Abstract
Adults with congenital heart disease represent a rapidly growing patient group. Dysfunction of the right ventricle is often present, and right heart failure constitutes the main cause of death. Heart failure therapies used in acquired left heart failure are often initiated in adults with right heart failure due to congenital heart disease, but the right ventricle differs substantially from the left ventricle, and the clinical evidence for this treatment strategy is lacking.
In this review, we identified existing clinical studies evaluating the effects of ACE inhibitors, angiotensin II receptor blockers and aldosterone antagonists in adults with congenital heart disease by a systematic literature search. From 13 identified studies no clear evidence of beneficial effects was found, but the design of the studies limits the validity of the results. The studies in general include low numbers of patients, have short follow-up periods and evaluate surrogate endpoints instead of hard clinical endpoints. Specific evaluation of symptomatic patients with a systemic right ventricle indicates that these patients may benefit from RAAS inhibitory treatments, but this requires further investigation.
To conclude, existing studies do not support the use of RAAS inhibitory treatments in right heart failure due to congenital heart disease but contain important limitations. Hence, there is a need for new well-designed trials including higher numbers of patients and validated endpoints to optimize and guide future treatment of this patient group.
Keywords
Congenital heart disease;Right ventricular failure;Renin angiotensin aldosterone system;Angiotensin-converting enzyme inhibitor;Angiotensin II receptor blocker
1. Background
Right heart failure is a frequent complication in adults with congenital heart disease (CHD) and constitutes the main cause of death in this patient group [1]. Right heart failure has been studied sparsely compared to left heart failure, and as a consequence, no treatment exists that effectively targets the failing right ventricle (RV). Left heart failure knowledge and treatment guidelines are often extrapolated to conditions with a failing right heart, but this generalization implies major issues and contrasts current recommendations. Hence, there is a fundamental need for thorough evaluation of current knowledge and treatment practice. This review evaluates the existing studies investigating the role of the renin–angiotensin–aldosterone-system (RAAS) in right heart failure due to CHD and the effects of RAAS inhibitory treatments.
1.1. The right ventricle versus the left ventricle
The RV differs substantially from the left ventricle (LV). They compose different anatomical structures, with the right ventricle being a crescent shaped, thin-walled compliant chamber contracting in the longitudinal direction, as opposed to the ellipsoid, thick-walled left ventricle, which primarily contracts with a radial motion [2]. Consequently the RV is more sensitive to changes in afterload than the LV [3]. The RV and the LV derive from different embryological cell lineages [4] and express distinctive gene patterns [5]. When subjected to pressure overload, the expression of genes essential to adaptive remodeling correlates with the degree of hypertrophy in the LV. In the RV, on the other hand, maladaptive factors related to apoptotic pathways are activated, and genes necessary for the contractile performance of the cardiomyocytes are relatively down regulated [6]. Adrenergic α1-receptor stimulation increases contractility in the LV, but in the RV contractility is impaired [7]. The ventricles may also respond very differently to the same therapeutic agent. Epoprostenol is life saving in pulmonary arterial hypertension and associated RV dysfunction but increases mortality in acquired left heart failure [8].
1.2. Right heart failure in congenital heart disease
Of all major congenital abnormalities, CHD accounts for approximately one-third [9], and the incidence of moderate to severe CHD is approximately 6 in 1000 live births, the majority with the need for structural and/or medical interventions. A few years ago many of these children died at an early age due to acute or chronic heart failure, but today, with better surgical and transcatheter techniques and intensive care, more patients with CHD survive into adulthood, forming a new and growing population of adults with CHD. Consequently, adults with CHD now outnumber children with CHD [10].
Adults with CHD predominantly develop failure of the RV, which may be induced by pressure overload or volume overload (Table 1). Surgical procedures e.g. atrial switch repair of transposition of the great arteries, after which the RV becomes the systemic ventricle, may also contribute to the pathology. In general the RV tolerates volume overload better than pressure overload [11], but all the defects listed in Table 1 may lead to deterioration of RV function and the development of symptomatic right heart failure with exercise intolerance, fatigue, fluid retention and dyspnea.
| Pressure overload | Right ventricular outflow obstruction |
| • Right ventricular outflow tract obstruction | |
| • Pulmonary valve stenosis | |
| • Pulmonary atresia | |
| • Pulmonary arterial stenosis | |
| Systemic right ventricle | |
| • Congenitally corrected transposition of the great arteries | |
| • After atrial switch repair of transposition of the great arteries | |
| • Right ventricle in a univentricular circulation | |
| Pulmonary arterial hypertension | |
| Volume overload | Left-to-right shunt |
| • Atrial septal defect □ | |
| • Atrioventricular septal defect | |
| • Total or partial anomalous pulmonary venous return | |
| Pulmonary regurgitation | |
| • After Fallot repair | |
| Tricuspid valve regurgitation | |
| • Ebstein´s anomaly | |
| • Due to right ventricular dilatation |
Right heart failure in adults with CHD is often associated with arrhythmias, pulmonary hypertension and the presence of remaining anatomical abnormalities including stenoses, valve regurgitations and residual shunts. Arteriosclerosis and subsequent myocardial ischemia is much less frequent in adults with CHD than adults with acquired left heart failure, but still a higher prevalence of traditional cardiovascular risk factors including hypertension, obesity and dyslipidemia has been observed in adults with CHD compared to the general population [12]. Together these factors add to the complexity of the management of this patient group and should be kept in mind when applying treatment strategies.
1.3. The renin–angiotensin–aldosterone-system and heart failure
Renin is released from the juxtaglomerular cells of the kidneys in response to low pressures. It is converted to angiotensin I by Angiotensinogen produced by the liver, which is then converted to angiotensin II by angiotensin converting enzyme (ACE) in the lungs. The hemodynamic effects of angiotensin II include stimulation of aldosterone production and systemic vasoconstriction and, consequently, fluid retention and increased systemic blood pressures. But it also has a number of direct cardiac effects. Angiotensin II induces hypertrophy and apoptosis of the cardiomyocytes, and it is the most important regulator of the development of myocardial fibrosis. These effects are main components of cardiac remodeling, which is a maladaptive response causing ventricular dilatation and cardiac dysfunction [13]. Aldosterone influences blood pressure regulation by increasing reabsorption of water and sodium in the kidneys, but it also promotes cardiac fibrosis and endothelial dysfunction. The improved survival in patients with left heart failure treated with the aldosterone antagonists is believed to be due to diuretic effects, reduced fibrosis and improved endothelial function [14] ; [15].
Large randomized controlled trials have demonstrated solid beneficial effects of RAAS inhibition in acquired left heart failure with an average reduction in 1-year mortality of 16% after treatment with ACE inhibitors [16]. Prevention of the deleterious effects induced by increased activation of the RAAS is now a keystone in the treatment of acquired left heart failure, but the role of the RAAS and the therapeutic potential of RAAS inhibition in right heart failure caused by CHD remain unclear.
1.4. The right ventricle and the renin–angiotensin–aldosterone-system
Studies indicate that the RAAS exerts effects on the RV along with its effects on the LV. In experimental studies, failure of the RV is associated with increased activation of the RAAS [17], but when investigating the effects of RAAS inhibition results are contradictory [18]; [19] ; [20]. In humans, the density of angiotensin II receptors is the same in the RV as in the LV of the healthy heart [21], and in failing hearts the angiotensin II receptor subtype 1 is selectively down regulated both in the failing RV and the failing LV [22]. In patients with mild hypertension RAAS inhibition improved RV myocardial performance index (an echocardiographic measure of combined systolic and diastolic function) unrelated to the reduction in blood pressure [23], and in a large cross sectional study the use of RAAS inhibitors was associated with changes in RV morphology independent of LV effects [24]. In adults with CHD, the activity of neurohormonal systems including the RAAS is increased. Bolger et al. studied 53 patients with CHD comprising 4 anatomical groups (single ventricle physiology, tetralogy of Fallot, systemic right ventricle and others (including septal defects and patent ductus arteriosus)) and compared them to healthy controls. Neurohormonal activation occurred in a similar way across different anatomical groups suggesting the molecular mechanisms involved in the development of heart failure in CHD to be independent of the anatomical defect. Furthermore, the neurohormonal activation was very alike the activation seen in adults with acquired left heart failure suggesting that adults with CHD may benefit from neurohormonal blocking treatments like patients with acquired left heart failure [25]. A more recent study compared 104 adults with CHD and RV dysfunction (primarily tetralogy of Fallot and pulmonary atresia) to healthy controls. They found no differences in angiotensin II and aldosterone levels between the CHD group and the controls [26]. This incongruence with previous findings may be explained by different compositions of the study populations. While Bolger et al. included patients with both left and right cardiac lesions; Lemmer et al. primarily investigated patients with lesions compromising the RV.
Thus, the role of RAAS activation in right heart failure associated with CHD remains unclear, specific studies are needed to evaluate the effects of RAAS inhibition in patients with CHD.
2. What do we know?
Using Pubmed, we performed a systematic literature search to identify studies investigating the effects of ACE inhibitors or angiotensin II receptor blockers in adults and older children (age > 13) with CHD and right heart failure conducted between 1995 and 2015. Additionally, all relevant reference lists were screened manually. Studies investigating adults with types of CHD that involves an increased load on the RV and thereby potentially the development of RV dysfunction and failure were included. The RV overloading condition could be due to the original defect or post-surgical. Case reports have been excluded from this review.
2.1. Existing clinical studies of RAAS inhibition in adult congenital heart disease
From the literature search, 13 studies investigating the effects of RAAS inhibition in patients with CHD were identified (Table 2 ; Table 3). Patients with a univentricular heart and a passive blood flow to support the pulmonary circulation after the Fontan procedure demonstrated no improvement of RV function after treatment with ACE inhibitors [27] ; [28]. An adverse reduction in the mean percent increase in cardiac index from rest to max exercise with enalapril treatment was observed [27].
| Reference | Design | Treatment | Duration | Population | N | Findings |
|---|---|---|---|---|---|---|
| Hopkins, 1996 [29] | Prospective cohort study | Enalapril (2.5 mg/day) Captopril (6.25 mg × 3/day) | 399 ± 313 days | Cyanotic CHD patients with:
|
10 | Treatment with enalapril/captopril caused:
|
| Kouatli, 1997 [27] | Randomized, double blind, placebo-controlled, crossover trial | Enalapril (0.2–0.3 mg/kg/day) | 10 weeks | Fontan patients (4–19 years after surgery) Mean age: 14.5 ± 6.2 years NYHA class: I | 18 | Compared to placebo treatment with enalapril caused:
|
| Hechter, 2001 [32] | Retrospective observational cohort study | Various ACE inhibitors and dosages | Minimum 6 months | Patients with systemic RV after atrial switch repair of TGA Mean age: 31 years (range 26–42 years) NYHA class: N/A | 14 | Treatment with ACE inhibitor caused:
|
| Lester, 2001 [31] | Randomized placebo-controlled crossover study | Losartan (25–50 mg/day) | 8 weeks | Patients with systemic RV after atrial switch repair of TGA Age: > 13 years NYHA class: N/A | 7 | Compared to placebo treatment with losartan caused:
|
| Ohuchi, 2001 [28] | Prospective cohort study (Subgroup analysis of case control study) | Enalapril (0.1 mg/kg/day) | 6.8 months | Fontan patients Mean age: 14 ± 3 years NYHA class: N/A | 18 | Treatment with enalapril caused:
|
| Robinson, 2002 [33] | Prospective cohort study | Enalapril (0.5 mg/kg/day) | 12 months | Patients with systemic RV after atrial switch repair of TGA Mean age: 13.8 ± 3 years NYHA class: I | 8 | Treatment with enalapril caused:
|
| Dore, 2005 [34] | Randomized double-blind, placebo-controlled, crossover, clinical trial | Losartan (50–100 mg/day) | 106 ± 6 days | Patients with systemic RV because of:
|
29 | Compared to placebo losartan treatment caused:
|
| Therrien, 2008 [35] | Randomized, double blind, placebo-controlled clinical trial | Ramipril (10 mg/day) | 12 months | Patients with systemic RV after atrial switch repair of TGA Mean age: 26 ± 2 years NYHA class: I–II | 17 | Compared to placebo ramipril treatment caused:
|
| Van der Bom, 2012 [36] | Randomized, double blind, placebo-controlled clinical trial | Valsartan (160 mg × 2 /day) | 3.2 years | Patients with systemic RV because of:
|
88 | Compared to placebo valsartan treatment caused:
|
| Babu-Narayan, 2012 [30] | Randomized, double-blind, placebo-controlled clinical trial | Ramipril (10 mg/day) | 26.3 ± 2.6 weeks | Patients with repaired ToF with moderate/severe pulmonary regurgitation and RV dilatation Mean age: 30.1 ± 10.3 years NYHA class: I–II | 64 | Compared to placebo ramipril treatment caused:
|
| Tutarel, 2012 [37] | Retrospective observational case control study | Enalapril (10 mg × 2/day) | 13.3 ± 4 months | Patients with systemic RV after atrial switch repair of TGA Mean age: 25.2 ± 3.5 years NYHA class: II | 14 | Enalapril treatment caused:
|
CHD: congenital heart disease; RVOT: right ventricular outflow tract; SAT: oxygen saturation; HR: heart rate; BP: blood pressure; NYHA: New York Heart Association; TGA: transposition of the great arteries; RV: right ventricle; LVEF: left ventricular ejection fraction; RVEF: right ventricular ejection fraction; AngII: angiotensin II; EDV: end diastolic volume; ToF: tetralogy of Fallot; LV: left ventricle; ESV: end systolic volume.
| Reference | Design | Treatment | Duration | Population | N | Findings |
|---|---|---|---|---|---|---|
| Mahle, 2009 [38] | Prospective cohort study | Spironolactone (50 mg/day) | 4 weeks | Fontan patients Mean age: 28 years NYHA class: I–II | 10 | Treatment with spironolactone caused:
|
| Dos, 2013 [39] | Randomized, double blind, placebo-controlled trial | Eplerenone (50 mg/day) | 12 months | Patients with systemic RV after atrial switch repair of TGA Mean age: 26.4 years NYHA class: I–II | 25 | Compared to placebo treatment with eplerenone caused:
|
NYHA: New York Heart Association; TGA: transposition of the great arteries; RV: right ventricle; TGA: transposition of the great arteries; RVEF: right ventricular ejection fraction.
In cyanotic CHD patients, ACE inhibition improved NYHA class in 8 out of 10 patients, but treatment was discontinued in 3 patients due to increased fatigue symptoms, although no decrease in arterial oxygen saturation was detected in any of the patients [29]. Compared to placebo, treatment with ramipril did not improve NYHA class in patients with repaired tetralogy of Fallot and subsequent pulmonary regurgitation and RV dilatation. An improvement in RV and LV long axis shortening was observed, but the treatment did not improve other measures of RV function or morphology [30].
A number of studies have investigated the effects of RAAS inhibition in patients with a systemic RV after atrial switch repair of transposition of the great arteries (Mustard or Senning operation). Although a small crossover study (n = 7) reported improved exercise duration and RV ejection fraction with losartan treatment [31], most studies have found no beneficial effect of RAAS inhibition in patients with systemic RV on neither RV function nor exercise capacity [32]; [33]; [34] ; [35]. One study even observed a decrease in maximal oxygen uptake with enalapril treatment [33]. A larger randomized controlled trial (n = 88) comparing valsartan to placebo found that while RV end diastolic volume and mass increased in the placebo group, there were no adverse changes in the treatment group [36]. In the subgroup of symptomatic patients, RV ejection fraction decreased in the placebo group but remained stable in the valsartan group. In a recent retrospective study evaluating only symptomatic patients (NYHA class II) with systemic RV, a decrease in plasma levels of NT-pro-BNP with enalapril treatment compared to an increase in the non-treated control group was observed [37]. Hard clinical endpoints were only evaluated by one study [36]. In patients with systemic RV, the overall risk of clinical events (composite endpoint of hospitalization due to heart failure, arrhythmias, reoperation or death) was the same for the treatment group and the placebo group. Treatment with aldosterone antagonists has only been investigated in very few studies (Table 3). Short-term treatment with spironolactone did not improve endothelial function in Fontan patients [38]. In patients with systemic right ventricular morphology, 12 months treatment with eplerenone did not change RV mass or function, nor did it affect heart failure or collagen turnover biomarker levels [39].
3. Discussion
Until now studies investigating the effects of RAAS inhibition in adults and older children with CHD have found no overall convincing beneficial effects in this patient group (Table 2). ACE inhibitors, angiotensin II receptor blockers and aldosterone antagonists seem to be well tolerated as the studies in general report no adverse effects of the treatments.
3.1. Limitations of clinical studies
The clinical studies performed so far may carry important limitations. Firstly, they include very few patients (between 7 and 88). This may have led to insufficient power to detect effects (beneficial or adverse) of the treatments. Despite being a growing patient group, the number of adults with CHD is still very low compared to the number of patients with acquired left heart failure. Conducting larger trials in adults with CHD is therefore challenging and requires broad multicenter collaborations. Additionally, adults with CHD are young and otherwise healthy people, who normally experience very few symptoms. This may decrease their motivation for participating in clinical studies further complicating the inclusion process [36].
Secondly, the treatment duration of the studies is often relatively short e.g. 12 months or less [27]; [28]; [30]; [31]; [38] ; [39]. The main benefit of RAAS inhibition is expected to be a result of long-term neurohormonal blockade and not short-term hemodynamic effects, and consequently beneficial effects may not be detectable if the follow-up period is too short. However, in severe left heart failure, enalapril improved NYHA class and exercise tolerance after two months of treatment [40], and in asymptomatic patients with reduced ejection fraction (LVEF < 35%) the incidence of development of heart failure was lower in the enalapril group compared to the placebo group after three months of treatment [41].
Thirdly, as a consequence of the low number of included patients and short follow-up periods, the randomized trials often evaluate surrogate endpoints (RV function, reported symptoms or level of NT-pro-BNP) instead of hard clinical endpoints (hospitalization or mortality) [27]; [30]; [31]; [34] ; [35]. Evaluation of RV function in adults with CHD implies a number of challenges. In adults with acquired left heart failure clear definitions of normal vs. abnormal function assessed by different imaging modalities are available, but in adults with CHD and right heart failure this is not the case. The complex shape of the RV makes it difficult to evaluate, and defining normal values of RV function, e.g. when the RV performs as the systemic ventricle, is complicated [42].
Furthermore, adults with CHD in general do not recognize or report symptoms of for example exercise intolerance, and often there is a high discrepancy between the patients' own assessments of their functional capacity and objective measures of RV function and exercise variables [26]; [27]; [33] ; [34]. This limits the use of self-reported symptoms in clinical studies with this patient group.
Enrolling a sufficient number of patients to evaluate clinical endpoints in studies of adults with CHD may, however, be very difficult considering the low number and the heterogeneity of the patient group. Consequently, prior identification and validation of suitable surrogate endpoints is essential for evaluation of safety and efficacy of heart failure therapies in adults with CHD in future studies [43].
3.2. The role of the renin–angiotensin–aldosterone-system in the development of right heart failure
The lack of demonstrated effects of RAAS inhibitory treatments in CHD and right heart failure in clinical trials may be explained by study limitations, but more intrinsic reasons ought to be considered too. The metabolic inactivation of ACE-inhibitory drugs is much more effective in the RV compared to the LV [44], and the use of ACE inhibitors did not affect neurohormone levels in adult patients with CHD [25]. The intracellular angiotensin signaling pathway is down regulated in the failing RV compared to the failing LV of children with CHD and RV or LV outflow obstructions, respectively [6]. In adults with an overloaded RV due to CHD, natriuretic peptides, endothelin-1, norepinephrine and epinephrine correlate closely with each other and with the dysfunction of the RV, while renin and aldosterone levels relate only to one another [25]. Thus, the RAAS may be a less important pathophysiological factor in the development of right heart failure compared to left heart failure.
Baseline levels of angiotensin II are only slightly elevated in patients with systemic RV [34], and in a larger group of patients with CHD (systemic RV, tetralogy of Fallot and single ventricle), RAAS was only activated in symptomatic patients contrary to other neurohormones, which were increased in both asymptomatic and symptomatic patients [25]. Also, aldosterone levels are significantly lower in asymptomatic patients with systemic RV compared to symptomatic patients. Accordingly, the RAAS does not seem to be an important factor in early asymptomatic stages of CHD. On the contrary, RAAS inhibition prevents RV hypertrophy and deterioration of RV function [36] and causes a decrease in NT-pro-BNP [37] in symptomatic patients.
The effects of RAAS inhibition also vary with the etiology of heart failure. In acquired left heart failure with preserved ejection fraction (diastolic heart failure), the effects of RAAS inhibition are less pronounced compared to heart failure with reduced ejection fraction (systolic heart failure) [45], and RAAS inhibitory therapies are in general not recommended in valvular heart disease [46]. Adults with CHD more often present with diastolic dysfunction and/or valvular defects than systolic dysfunction, which may reduce the potential effects of RAAS inhibition in this patient group.
Also, beneficial treatment strategies of one condition of CHD with right heart failure may not apply to other conditions. Theoretically, the vasodilatation induced by RAAS inhibition may be beneficial in conditions with a septal defect where decreased systemic vascular resistance may reduce the magnitude of the shunt [47], but detrimental in patients after the Fontan procedure where venous vasodilatation may reduce the ability to mobilize blood from the venous reserve during exercise [27].
3.3. Current recommendations and clinical practice
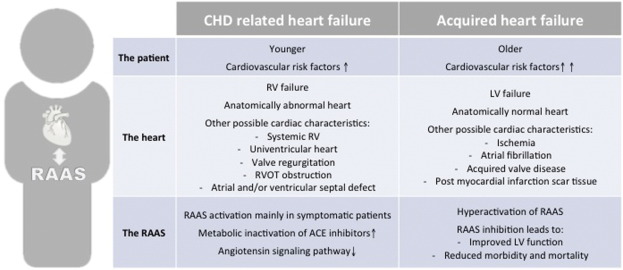
With no effective right heart failure treatments available, the dilemma of extrapolating recommendations from the treatment guidelines for acquired left heart failure persists. But despite some parallels in the remodeling processes and neurohormonal activation in heart failure regardless of the etiology, the differences between acquired left heart failure and right heart failure associated with CHD are considerable (Fig. 1). Recently, the American Heart Association have stated that older adults with CHD (age > 40) should only be treated at specialized centers, as they cannot be directly compared to patients with acquired heart failure [48].
|
|
|
Fig. 1. Differences between CHD related heart failure and acquired heart failure. CHD: congenital heart disease; RV: right ventricle; LV: left ventricle; RVOT: right ventricular outflow tract; RAAS: renin–angiotensin–aldosterone-system; ACE: angiotensin converting enzyme. |
Todays treatment guidelines in general include very few recommendations for medical treatment of heart failure in adults with CHD. According to the ACC/AHA 2008 Guidelines for the Management of Adults With Congenital Heart Disease [49], the use of ACE inhibitors are restricted to 1) patients with atrioventricular septal defects and symptoms of chronic heart failure and 2) Fontan patients with dysfunction of the systemic ventricle. Aldosterone antagonism may improve the protein losing enteropathy syndrome of Fontan patients, although the effects have only been reported by case reports. Diuretics in general are only recommended in patients with signs of congestion.
Despite the fact that the guidelines for the management of adults with CHD in general advise against the use of traditional left heart failure therapies in CHD, treatment with ACE inhibitors, angiotensin II receptor blockers and aldosterone antagonists is often initiated nonetheless. Data from clinical studies reveal that patients with CHD are often empirically treated with RAAS inhibitory drugs [32]; [34]; [35] ; [39]. Of the 53 patients with CHD evaluated by Bolger et al., 14 were taking ACE inhibitors, 3 were treated with angiotensin II receptor blockers, and 6 were treated with spironolactone [25]. In a more recent study, only 1 of 104 adult patients with CHD and RV dysfunction were treated with an angiotensin II receptor blocker [26], indicating that treatment practices may have changed during the past years. A retrospective evaluation of patients with systemic RV after atrial switch repair of TGA revealed that in the majority treatment with ACE inhibitor was initiated based solely on the belief that the therapy might be helpful. Only a few were treated because of dyspnea or high blood pressures [32].
4. Conclusion
Adults with CHD constitute a growing patient group often presenting with dysfunction and eventually failure of the RV. Traditionally, they have been excluded from the large heart failure trials, and consequently no clear evidence of effective medical heart failure therapies are available for these patients. Clinical studies investigating heart failure therapies, including RAAS inhibitory treatments, specifically in adults with CHD have failed to demonstrate beneficial effects, but may have been underpowered. Therefore, current clinical practice regarding these patients is primarily based on empirical extrapolations and expert opinions. This may include initiation of treatment with ACE inhibitors, angiotensin II receptor blockers and aldosterone antagonists despite the lack of clinical evidence to support this strategy. Thus, there is an urgent need for well-designed trials including higher numbers of patients and validated endpoints to optimize and guide future treatment of adults with CHD.
Conflicts of interest
None.
References
- [1] C.L. Verheugt, C.S.P.M. Uiterwaal, E.T. van der Velde, F.J. Meijboom, P.G. Pieper, A.P.J. van Dijk, H.W. Vliegen, D.E. Grobbee, B.J.M. Mulder; Mortality in adult congenital heart disease; Eur. Heart J., 31 (2010), pp. 1220–1229
- [2] M.K. Friedberg, A.N. Redington; Right versus left ventricular failure: differences, similarities, and interactions; Circulation, 129 (2014), pp. 1033–1044
- [3] W. MacNee; Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part one; Am. J. Respir. Crit. Care Med., 150 (1994), pp. 833–852
- [4] S. Zaffran; Right ventricular myocardium derives from the anterior heart field; Circ. Res., 95 (2004), pp. 261–268
- [5] J.I. Drake, H.J. Bogaard, S. Mizuno, B. Clifton, B. Xie, Y. Gao, C.I. Dumur, P. Fawcett, N.F. Voelkel, R. Natarajan; Molecular signature of a right heart failure program in chronic severe pulmonary hypertension; Am. J. Respir. Cell Mol. Biol., 45 (2011), pp. 1239–1247
- [6] B.D. Kaufman, M. Desai, S. Reddy, J.C. Osorio, J.M. Chen, R.S. Mosca, A.W. Ferrante, S. Mital; Genomic profiling of left and right ventricular hypertrophy in congenital heart disease; J. Card. Fail., 14 (2008), pp. 760–767
- [7] G.-Y. Wang, D.T. McCloskey, S. Turcato, P.M. Swigart, P.C. Simpson, A.J. Baker; Contrasting inotropic responses to alpha1-adrenergic receptor stimulation in left versus right ventricular myocardium; Am. J. Physiol. Heart Circ. Physiol., 291 (2006), pp. H2013–H2017
- [8] R.M. Califf, K.F. Adams, W.J. McKenna, M. Gheorghiade, B.F. Uretsky, S.E. McNulty, H. Darius, K. Schulman, F. Zannad, E. Handberg-Thurmond, F.E. Harrell, W. Wheeler, J. Soler-Soler, K. Swedberg; A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST); Am. Heart J., 134 (1997), pp. 44–54
- [9] D. van der Linde, E.E.M. Konings, M.A. Slager, M. Witsenburg, W.A. Helbing, J.J.M. Takkenberg, J.W. Roos-Hesselink; Birth prevalence of congenital heart disease worldwide; Jac, 58 (2011), pp. 2241–2247
- [10] C.L. Webb, K.J. Jenkins, P.P. Karpawich, A.F. Bolger, R.M. Donner, H.D. Allen, R.J. Barst; Congenital Cardiac Defects Committee of the American Heart Association Section on Cardiovascular Disease in the Young. Collaborative care for adults with congenital heart disease; Circulation, 105 (2002), pp. 2318–2323
- [11] P.A. Davlouros, K. Niwa, G. Webb, M.A. Gatzoulis; The right ventricle in congenital heart disease; Heart, 92 (Suppl. 1) (2006), pp. i27–i38
- [12] P. Moons, K. Van Deyk, D. Dedroog, E. Troost, W. Budts; Prevalence of cardiovascular risk factors in adults with congenital heart disease; Eur. J. Cardiovasc. Prev. Rehabil., 13 (2006), pp. 612–616
- [13] J.N. Cohn, R. Ferrari, N. Sharpe; Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling; J. Am. Coll. Cardiol., 35 (2000), pp. 569–582
- [14] F. Zannad, B. Dousset, F. Alla; Treatment of congestive heart failure: interfering the aldosterone–cardiac extracellular matrix relationship; Hypertension, 38 (2001), pp. 1227–1232
- [15] C.A. Farquharson, A.D. Struthers; Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure; Circulation, 101 (2000), pp. 594–597
- [16] I. Vonder Muhll, P. Liu, G. Webb; Applying standard therapies to new targets: the use of ACE inhibitors and B-blockers for heart failure in adults with congenital heart disease; Int. J. Cardiol., 97 (2004), pp. 25–33
- [17] L. Watkins, J.A. Burton, E. Haber, J.R. Cant, F.W. Smith, A.C. Barger; The renin–angiotensin–aldosterone system in congestive failure in conscious dogs; J. Clin. Invest., 57 (1976), pp. 1606–1617
- [18] S. Andersen, J.G. Schultz, A. Andersen, S. Ringgaard, J.M. Nielsen, S. Holmboe, M.D. Vildbrad, F.S. de Man, H.J. Bogaard, A. Vonk Noordegraaf, J.E. Nielsen-Kudsk; Effects of bisoprolol and losartan treatment in the hypertrophic and failing right heart; J. Card. Fail., 20 (2014), pp. 864–873
- [19] F.S. de Man, L. Tu, M.L. Handoko, S. Rain, G. Ruiter, C. François, I. Schalij, P. Dorfmüller, G. Simonneau, E. Fadel, F. Perros, A. Boonstra, P.E. Postmus, J. van der Velden, A. Vonk Noordegraaf, M. Humbert, S. Eddahibi, C. Guignabert; Dysregulated renin–angiotensin–aldosterone system contributes to pulmonary arterial hypertension; Am. J. Respir. Crit. Care Med., 186 (2012), pp. 780–789
- [20] M.K. Friedberg, M.-Y. Cho, J. Li, R.S. Assad, M. Sun, S. Rohailla, O. Honjo, C. Apitz, A.N. Redington; Adverse biventricular remodeling in isolated right ventricular hypertension is mediated by increased TGFβ1 signaling and is abrogated by angiotensin receptor blockade; Am. J. Respir. Cell Mol. Biol., 130710135108004 (2013)
- [21] H. Urata, B. Healy, R.W. Stewart, F.M. Bumpus, A. Husain; Angiotensin II receptors in normal and failing human hearts; J. Clin. Endocrinol. Metab., 69 (1989), pp. 54–66
- [22] K. Asano, D.L. Dutcher, J.D. Port, W.A. Minobe, K.D. Tremmel, R.L. Roden, T.J. Bohlmeyer, E.W. Bush, M.J. Jenkin, W.T. Abraham, M.V. Raynolds, L.S. Zisman, M.B. Perryman, M.R. Bristow; Selective downregulation of the angiotensin II AT1-receptor subtype in failing human ventricular myocardium; Circulation, 95 (1997), pp. 1193–1200
- [23] G. Pechlivanidis, L. Mantziari, G. Giannakoulas, H. Dimitroula, H. Styliadis, H. Karvounis, I.H. Styliadis, G. Parharidis; Effects of renin–angiotensin system inhibition on right ventricular function in patients with mild essential hypertension; J. Renin-Angiotensin-Aldosterone Syst., 12 (2011), pp. 358–364
- [24] C.E. Ventetuolo, J.A.C. Lima, R.G. Barr, M.R. Bristow, E. Bagiella, H. Chahal, J.R. Kizer, D.J. Lederer, D.A. Bluemke, S.M. Kawut; The renin–angiotensin system and right ventricular structure and function: the MESA-right ventricle study; Pulm. Circ., 2 (2012), pp. 379–386
- [25] A.P. Bolger, R. Sharma, W. Li, M. Leenarts, P.R. Kalra, M. Kemp, A.J.S. Coats, S.D. Anker, M.A. Gatzoulis; Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease; Circulation, 106 (2002), pp. 92–99
- [26] J. Lemmer, G. Heise, A. Rentzsch, P. Boettler, T. Kuehne, K.O. Dubowy, B. Peters, B. Lemmer, A. Hager, B. Stiller, German Competence Network for Congenital Heart Defects; Right ventricular function in grown-up patients after correction of congenital right heart disease; Clin. Res. Cardiol., 100 (2011), pp. 289–296
- [27] A.A. Kouatli, J.A. Garcia, T.M. Zellers, E.M. Weinstein, L. Mahony; Enalapril does not enhance exercise capacity in patients after Fontan procedure; Circulation, 96 (1997), pp. 1507–1512
- [28] H. Ohuchi, S. Hasegawa, K. Yasuda, O. Yamada, Y. Ono, S. Echigo; Severely impaired cardiac autonomic nervous activity after the Fontan operation; Circulation, 104 (2001), pp. 1513–1518
- [29] W.E. Hopkins, D.P. Kelly; Angiotensin-converting enzyme inhibitors in adults with cyanotic congenital heart disease; Ajc, 77 (1996), pp. 439–440
- [30] S.V. Babu-Narayan, A. Uebing, P.A. Davlouros, M. Kemp, S. Davidson, K. Dimopoulos, S. Bayne, D.J. Pennell, D.G. Gibson, M. Flather, P.J. Kilner, W. Li, M.A. Gatzoulis; Randomised trial of ramipril in repaired tetralogy of Fallot and pulmonary regurgitation; Int. J. Cardiol., 154 (2012), pp. 299–305
- [31] S.J. Lester, D.B. McElhinney, E. Viloria, G.P. Reddy, E. Ryan, W. Tworetzky, N.B. Schiller, E. Foster; Effects of losartan in patients with a systemically functioning morphologic right ventricle after atrial repair of transposition of the great arteries; Ajc, 88 (2001), pp. 1314–1316
- [32] S.J. Hechter, P.M. Fredriksen, P. Liu, G. Veldtman, N. Merchant, M. Freeman, J. Therrien, L. Benson, S. Siu, G. Webb; Angiotensin-converting enzyme inhibitors in adults after the Mustard procedure; Ajc, 87 (2001), pp. 660–663 (A11)
- [33] B. Robinson, C.T. Heise, J.W. Moore, J. Anella, M. Sokoloski, E. Eshaghpour; Afterload reduction therapy in patients following intraatrial baffle operation for transposition of the great arteries; Pediatr. Cardiol., 23 (2002), pp. 618–623
- [34] A. Dore, C. Houde, K.-L. Chan, A. Ducharme, P. Khairy, M. Juneau, F. Marcotte, L.-A. Mercier; Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial; Circulation, 112 (2005), pp. 2411–2416
- [35] J. Therrien, Y. Provost, J. Harrison, M. Connelly, H. Kaemmerer, G.D. Webb; Effect of angiotensin receptor blockade on systemic right ventricular function and size: a small, randomized, placebo-controlled study; Int. J. Cardiol., 129 (2008), pp. 187–192
- [36] T. van der Bom, M.M. Winter, B.J. Bouma, M. Groenink, H.W. Vliegen, P.G. Pieper, A.P.J. van Dijk, G.T. Sieswerda, J.W. Roos-Hesselink, A.H. Zwinderman, B.J.M. Mulder; The effect of valsartan on the systemic right ventricular function: a double-blind randomized placebo-controlled pilot trial; Circulation (2012)
- [37] O. Tutarel, G.P. Meyer, H. Bertram, A. Wessel, B. Schieffer, M. Westhoff-Bleck; Safety and efficiency of chronic ACE inhibition in symptomatic heart failure patients with a systemic right ventricle; Int. J. Cardiol., 154 (2012), pp. 14–16
- [38] W.T. Mahle, A. Wang, A.A. Quyyumi, M.E. McConnell, W.M. Book; Impact of spironolactone on endothelial function in patients with single ventricle heart; Congenit. Heart Dis., 4 (2009), pp. 12–16
- [39] L. Dos, S. Pujadas, M. Estruch, A. Mas, I. Ferreira-González, A. Pijuan, R. Serra, J. Ordóñez-Llanos, M. Subirana, G. Pons-Lladó, J.R. Marsal, D. García-Dorado, J. Casaldàliga; Eplerenone in systemic right ventricle: double blind randomized clinical trial. The evedes study; Int. J. Cardiol., 168 (2013), pp. 5167–5173
- [40] J.G. Cleland, H.J. Dargie, S.G. Ball, G. Gillen, G.P. Hodsman, J.J. Morton, B.W. East, I. Robertson, I. Ford, J.I. Robertson; Effects of enalapril in heart failure: a double blind study of effects on exercise performance, renal function, hormones, and metabolic state; Br. Heart J., 54 (1985), pp. 305–312
- [41] Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD InvestigatorsN. Engl. J. Med., 327 (1992), pp. 685–691
- [42] W.M. Book; Heart failure in the adult patient with congenital heart disease; J. Card. Fail., 11 (2005), pp. 306–312
- [43] W.M. Book, R.E. Shaddy; Medical therapy in adults with congenital heart disease; Heart Fail. Clin., 10 (2014), pp. 167–178
- [44] T. Thum, J. Borlak; Gene expression in distinct regions of the heart; Lancet, 355 (2000), pp. 979–983
- [45] M. Fu, J. Zhou, A. Sun, S. Zhang, C. Zhang, Y. Zou, M. Fu, J. Ge; Efficacy of ACE inhibitors in chronic heart failure with preserved ejection fraction — a meta analysis of 7 prospective clinical studies; Int. J. Cardiol., 155 (2012), pp. 33–38
- [46] A. Vahanian, O. Alfieri, F. Andreotti, M.J. Antunes, G. Baron-Esquivias, H. Baumgartner, M.A. Borger, T.P. Carrel, M. De Bonis, A. Evangelista, V. Falk, B. Iung, P. Lancellotti, L. Pierard, S. Price, H.J. Schafers, G. Schuler, J. Stepinska, K. Swedberg, J. Takkenberg, U.O. Oppell Von, S. Windecker, J.L. Zamorano, Zembala M.; Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); Eur. J. Cardiothorac. Surg., 42 (2012), pp. S1–S44
- [47] M. Montigny, A. Davignon, J.C. Fouron, P. Biron, A. Fournier, R. Elie; Captopril in infants for congestive heart failure secondary to a large ventricular left-to-right shunt; Ajc, 63 (1989), pp. 631–633
- [48] A.B. Bhatt, E. Foster, K. Kuehl, J. Alpert, S. Brabeck, S. Crumb, W.R. Davidson, M.G. Earing, B.B. Ghoshhajra, T. Karamlou, S. Mital, J. Ting, Z.H. Tseng, American Heart Association Council on Clinical Cardiology; Congenital heart disease in the older adult: a scientific statement from the American Heart Association; Circulation, 131 (2015), pp. 1884–1931
- [49] C.A. Warnes, R.G. Williams, T.M. Bashore, J.S. Child, H.M. Connolly, J.A. Dearani, P. del Nido, J.W. Fasules, T.P. Graham, Z.M. Hijazi, S.A. Hunt, M.E. King, M.J. Landzberg, P.D. Miner, M.J. Radford, E.P. Walsh, G.D. Webb; ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease); Circulation, 118 (2008), pp. e714–e833
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?