μg) was prepared by reverse transcription. The expression of Wnt3a, Wnt8a, LRP5, glycogen synthase kinase 3β (GSK3β), CK1α, β-catenin, APC, Axin, PTEN, lymphoid enhancer-binding factor 1 (LEF1), Dkk1, Dkk4, Myogenin, Neurogenin1, Hes1, and Hes5 was examined by qPCR. Briefly, 40 ng cDNA were incubated with SYBR Green (Qiagen, Alameda, CA) and gene specific primers (Table S1) to examine levels of transcripts. All samples were run in triplicate. A standard curve (10−3 ng–10−7 ng) was constructed for each gene, and expression in each sample was normalized against GADPH as the housekeeper.
2.6. Immunoblotting
Cells were lysed in RIPA buffer containing protease and phosphatase inhibitors. The cell suspension was centrifuged at 15,000g for 15 min to remove debris, and protein levels in the supernatant quantified with BioRad’s DC protein assay. Proteins (5–10 μg) were electrophoresed and transferred per standard methods, prior to blocking in 5% milk/TBST. All primary antibodies were diluted 1:1000 in TBST and were incubated overnight at 4 °C. The antibodies included anti-β-catenin (Cell Signaling no. 9562), anti-GSK3β (Cell Signaling no. 9315), anti-Wnt3a (R&D Systems no. MAB1324-050), and anti-Wnt8a (R&D Systems no. AF2248). Anti-GAPDH antibody (Genetex no. GT-239) was used as a loading control. The appropriate secondary HRP-conjugated antibody (1:2000 dilution, Santa Cruz) was incubated with the blot and proteins were detected using Luminol reagent (Santa Cruz). Band intensity was determined by densitometry.
2.7. Immunoprecipitation for phosphorylated β-catenin and GSK3β
Cell lysates (50 μg) were incubated overnight at 4 °C with either anti-β-catenin or GSK3β at a 1:100 dilution. Dynabeads Protein G (Life Technologies) were added to the suspension for 2 h at 4 °C and the specific protein immunoprecipitated. After denaturing, the proteins were electrophoresed, transferred, and blocked. Primary antibodies (anti-phospho-GSK3β (Ser9), Cell Signaling no. 9323, anti-phospho-β-catenin (Thr41/Ser45), Cell Signaling no. 9565, and anti-phospho-β-catenin (Ser33/37/Thr41, Cell Signaling no. 9561)) were diluted 1:1000 in TBST and incubated with the blot overnight at 4 °C. The anti-rabbit HRP secondary antibody (1:2000) was incubated with the blot and proteins were detected using Luminol reagent (Santa Cruz). Band intensity was determined by densitometry, with MHC used as the loading control.
2.8. Statistics
For transcript levels, the replicates (n = 3) of each exposure group were averaged together and statistical significance determined by ANOVA followed by Tukey’s (p < 0.05). Protein levels were assessed by immunoblotting followed by densitometry. These values were converted into a relative intensity for each group (n = 2–3; replicated at least twice), and statistical significance determined by ANOVA followed by Tukey’s (p < 0.05).
3. Results
3.1. Arsenic exposure during embryoid body formation reduces β-catenin transcripts
Previous research indicated that arsenic exposure reduced differentiation of stem cells into skeletal muscles and sensory neurons by reducing the expression of key transcription factors, such as myogenin and neurogenin [18] . Since β-catenin expression was also reduced, we hypothesized that arsenic targeted proteins involved in the canonical Wnt signaling pathway during early cell fate determination. Thus, transcript levels of several genes important in this pathway were examined. At day 5 of embryoid body formation, β-catenin and LEF expression were both significantly reduced (Table 1 ), while Lrp5 expression was significantly increased. Although expression of GSK3β was lower in the arsenic-exposed cells, this was not statistically significant (p = 0.1).
| Transcript name | Day 5 fold changea |
|---|---|
| LRP5 | 1.42±0.10* |
| APC | 1.15±0.18 |
| Axin | 1.08±0.09 |
| GSK3β | 0.80±0.13 |
| CK1α | 1.04±0.14 |
| β-catenin | 0.61±0.09* |
| LEF | 0.79±0.03* |
| PTEN | 1.18±0.13 |
| DKK1 | 0.90±0.18 |
| DKK4 | 1.30±0.38 |
a. Fold change is expressed as transcript number in the arsenic-exposed embryoid bodies divided by transcript number in the control embryoid bodies (n = 3; average ± S.D.).
- . p < 0.05.
3.2. Exogenous Wnt3a rescues the arsenic-induced inhibition of differentiation, but does not alter β-catenin levels
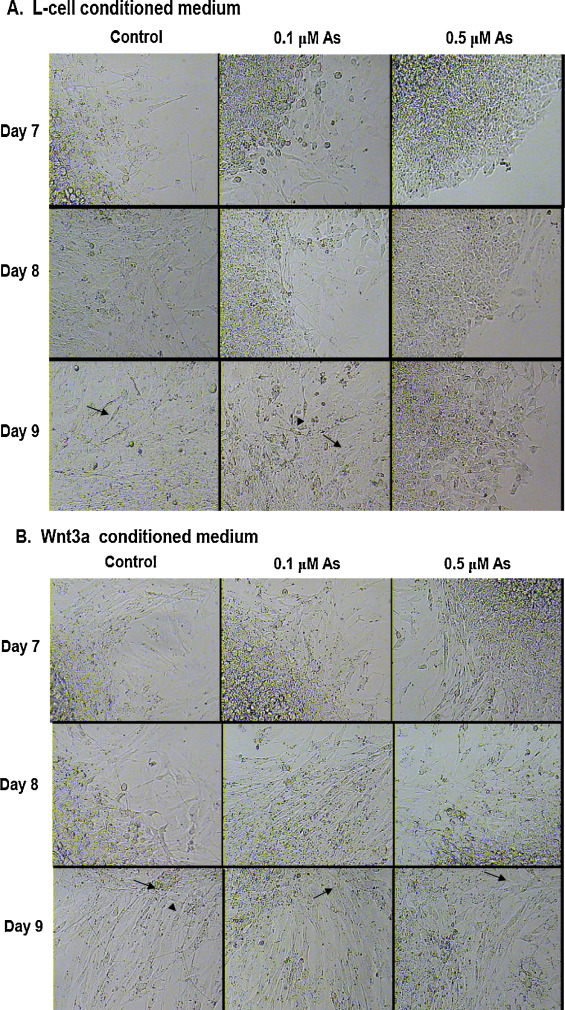
Exogenous Wnt3a was added to the cells to determine whether this protein could rescue the arsenic-mediated reduction in cell differentiation. Conditioned, filtered medium from L- cells and L/Wnt3a cells (Fig. S1) were used to co-expose cells treated with 0, 0.1, or 0.5 μM arsenic for either 5 days (embryoid body formation) or 9 days (differentiated skeletal myotubes and neurons). Arsenic reduced the formation of neurons and skeletal myocytes in a dose-responsive manner after 7–9 days of exposure in L-cell containing medium (Fig. 1 A). However, when cells were treated with Wnt3a conditioned medium, both the control and arsenic exposed cells had many more myotubes (Fig. 1 B), indicating that Wnt3a co-exposure can rescue the effects of arsenic exposure on cellular differentiation.
|
|
|
Fig. 1. Wnt3a co-exposure rescues the arsenic-induced inhibition of stem cell differentiation. P19 cells were aggregated to form embryoid bodies that were then allowed to differentiate for 7–9 days in the presence of 0, 0.1, or 0.5 μM arsenic. Cells were grown in either L-cell conditioned medium (A) or in Wnt3a-conditioned medium (B). Elongated and multinucleated myotubes are indicated by arrows and neurons with dendritic processes are indicated by arrowheads. |
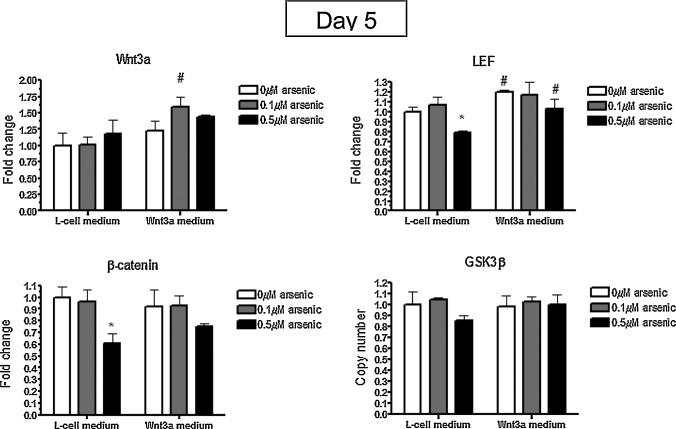
After 5 days of embryoid body formation, the transcript levels of a Wnt3a target gene, LEF, was significantly increased in the Wnt3a-conditioned medium groups over the L-cell groups (Fig. 2 ). These findings indicate that Wnt3a was available and active in the exposures. Interestingly, Wnt8a levels were reduced in the Wnt3-conditioned medium groups by 1.6- to 2-fold, but arsenic had no effect on its expression (data not shown). Similar to Table 1 , arsenic exposure significantly reduced β-catenin mRNA expression to 61% of control levels (Fig. 2 ), but this was not rescued with Wnt3a treatment.
|
|
|
Fig. 2. Arsenic reduces β-catenin transcripts in day 5 embryoid bodies. P19 cells were cultured in L-cell or Wnt3a-conditioned medium during the 5 days of embryoid body formation while being co-exposed to 0, 0.1, or 0.5 μM arsenite. The levels of LEF, β-catenin, GSK3β, and Wnt3a transcripts were quantified by qPCR. Each sample was run in triplicate (n = 3), results were normalized to GAPDH, and are expressed as the average normalized number of molecules. Statistical differences were determined by ANOVA followed by Tukey’s post hoc test (p < 0.05) to determine statistical differences between arsenic concentrations (*) and between L- and Wnt3a-conditioned medium (#). |
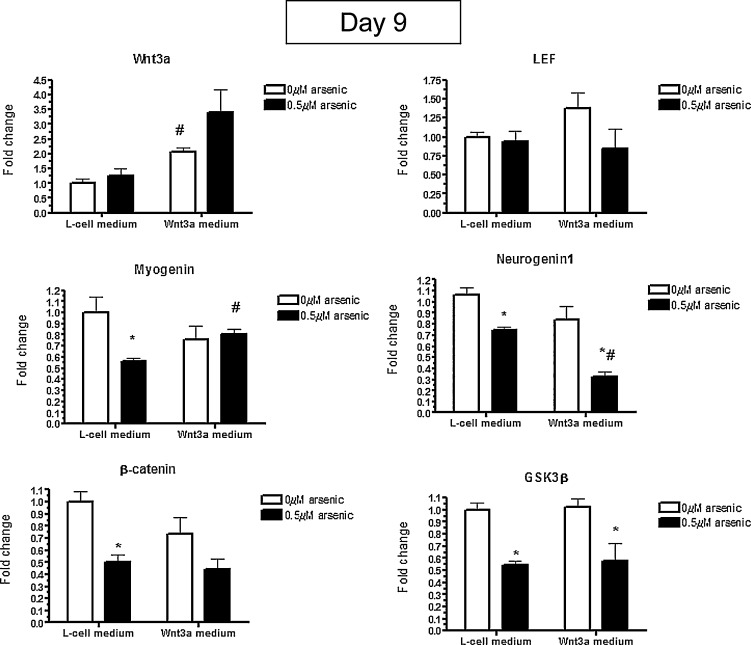
To understand the mechanisms responsible for the Wnt3a-mediated rescue, mRNA expression was examined in the day 9 differentiated cells seen in Fig. 1 . Wnt3a mRNA levels were still increased after exposure to Wnt3a-conditioned medium for 9 days, while LEF levels were not changed anymore (Fig. 3 ), unlike what was seen after 5 days of exposure. To confirm the rescue of differentiation with Wnt3a co-exposure, the levels of Myogenin and Neurogenin 1 were assessed. Myogenin levels were significantly reduced to 56% of the controls in the 0.5 μM arsenic exposed cells grown in L-cell conditioned medium, which was partially rescued with the Wnt3a exposure, going to back to 83% of the controls (Fig. 3 ). However, while Neurogenin1 was also significantly reduced in the arsenic-exposed L-cell medium, Wnt3a co-exposure further reduced Neurogenin1 levels, down to 33% of the controls (Fig. 3 ). While arsenic exposure significantly reduced the expression of both β-catenin and GSK3β mRNA to ∼55% of control levels, this was irrespective of whether the cells were cultured in L-cell or Wnt3a-conditioned medium (Fig. 3 ). The transcript data indicates that although Wnt3a rescues the muscle phenotype of arsenic-induced differentiation inhibition, it does not do so by altering transcript levels of GSK3β or β-catenin.
|
|
|
Fig. 3. Wnt3a co-exposure rescues the levels of Myogenin and Neurogenin1, but does not rescue the reduction in β-catenin and GSK3β transcripts in day 9 differentiated cells treated with arsenic. P19 cells were cultured in L-cell or Wnt3a-conditioned medium during the 9 days of differentiation while being co-exposed to 0 or 0.5 μM arsenite. The levels of Wnt3a, LEF, Myogenin, Neurogenin1, β-catenin, and GSK3β transcripts were quantified by qPCR. Each sample was run in triplicate (n = 3), results were normalized to GAPDH, and are expressed as the average normalized number of molecules. Statistical differences were determined by ANOVA followed by Tukey’s post hoc test (p < 0.05) to determine statistical differences between arsenic concentrations (*) and between L- and Wnt3a-conditioned medium (#). |
3.3. Arsenic does not alter protein levels nor the phosphorylation status of GSK3β or β-catenin
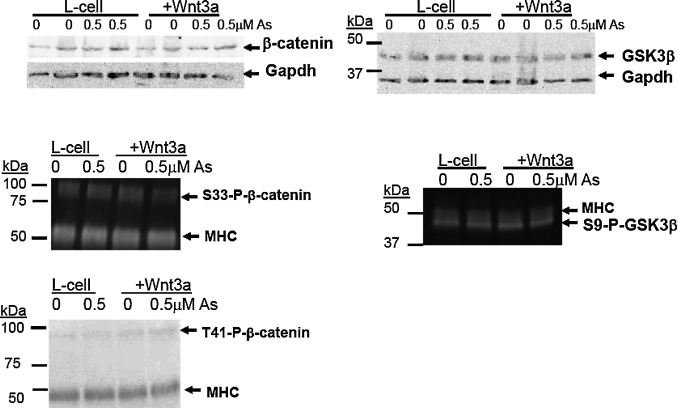
Next, protein levels and phosphorylation status of GSK3β and β-catenin were examined. In the absence of Wnt signaling, β-catenin is first phosphorylated at S45 by CK1α and then phosphorylated at S33/S37 by GSK3β, which results in its degradation [45] . There were no changes in total β-catenin protein in day 9 cells exposed to arsenic, nor were there difference between the L-cell and Wnt3a-conditioned medium treatments (Fig. 4 ). Similarly, phosphorylation of β-catenin at S33/S37, T41/S45, total GSK3β, or S9-phospho- GSK3β levels were not significantly altered by arsenic (Fig. 4 ). These results indicate that arsenic does not impact protein expression of GSK3β or β-catenin [32] , nor does it alter the phosphorylation status of either GSK3β or β-catenin.
|
|
|
Fig. 4. Arsenic does not alter protein levels of β-catenin and GSK3β in day 9 differentiated cells. P19 cells were cultured in L-cell or Wnt3a-conditioned medium during the 9 days of differentiation while being co-exposed to 0, 0.1, or 0.5 μM arsenite. The levels of β-catenin and GSK3β were determined by immunoblotting, with representative blots (n = 3) shown. To examine the phosphorylation state of β-catenin and GSK3β, immunoprecipatation was conducted using antibodies that recognize the total protein. Phospho-specific antibodies were used for immunoblotting, with representative images (n = 2) shown. Myosin heavy chain (MHC) provides a loading control. |
3.4. Neither Wnt3a nor Wnt8a levels are changed by arsenic
There are several other mechanistic possibilities for how exogenous Wnt3a could rescue the phenotype of reduced differentiation due to arsenic exposure, such as altering the noncanonical Wnt signaling pathway. The levels of Wnt3a in day 9 cells and exosomes were not changed due to arsenic exposure (Fig. S2). While there is robust expression of Wnt8a in exosomes (Fig. S2), its level is not changed by arsenic. There is little expression of Wnt3a and Wnt8a in exosome-depleted medium (data not shown). While Wnt3a is expressed in cells, its levels are unchanged by arsenic (Fig. S2). Wnt8a levels in the cells were not detectable (data not shown).
3.5. Arsenic reduces Mash1 levels, which cannot be rescued by exogenous Wnt3a
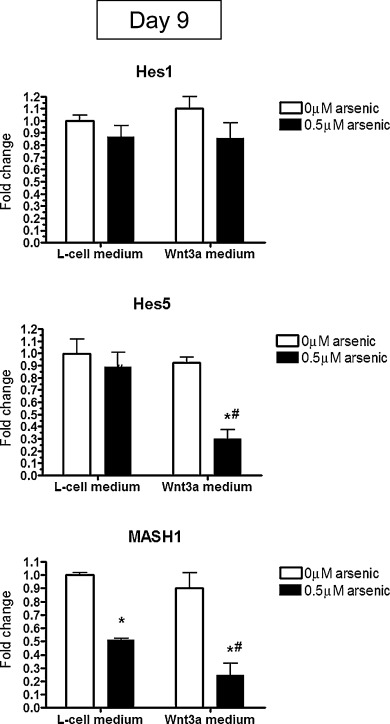
Another mechanistic possibility for the Wnt3a rescue is to alter cross-talk between multiple pathways. For example, the Wnt and Notch pathways are required to work together to regulate many aspects of cell fate determination during development [8] and [42] . We examined the levels of two target genes in the Notch signaling pathway, Hes1 and Hes5 [25] , in day 9 differentiated cells. There were no differences in Hes1 transcript levels in arsenic- and/or Wnt3a-exposed cells (Fig. 5 ). Typically, decreases in the expression of Hes5, a Notch signaling pathway target gene, lead to increases in MASH1 and therefore lead to cellular differentiation [43] . We found that arsenic exposure to day 9 differentiated cells does just the opposite, by keeping Hes5 levels high and reducing MASH1 transcript levels by 2.2-fold (Fig. 5 ). When arsenic-exposed cells are cultured in Wnt3a-containing medium, Hes5 levels are reduced in order to rescue the phenotype, but MASH1 levels still remain repressed (Fig. 5 ). Thus, while Wnt3a can partially rescue the inhibition of differentiation due to arsenic exposure, it appears to do so by modulating Notch target genes, such as Hes5, rather than working through the canonical Wnt signaling pathway.
|
|
|
Fig. 5. Changes in Hes5 mRNA expression due to arsenic exposure can be rescued by exogenous Wnt3a. mRNA was extracted from P19 cells co-exposed to Wnt3a conditioned medium and 0.5 μM arsenic for 9 days. The levels of Hes1, Hes5, and MASH1 were assessed by qPCR. Each sample was run in triplicate (n = 3), results were normalized to GAPDH, and are expressed as the average normalized number of molecules. Statistical differences were determined by ANOVA followed by Tukey’s post hoc test (p < 0.05) to determine statistical differences between arsenic concentrations (*) and between L- and Wnt3a-conditioned medium (#). |
4. Discussion
This study shows that arsenic exposure inhibits stem cell differentiation into skeletal myotubes and sensory neurons, in part, by reducing the expression of β-catenin, GSK3β, Hes5, and MASH1/Ascl1 transcripts. The cellular phenotype can effectively be rescued by exogenous Wnt3a, but interestingly, while Hes5 expression can be inhibited and myogenin expression can be increased with Wnt3a co-exposure, Wnt3a does not change the expression of MASH1, GSK3β, or β-catenin. The results highlight how chemicals, such as arsenic, can impair the interplay between the Wnt and Notch pathways in cellular differentiation.
We have previously shown that low levels of arsenic inhibit stem cell differentiation into sensory neurons and skeletal muscles, and hypothesized that these morphological changes were due to impaired Wnt signaling. Others have also shown that arsenic can impair activity or reduce transcripts of β-catenin and GSK3β [44] , [49] and [71] , which are key proteins in the Wnt3a signaling pathway. In the present study, we confirm that β-catenin transcripts are reduced due to arsenic exposure at both the early phases of embryoid body formation [18] and [38] and show that this repression continues throughout the differentiation period, up to day 9 in which sensory neurons and skeletal myotubes have formed.
Both β-catenin and GSK3β play an important role in the canonical Wnt3a signaling pathway. Indeed, Wnt3a can increase the skeletal muscle-specific transcript MyoD by targeting its distal enhancer [46] , and Wnt3a is important is sensory neuron formation during development [48] . Studies have shown that in myogenic and neurogenic precursor cells, exogenous Wnt3a enhances differentiation [2] , [6] and [21] . During the co-exposure of P19 cells, exogenous Wnt3a was able to rescue the arsenic-induced reduction of differentiation. However, neither β-catenin nor GSK3β transcript levels were not increased with the Wnt3a co-exposure at either day 5 or day 9.
Recent evidence indicates that exogenous Wnt3a increases the differentiation of stem cells by the using both canonical and noncanonical signaling pathways [56] . For example, when Wnt3a was exposed to iPS-induced neural progenitor cells, neurogenesis increased, but these effects could not be mimicked with stabilized β-catenin [21] . Similarly, neuronal differentiation of either embryonic stem cells or induced pluripotent stem cells can be blocked if Wnt3a is inhibited or silenced, but not when β-catenin signaling is inhibited [2] . Thus, β-catenin-independent Wnt signaling may be driving stem cell differentiation. Indeed, it appears that there is a switch between canonical and non-canonical Wnt signaling part way through neuronal differentiation. The earlier time points appear to work through the Wnt3a/β-catenin pathway, while in later stages of neuronal differentiation, Wnt3a/JNK signaling activates the AP-1 family of proteins [2] . This may explain why transcript levels of β-catenin are altered without a change in protein levels in day 9 differentiated cells. Other investigators have shown that arsenic can activate the ERK and JNK pathways in several different cells types [20] and [34] . The Wnt/PCP and Wnt/calcium signaling pathways do not appear to play a major role in neuronal differentiation, at least in vitro[2] .
There is also cross-talk between the canonical Wnt3a signaling pathways and others. For example, membrane-bound Notch can bind to unphosphorylated β-catenin in stem cells and prevent it from accumulating in the nucleus, a process that does not require GSK3β activity [29] . Previous studies have indicates that arsenic exposure does not alter either total or S9-phospho-GSK3β levels in day 5 embryoid bodies [32] . Our current results indicate that in day 9 differentiated cells, arsenic does not alter either total or phospho-GSK3β, nor are these levels altered by the addition of Wnt3a. While we have previously shown that β-catenin nuclear translocation is significantly reduced in arsenic-exposed day 2 and day 5 embryoid bodies [18] , this may be in part due to a switch from canonical to non-canonical pathways during the differentiation process, and may also be due to another signaling pathway.
The Notch signaling pathway plays an opposing role to Wnt3a in stem cell differentiation to neurons and myocytes [9] and [75] . To enhance differentiation, Wnt3a target proteins are kept at high levels, while Notch target transcription factors, such as Hes1 and Hes5 are reduced [25] and [43] , likely in part because β − catenin can bind to the Hes1 promoter [30] . Hes proteins work to repress neurogenesis by actively binding to N-boxes on the promoters of neurogenic transcription factors, such as the neurogenins and Ascl1/MASH1, and prevent their transcription. They can also passively inhibit myogenesis by forming heterodimers with MASH1 and prevent its binding to E-boxes on the promoters of myogenic transcription factors, such as MyoD (reviewed in Ref. [27] ).
In the central nervous system, Hes1 is expressed in the earliest neuroepithelial cell types, while Hes5 expression occurs in more differentiated cell types [27] . Indeed, in human and mouse muscle stem cells, Hes5 rather than Hes1 drives the expression of the muscle-specific transcription factors MyoD and myogenin [50] and [54] . Once Hes5 levels drop, the levels of the pro-myogenic and pro-neurogenic protein MASH1 increase to drive cellular differentiation [3] , [39] , [43] , [53] and [76] . We find that undifferentiated day 5 embryoid bodies express 3.8- to 4-fold higher Hes1 and Hes5 levels (Fig. S3) than do day 9 differentiated cells. Due to the high Hes levels in day 5 embryoid bodies, MASH1 levels are 2.5-fold lower (Fig. S3) than in day 9 differentiated cells.
By day 9, Hes1 and Hes5 transcripts are both reduced by ∼4-fold, which, in control cells, is accompanied by an increase is MASH1 levels, thus allowing neurogenesis and myogenesis to occur. Interestingly, arsenic exposure does not alter the levels of Hes1 or Hes5, but it does decrease MASH1 levels by 2-fold. During the early stages of differentiation, Hes5 necessary to start production of MASH1 [47] , but then later, MASH1 turns off Hes5 production by binding to an E-box region within the Hes5 promoter [64] . When exogenous Wnt3a is co-cultured with these cells up to day 9, Hes1 levels do not change, whether the cells are exposed to arsenic of not. With the co-exposure to Wnt3a, Hes5 levels are reduced by 3-fold, which implies that Wnt3a can rescue differentiation. Indeed, when Wnt3a was exposed to iPS-induced neural progenitor cells, Hes5 levels were dramatically reduced, MASH1 expression was increased [43] , and neurogenesis increased [21] . Interestingly though, the co-exposure of Wnt3a and arsenic keep the levels of MASH1 significantly reduced. Taken together, our data suggests that the feedback loop between Hes5 and MASH1 is somehow disrupted by arsenic.
How arsenic disrupts the feedback loops between the Hes proteins and MASH1 is not known. One possibility is through epigenetic regulation. Hes1 expression is induced after Notch activation, and then oscillates due to a negative feedback loop, as the Hes1 protein binds to N-box sequences in its promoter and represses its expression. Furthermore, both Hes1 mRNA and protein are unstable [17] . We only examined Hes1 levels on a day-to-day basis, and so whether arsenic is disrupting the appropriate oscillation patterns is unknown. However, microRNA-9 (miR-9) has been proposed to regulate the oscillatory expression of Hes1 since the oscillation pattern is dampened in miR-9 knockouts [4] and [61] . We have shown that arsenic exposure in day 5 and day 9 P19 cells decreases the levels of miR-9 (data not shown), which might indicate that Hes1 is not oscillating appropriately. Thus, while Wnt3a can partially rescue the inhibition of differentiation due to arsenic exposure, it appears to do so by modulating Notch target genes rather than working through the canonical Wnt signaling pathway. Overall, this study indicates a potential mechanism for the arsenic-induced reduction in stem cell differentiation, due to the inhibition of MASH1. These results further support that arsenic can impact the feedback loops between the Wnt and Notch pathways during cellular differentiation.
Transparency document
Transparency Document.
Acknowledgements
Funding for this study was provided by NIH (ES023065 ).
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- [1] K. Anakwe, L. Robson, J. Hadley, P. Buxton, V. Church, S. Allen, C. Hartmann, B. Harfe, T. Nohno, A.M. Brown, D.J. Evans, P. Francis-West; Wnt signalling regulates myogenic differentiation in the developing avian wing; Development, 130 (2003), pp. 3503–3514
- [2] N. Bengoa-Vergniory, I. Gorroño-Etxebarria, I. González-Salazar, R.M. Kypta; A switch from canonical to noncanonical Wnt signaling mediates early differentiation of human neural stem cells; Stem Cells, 32 (2014), pp. 3196–3208
- [3] N. Bertrand, D.S. Castro, F. Guillemot; Proneural genes and the specification of neural cell types; Nat. Rev. Neurosci., 3 (2002), pp. 517–530
- [4] B. Bonev, P. Stanley, N. Papalopulu; MicroRNA-9 modulates Hes1 ultradian oscillations by forming a double-negative feedback loop; Cell Rep., 2 (2012), pp. 10–18
- [5] U. Borello, B. Berarducci, P. Murphy, L. Bajard, V. Buffa, S. Piccolo, M. Buckingham, G. Cossu; The Wnt/beta-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis; Development, 133 (2006), pp. 3723–3732
- [6] A.S. Brack, I.M. Conboy, M.J. Conboy, J. Shen, T.A. Rando; A temporal switch from Notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis; Cell Stem Cell, 2 (2008), pp. 50–59
- [7] N. Cherry, K. Shaikh, C. McDonald, Z. Chowdhury; Stillbirth in rural Bangladesh: arsenic exposure and other etiological factors: a report from Gonoshasthaya Kendra; Bull. World Health Organ., 86 (2008), pp. 172–177
- [8] G.M. Collu, A. Hidalgo-Sastre, K. Brennan; Wnt-Notch signalling crosstalk in development and disease; Cell. Mol. Life Sci., 71 (2014), pp. 3553–3567
- [9] I.M. Conboy, M.J. Conboy, G.M. Smythe, T.A. Rando; Notch-mediated restoration of regenerative potential to aged muscle; Science, 302 (2003), pp. 1575–1577
- [10] G. Concha, G. Vogler, D. Lezcano, B. Nermell, M. Vahter; Exposure to inorganic arsenic metabolites during early human development; Toxicol. Sci., 44 (1998), pp. 185–190
- [11] M.A. Davis, T.A. Mackenzie, K.L. Cottingham, D. Gilbert-Diamond, T. Punshon, M.R. Karagas; Rice consumption and urinary arsenic concentrations in U.S. children; Environ. Health Perspect., 120 (2012), pp. 1418–1424
- [12] P. Dhar, N. Mohari, R.D. Mehra; Preliminary morphological and morphometric study of rat cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period; Toxicology, 234 (2007), pp. 10–20
- [13] R.I. Dorsky, R.T. Moon, D.W. Raible; Control of neural crest cell fate by the Wnt signalling pathway; Nature, 396 (1998), pp. 370–373
- [14] S. Frankel, J. Concannon, K. Brusky, E. Pietrowicz, S. Giorgianni, W.D. Thompson, D.A. Currie; Arsenic exposure disrupts neurite growth and complexity in vitro; Neurotoxicology, 30 (2009), pp. 529–537
- [15] K.M. Gaworecki, R.W. Chapman, M.G. Neely, A.R. D’Amico, L.J. Bain; Arsenic exposure to killifish during embryogenesis alters muscle development; Toxicol. Sci., 125 (2012), pp. 522–531
- [16] H. Guan, F. Piao, X. Zhang, X. Li, Q. Li, L. Xu, F. Kitamura, K. Yokoyama; Prenatal exposure to arsenic and its effects on fetal development in the general population of Dalian; Biol. Trace Elem. Res., 149 (2012), pp. 10–15
- [17] H. Hirata, S. Yoshiura, T. Ohtsuka, Y. Bessho, T. Harada, K. Yoshikawa, R. Kageyama; Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop; Science, 298 (2002), pp. 840–843
- [18] G.-M. Hong, L.J. Bain; Arsenic exposure inhibits myogenesis and neurogenesis in P19 stem cells through repression of the b-catenin signaling pathway; Toxicol. Sci., 129 (2012), pp. 146–156
- [19] C. Hopenhayn, C. Ferreccio, S. Browning, B. Huang, C. Peralta, H. Gibb, I. Hertz-Picciotto; Arsenic exposure from drinking water and birth weight; Epidemiology, 14 (2003), pp. 593–602
- [20] Y. Hou, Y. Wang, H. Wang, Y. Xu; Induction of glutathione synthesis in human hepatocytes by acute and chronic arsenic exposure: differential roles of mitogen-activated protein kinases; Toxicology, 325 (2014), pp. 96–106
- [21] R. Hubner, A.C. Schmole, A. Liedmann, M.J. Frech, A. Rolfs, J. Luo; Differentiation of human neural progenitor cells regulated by Wnt-3a; Biochem. Biophys. Res. Commun., 400 (2010), pp. 358–362
- [22] Y. Hwang, S. Suk, Y.R. Shih, T. Seo, B. Du, Y. Xie, Z. Li, S. Varghese; Wnt3a promotes myogenesis of human embryonic stem cells and enhances in vivo engraftment; Sci. Rep., 4 (2014), p. 5916
- [23] M. Ikeya, S. Takada; Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome; Development, 125 (1998), pp. 4969–4976
- [24] Y. Jin, S. Xi, X. Li, C. Lu, G. Li, Y. Xu, C. Qu, Y. Niu, G. Sun; Arsenic speciation transported through the placenta from mother mice to their newborn pups; Environ. Res., 101 (2006), pp. 349–355
- [25] R. Kageyama, T. Ohtsuka, J. Hatakeyama, R. Ohsawa; Roles of bHLH genes in neural stem cell differentiation; Exp. Cell Res., 306 (2005), pp. 343–348
- [26] A. Kikuchi, H. Yamamoto, A. Sato, S. Matsumoto; Wnt5a: its signalling, functions and implication in diseases; Acta Physiol. (Oxf), 204 (2012), pp. 17–33
- [27] T. Kobayashi, R. Kageyama; Expression dynamics and functions of Hes factors in development and diseases; Curr. Top. Dev. Biol., 110 (2014), pp. 263–288
- [28] M. Kurzius-Spencer, J.L. Burgess, R.B. Harris, V. Hartz, J. Roberge, S. Huang, C.H. Hsu, M.K. O'Rourke; Contribution of diet to aggregate arsenic exposures-An analysis across populations; J. Expo. Sci. Environ. Epidemiol., 24 (2014), pp. 156–162
- [29] C. Kwon, P. Cheng, I. King, V. Nigam, D. Srivastava; Notch post-translationally regulates β-catenin protein in stem and progenitor cells; Nat. Cell Biol., 13 (2011), pp. 1244–1251
- [30] B. Li, Z. Jia, T. Wang, W. Wang, C. Zhang, P. Chen, K. Ma, C. Zhou; Interaction of Wnt/β-catenin and notch signaling in the early stage of cardiac differentiation of P19CL6 cells; J. Cell. Biochem., 113 (2012), pp. 629–639
- [31] W.H. Lien, E. Fuchs; Wnt some lose some: transcriptional governance of stem cells by Wnt/β-catenin signaling; Genes Dev., 28 (2014), pp. 1517–1532
- [32] J.-T. Liu, L.J. Bain; Arsenic inhibits hedgehog signaling during P19 cell differentiation; Toxicol. Appl. Pharm., 281 (2014), pp. 243–253
- [33] P. Liu, M. Wakamiya, M.J. Shea, U. Albrecht, R.R. Behringer, A. Bradley; Requirement for Wnt3 in vertebrate axis formation; Nat. Genet., 22 (1999), pp. 361–365
- [34] S. Liu, F. Wang, L. Yan, L. Zhang, Y. Song, S. Xi, J. Jia, G. Sun; Oxidative stress and MAPK involved into ATF2 expression in immortalized human urothelial cells treated by arsenic; Arch. Toxicol., 87 (2013), pp. 981–989
- [35] M.N. Llanos, A.M. Ronco; Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities; Reprod. Toxicol., 27 (2009), pp. 88–92
- [36] J. Lyu, F. Costantini, E.H. Jho, C.K. Joo; Ectopic expression of Axin blocks neuronal differentiation of embryonic carcinoma P19 cells; J. Biol. Chem., 278 (2003), pp. 13487–13495
- [37] B. Mandal, T. Suzuki; Arsenic around the world: a review; Talanta, 58 (2002), pp. 201–235
- [38] C.R. McCoy, B.S. Stadelman, J.L. Brumaghim, J.T. Liu, L.J. Bain; Arsenic and its methylated metabolites inhibit the differentiation of neural plate border specifier cells; Chem. Res. Toxicol., 28 (2015), pp. 1409–1421
- [39] C. McFarlane, G.Z. Hui, W.Z. Amanda, H.Y. Lau, S. Lokireddy, G. Xiaojia, V. Mouly, G. Butler-Browne, P.D. Gluckman, M. Sharma, R. Kambadur; Human myostatin negatively regulates human myoblast growth and differentiation; Am. J. Physiol. Cell Physiol., 301 (2011), pp. C195–203
- [40] S.G. Megason, A.P. McMahon; A mitogen gradient of dorsal midline Wnts organizes growth in the CNS; Development, 129 (2002), pp. 2087–2098
- [41] A.A. Meharg, P.N. Williams, E. Adomako, Y.Y. Lawgali, C. Deacon, A. Villada, R.C. Cambell, G. Sun, Y.G. Zhu, J. Feldmann, A. Raab, F.J. Zhao, R. Islam, S. Hossain, J. Yanai; Geographical variation in total and inorganic arsenic content of polished (white) rice; Environ. Sci. Technol., 43 (2009), pp. 1612–1617
- [42] S. Munoz-Descalzo, J. de Navascues, A.M. Arias; Wnt-Notch signalling: an integrated mechanism regulating transitions between cell states; Bioessays, 34 (2011), pp. 110–118
- [43] C. Mußmann, R. Hubner, M. Trilck, A. Rolfs, M.J. Frech; HES5 is a key mediator of Wnt-3a-induced neuronal differentiation; Stem Cells Dev., 23 (2014), pp. 1328–1339
- [44] K. Nohara, K. Okamura, T. Suzuki, H. Murai, T. Ito, K. Shinjo, S. Takumi, T. Michikawa, Y. Kondo, K. Hata; Augmenting effects of gestational arsenite exposure of C3H mice on the hepatic tumors of the F2 male offspring via the F1 male offspring; J. Appl. Toxicol. (2015) Epub
- [45] K. Orford, C. Crockett, J.P. Jensen, A.M. Weissman, S.W. Byers; Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin; J. Biol. Chem., 272 (1997), pp. 24735–24738
- [46] Y.C. Pan, X.W. Wang, H.F. Teng, Y.J. Wu, H.C. Chang, S.L. Chen; Wnt3a signal pathways activate MyoD expression by targeting cis-elements inside and outside its distal enhancer; Biosci. Rep., 35 (2015), p. e00180
- [47] C.M. Parras, C. Hunt, M. Sugimori, M. Nakafuku, D. Rowitch, F. Guillemot; The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes; J. Neurosci., 27 (2007), pp. 4233–4242
- [48] A. Patapoutian, L.F. Reichardt; Roles of Wnt proteins in neural development and maintenance; Curr. Opin. Neurobiol., 10 (2000), pp. 392–399
- [49] J.S. Petrick, F.M. Blachere, O. Selmin, R.C. Lantz; Inorganic arsenic as a developmental toxicant: In utero exposure and alterations in the developing rat lungs ; Mol. Nutr. Food Res., 53 (2009), pp. 583–591
- [50] L. Qin, J. Xu, Z. Wu, Z. Zhang, J. Li, C. Wang, Q. Long; Notch1-mediated signaling regulates proliferation of porcine satellite cells (PSCs); Cell Signal., 25 (2013), pp. 561–569
- [51] A. Rahman, M. Vahter, A.H. Smith, B. Nermell, M. Yunus, S. El Arifeen, L.A. Persson, E.C. Ekström; Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh; Am. J. Epidemiol., 169 (2009), pp. 304–312
- [52] J.L. Rosado, D. Ronquillo, K. Kordas, O. Rojas, J. Alatorre, P. Lopez, G. Garcia-Vargas, M. Del Carmen Caamaño, M.E. Cebrián, R.J. Stoltzfus; Arsenic exposure and cognitive performance in Mexican school children; Environ. Health Perspect., 115 (2007), pp. 1371–1375
- [53] K. Schuster-Gossler, R. Cordes, A. Gossler; Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants; Proc. Natl. Acad. Sci. U. S. A., 104 (2007), pp. 537–542
- [54] C. Shawber, D. Nofziger, J.J. Hsieh, C. Lindsell, O. Bogler, D. Hayward, G. Weinmaster; Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway; Development, 122 (1996), pp. 3765–3773
- [55] J.S. Sidhu, R.A. Ponce, M.A. Vredevoogd, X. Yu, E. Gribble, S.W. Hong, E. Schneider, E.M. Faustman; Cell cycle inhibition by sodium arsenite in primary embryonic rat midbrain neuroepithelial cells; Toxicol. Sci., 89 (2006), pp. 475–484
- [56] M. Simonetti, N. Agarwal, S. Stösser, K.K. Bali, E. Karaulanov, R. Kamble, B. Pospisilova, M. Kurejova, W. Birchmeier, C. Niehrs, P. Heppenstall, R. Kuner; Wnt-Fzd signaling sensitizes peripheral sensory neurons via distinct noncanonical pathways; Neuron, 83 (2014), pp. 104–121
- [57] I.S. Skerjanc; Cardiac and skeletal muscle development in P19 embryonal carcinoma cells; Trends Cardiovasc. Med., 9 (1999), pp. 139–143
- [58] P. Smedley, D. Kinniburgh; A review of the source, behaviour and distribution of arsenic in natural waters; Appl. Geochem., 17 (2002), pp. 517–568
- [59] A.A. Steffens, G.-M. Hong, L.J. Bain; Sodium arsenite delays the differentiation of C2C12 mouse myoblast cells and alters methylation patterns on the transcription factor myogenin; Toxicol. Appl. Pharm., 250 (2011), pp. 154–161
- [60] S. Takada, K.L. Stark, M.J. Shea, G. Vassileva, J.A. McMahon, A.P. McMahon; Wnt-3a regulates somite and tailbud formation in the mouse embryo; Genes Dev., 8 (1994), pp. 174–189
- [61] S.-L. Tan, T. Ohtsuka, A. González, R. Kageyama; MicroRNA9 regulates neural stem cell differentiation by controlling Hes1 expression dynamics in the developing brain; Genes Cells, 17 (2012), pp. 952–961
- [62] D. ten Berge, W. Koole, C. Fuerer, M. Fish, E. Eroglu, R. Nusse; Wnt signaling mediates self-organization and axis formation in embryoid bodies; Cell Stem Cell, 3 (2008), pp. 508–518
- [63] S.Y. Tsai, H.Y. Chou, H.W. The, C.M. Chen, C.J. Chen; The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence; Neurotoxicology, 24 (2003), pp. 747–753
- [64] T. Ueno, J. Ito, S. Hoshikawa, Y. Ohori, S. Fujiwara, S. Yamamoto, T. Ohtsuka, R. Kageyama, M. Akai, K. Nakamura, T. Ogata; The identification of transcriptional targets of Ascl1 in oligodendrocyte development; Glia, 60 (2012), pp. 1495–1505
- [65] M. Vahter; Effects of arsenic on maternal and fetal health; Annu. Rev. Nutr., 29 (2009) (12. 11–12.19)
- [66] O.S. von Ehrenstein, S. Poddar, Y. Yuan, D.G. Mazumder, B. Eskenazi, A. Basu, M. Hira-Smith, N. Ghosh, S. Lahiri, R. Haque, A. Ghosh, D. Kalman, S. Das, A.H. Smith; Childrens intellectual function in relation to arsenic exposure; Epidemiology, 18 (2007), pp. 44–51
- [67] X. Wang, P. Yang; In vitro differentiation of mouse embryonic etem (mES) cells using the hanging drop method; JoVE, 17 (2008) http://www.jove.com/index/details.stp?id=825,810.3791/3825
- [68] X. Wang, D. Meng, Q. Chang, J. Pan, Z. Zhang, G. Chen, Z. Ke, J. Luo, X. Shi; Arsenic inhibits neurite outgrowth by inhibiting the LKB1-AMPK signaling pathway; Environ. Health Perspect., 118 (2010), pp. 627–634
- [69] G.A. Wasserman, X. Liu, F. Parvez, H. Ahsan, P. Factor-Litvak, A. van Geen, V. Slavkovich, N.J. Loiacono, Z. Cheng, I. Hussain, H. Momotaj, J.H. Graziano; Water arsenic exposure and childrens intellectual function in Araihazar, Bangladesh; Environ. Health Perspect., 112 (2004), pp. 1329–1333
- [70] G.A. Wasserman, X. Liu, F. Parvez, H. Ahsan, P. Factor-Litvak, J. Kline, A. van Geen, V. Slavkovich, N.J. Loiacono, D. Levy, Z. Cheng, J.H. Graziano; Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh; Environ. Health Perspect., 115 (2007), pp. 285–289
- [71] P. Watcharasit, S. Suntararuks, D. Visitnonthachai, A. Thiantanawat, J. Satayavivad; β-catenin involvement in arsenite-induced VEGF expression in neuroblastoma SH-SY5Y cells; Environ. Toxicol., 29 (2014), pp. 672–678
- [72] Y. Xie, J. Liu, L. Benbrahim-Tallaa, J.M. Ward, D. Logsdon, B.A. Diwan, M.P. Waalkes; Aberrant DNA methylation and gene expression in livers of newborn mice transplacentally exposed to a hepatocarcinogenesis dose of inorganic arsenic; Toxicology, 236 (2007), pp. 7–15
- [73] Y.P. Yen, K.S. Tsai, Y.W. Chen, C.F. Huang, R.S. Yang, S.H. Liu; Arsenic inhibits myogenic differentiation and muscle regeneration; Environ. Health Perspect., 18 (2010), pp. 949–956
- [74] Y. Yoshikawa, T. Fujimori, A.P. McMahon, S. Takada; Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse; Dev. Biol., 183 (1997), pp. 234–242
- [75] L. Yuan, B.A. Hassan; Neurogenins in brain development and disease: an overview; Arch. Biochem. Biophys., 558 (2014), pp. 10–13
- [76] A. Zalc, S. Hayashi, F. Aurade, D. Bröhl, T. Chang, D. Mademtzoglou, P. Mourikis, Z. Yao, Y. Cao, C. Birchmeier, F. Relaix; Antagonistic regulation of p57kip2 by Hes/Hey downstream of Notch signaling and muscle regulatory factors regulates skeletal muscle growth arrest; Development, 141 (2014), pp. 2780–2790
Document information
Published on 02/05/17
Accepted on 02/05/17
Submitted on 02/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?