Abstract
Toxoplasmosis is a worldwide zoonosis with high impact on human and animal health. Consumption of unpasteurized milk is a risk factor of human toxoplasmosis. The aim of this study was to estimate the seroprevalence and molecular prevalence of T. gondii in goats’ milk in Northwest of Tunisia (Jendouba Governorate). A total number of 77 blood samples were collected from six herds were screened with a commercial ELISA kit for T. gondii antibodies. For the same goats’ samples, a nested PCR was performed to detect T. gondii DNA in milk. The seroprevalence of T. gondii infection was 31.2% (±0.05) while the molecular prevalence of this parasite in milk was estimated to 7.8% (±0.03). A very low value of kappa showed that there is not agreement between seroprevalence and parasite prevalence in milk. These results suggest that the consumption of raw milk from naturally infected goats is a potential source of human infection. An extension programme should be implemented to decrease related to goats’ raw milk consumption.
Introduction
Toxoplasmosis is a zoonotic infection caused by Toxoplasma gondii, a protozoan parasite belonging to the order Coccidia and the Apicomplexa phylum. T. gondii infects warm-blooded animals including humans with felines (mainly cats) as definitive hosts (Dubey 2010). Toxoplasmosis is a serious risk for seronegative pregnant women and immunocompromised persons (Montoya & Liesenfeld 2004; Tenter 2009). Infection with T. gondii is frequently asymptomatic, but remains serious even fatal for specific groups including congenitally infected fetuses and newborns, immunocompromised individuals (AIDS patients), and transplanted persons (Saadatnia & Golkar 2012). It is estimated that T. gondii infects up to one-third of the human population in the world (Dubey & Jones 2008). In Tunisia, human seroprevalence is high; it has been estimated to 70% in female Tunisian aged of 30 years in the northwest of the country (Bouratbine et al. 2001).
Among the food-borne diseases, toxoplasmosis is a real burden in several countries (EFSA, 2007; Havelaar et al. 2010; Scallan et al. 2011). For example, in USA, toxoplasmosis is the second cause of mortality after salmonellosis, and the fourth cause of hospitalization after salmonellosis, campylobacteriosis and norovirus infections (Scallan et al. 2011). Many foods are a reservoir of T. gondii. The main concerned foods are raw and undercooked meat infected with T. gondii, unpasteurized milk from infected animals, infected vegetables and water contaminated with oocysts (Dubey 1994, 1996; Baril et al. 1995). Among food animals, sheep and goats are the main sources of human infection (Dubey 2010). Infection with T. gondii in goats is ranging between 3.7 and 81.8% (Dubey & Adams 1990; Costa et al. 2012). In North Africa, studies about T. gondii infection in goats are scarce. In Egypt, positive ELISA and PCR results were found in 41.7 and 25% of goats, respectively (Ghoneim et al. 2010). In Tunisia, a single study was performed by Ben (1986), the seroprevalence in goats was estimated to 16%. Recently, a serological survey carried out in Morocco, reported that 8.5% (9/106) of goats were positive to toxoplasmosis (Benkirane et al. 2015). Several studies showed that drinking unpasteurized goats’ milk could cause clinical and even fatal toxoplasmosis infections in humans (Riemann et al. 1975; Patton et al. 1990; Skinner et al. 1990). When excreted in milk, T. gondii tachyzoites are infective (Skinner et al. 1990). Recently, few studies indicated the presence of T. gondii in goats milk and reported low prevalence ranging between 6 and 9.4% (Bezerra et al. 2013; Dehkordi et al. 2013).
Among the reliable methods, polymerase chain reaction (PCR) has the highest accuracy, sensitivity and specificity compared with conventional diagnostic methods (Held et al. 2000; Kompalic-Cristo et al. 2007). This study aimed to estimate the seroprevalence of T. gondii infection and the molecular prevalence of T. gondii DNA in milk samples to provide a preliminary assess of contamination risk by T. gondii in Tunisian goats’ milk.
Materials and methods
Study area
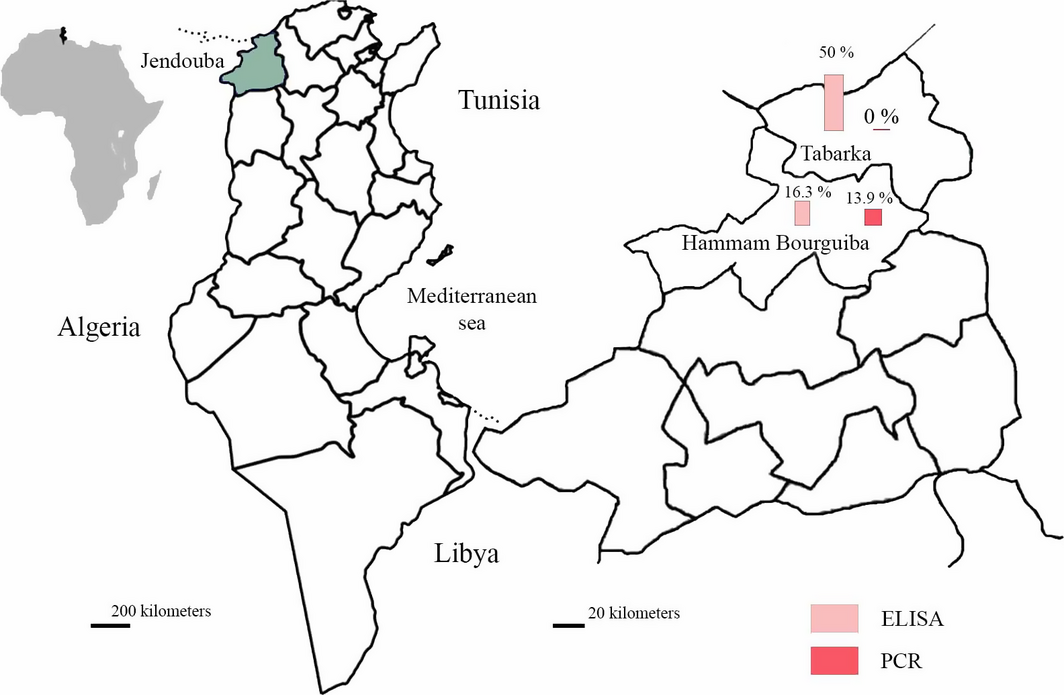
This study was carried out in goat farms located in Northwest Tunisia (localities of Tabarka and Hammam Bourguiba, Jendouba Governorate (Fig. 1)). These localities are situated between 36°45′ and 36°57′ North latitudes and between 8°35′ and 8°45′ East longitudes with an altitude ranging between 20 and 150 m. The governorate of Jendouba has the most temperate climate in Tunisia with a mean annual rainfall of 1000 mm. The mean minimal and maximal temperature are 5 and 30°C in winter and summer, respectively (National Institute of Meteorology, Tunisia).
|
|
|
Figure 1. Goat ELISA and PCR infection prevalence of Toxoplasma gondii in the governorate of Jendouba (districts of Hammam Bourguiba and Tabarka, Northwest Tunisia) |
Samples collection
This study was carried out from February to May 2014 in six herds totalling 77 lactating goats of different ages and breeds (Table 1). Blood samples were collected in dry tubes from the jugular vein; the sera were separated and stored at −20°C until used. Simultaneously, 50 mL of milk was collected from each goat and kept frozen in sterile tubes for molecular study.
| Parameter | ELISA | PCR | |||
|---|---|---|---|---|---|
| Positive/examined (%±SE) | OR [95% CI] | Positive/examined (%±SE) | OR [95% CI] | ||
| Locality |
Tabarka Hammam Bourguiba
|
17/34 (50 ± 9) 7/43 (16.3 ± 6)
|
5.14 [1.61; 16.99]* |
0/34 (0)* 6/43 (13.9 ± 5)
|
NA |
| Age group (years) |
≤3 4 to 5 ≥6
|
9/19 (47.4 ± 11) 9/42 (21.4 ± 6) 6/16 (37.5 ± 12)
|
3.3 [0.89; 12.49] 1.5 [0.32; 7.26]
|
2/19 (10.5 ± 7) 3/42 (7.1 ± 4) 1/16 (6.2 ± 6)
|
1.53 [0.16; 12.89] 1.76 [0.11; 54.96]
|
| Breed |
Local breed Cross bred Exotic breeds*
|
5/22 (22.7 ± 9) 10/31 (32.2 ± 8) 9/24 (37.5 ± 9)
|
0.62 [0.15; 2.51] 0.49 [0.11; 2.12]
|
2/22 (9.1 ± 6) 4/31 (12.9 ± 6) 0/24
|
0.68 [0.08; 4.99] NA
|
| Abortion history |
Yes No
|
5/19 (26.3 ± 10) 19/58 (32.7 ± 6)
|
0.73 [0.2; 2.64] |
4/19 (21 ± 9) 2/58 (3.4 ± 2)
|
7.47 [1.02; 66.13]* |
| Number of cats |
0 1 ≥2
|
6/8 8/49 (16.3 ± 5) 10/20 (50 ± 11)
|
15.38 [2.16; 137.78]* 3 [0.38; 28.43]
|
0/8 6/49 (12.2 ± 5) 0/20
|
NA |
SE, Standard Error; CI, 95% Confidence Interval; NA, Not Applicable. *P ≤ 0.05. *Alpine and Boer goats. | |||||
Serology
Samples of 10 μL of sera were examined for IgG antibodies against T. gondii using an ELISA kit (ID Screen® Toxoplasmosis Indirect, Montpellier, France) in accordance to the manufacturers instructions.
Molecular study
DNA was extracted from milk as described by Mancianti et al. (2013). Briefly, the milk samples were centrifuged at 2200 g for 5 min. To avoid interference with casein, 1 mL of the pellet was treated with 200 μL of TE (1 mM EDTA, 10 mM Tris-HCl (pH = 7.6)) and 300 μL 0.5 M EDTA (pH 8). The solution was resuspended then centrifuged at 3000 g for 10 min and finally diluted in 200 μL of PBS (Psifidi et al. 2010). DNA was isolated from the resuspended pellet using Wizard Genomics DNA (Promega, Madisson, Wisconsin, USA) extraction kit according to manufacturers instructions; the DNA was stored at −20°C until used.
To evaluate the efficiency of DNA extraction protocol, a PCR assay targeting the hypervariable regions V1-V3 coding for 18S rRNA was performed using two forward primers AACCTGGTTGATCCTGCCAGT (1 A) and reverse primers GGCACCAGACTTGCCCTC (564 R). The PCR reaction was carried out in a mixture of 1× PCR buffer, 2 mM MgCl2, 10 μM of each primer, 0.2 mM of each dNTP, 2 U Taq polymerase, 1.5 μL of DNA template and distilled water to a total volume of 25 μL (Wang et al., 2014). The PCR conditions were 5 min at 94°C followed by 25 cycles of denaturation at 94°C for 50 s, annealing at 58°C for 50 s and a final extension at 72°C for 10 min. A nested PCR was performed to amplify a T. gondii DNA fragment of 227 bp belonging to ITS1 gene and coding for the 18S – 5.8S rRNA according to a modified protocol of Hurtado et al. (2001). Two external primers were used, namely, NN1 (5′-CCTTTGAATCCCAAGCAAAACATGAG-3′) and NN2 (5′-GCGAGCCAAGACATCCATTGCTGA-3′). After the first PCR, nested PCR was performed using 2 μL of the first PCR product as template and T. gondii-specific primers Tg-NP1 (5′-GTGATAGTATCGAAAGGTAT-3′) and Tg-NP2 (5′-ACTCTCTCTCAAATGTTCCT-3′). Each amplification was performed in 25 μL of total reaction volume consisting of 0.1 μM of each primer, Taq polymerase buffer (1x) supplemented with MgCl2 (2 mM), 0.2 mM dNTPs, 0.5 U Taq DNA polymerase (R GoTaq, Promega). Thermal amplification programme was 3 min at 94°C followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 67°C for 45 s and extension at 72°C for 1 min with an elongation step of 5 min at 72°C. For nested PCR, all thermal cycler conditions were the same, except the annealing temperature (53°C for 30 s). Positive controls (including T. gondii DNA) and negative controls (distilled water) were included in each PCR run. The PCR products were visualized by electrophoresis in 1.5% (w/v) agarose gel (Promega®) supplemented with 0.05% ethidium bromide (Promega®) in TBE buffer (Tris-Borate-EDTA) (Promega®).
Statistical analysis
A chi-square Mantel–Haenszel test was performed for comparison of infection prevalence with Epi Info 6 at a threshold of 5% (Schwartz 1993). The concordance between PCR and ELISA was estimated with the Kappa test (Toma et al. 2007).
Results
Seroprevalence
The seroprevalence in goats was 31.2% (±5%). It was significantly higher in Tabarka (17/34; 50 ± 9%) than in Hammam Bourguiba (7/43; 16.3 ± 6%) (P = 0.001) (Table 1). There was no significant difference in the seroprevalence of T. gondii in different age categories. The infection rate by T. gondii was higher in herds containing more than one cat (P = 0.004).
Molecular detection in milk
A total number of six samples milk were positive (7.8 ± 3%). The T. gondii molecular prevalence was significantly higher in Hammam Bourguiba (6/43; 13.9 ± 5%) than in Tabarka (0/34) (P = 0.02). The molecular prevalence of T. gondii was higher for goats with history of abortion (21 ± 9.4%) (P = 0.01). Among seropositive goats (n = 24), two milk samples were positive to n-PCR (8.33 ± 5%). The kappa coefficient between n-PCR and ELISA was very low (Ƙ = 0.01).
Discussion and conclusion
In this study, we used an ELISA and a nPCR in milk for detection of T. gondii from lactating goats in North-western Tunisia. Anti-T. gondii antibodies were found in 31.2% of serum samples, only two of them were also positive for milk-PCR. Our results were comparable with those reported in China (29.54% by ELISA), Thailand and Pakistan (27.9 and 25.5% by Latex Agglutination Test, respectively) (Jittapalapong et al. 2005; Ramzan et al. 2009; Liu et al. 2015). In Europe, T. gondii is widespread with high infection rates. In Italy, 60.6% of goats’ serum samples were positive by micro-agglutination test (Mancianti et al. 2013). Similar findings were reported in Bulgaria, where 59.8% of the samples were positive by inhibition of haemagglutination test (Prelozov et al. 2008). In Romania, the seroprevalences were 33.1 and 52.8% in kids and dairy goats, respectively (Lovu et al. 2012; Paştiu et al. 2015). In USA, Korea and Nigeria lower rates were reported (6.8; 5.1 and 4.6%, respectively) (Kamani et al. 2010; Jung et al. 2014; Yaglom et al. 2014).
Consuming raw goats’ milk represents a real risk factor for human toxoplasmosis (Skinner et al. 1990). Detection of toxoplasmosis in different lactating species was recently studied by Dehkordi et al. (2013), with different techniques. They showed that raw milk from all domestic species (goats, sheep, buffalos, cattle and camel) could be contaminated. The prevalence of T. gondii in the milk of naturally infected goats is relatively low. We found that 7.8% of milk samples were shedding Toxoplasma DNA. Similar results were reported by Mancianti et al. (2013) in Italy where 7.9% of the samples were positive. A slightly lower rate was reported in Brazil where the parasite was detected in 6% of the milk samples (15/248) (Bezerra et al. 2013). The environmental conditions, diagnostic methods and the presence of cats in the herds could explain this discrepancy. In this study, the molecular prevalence of T. gondii was significantly higher in Hammam Bourguiba than in Tabarka (P = 0.02). This might be attributed to its climate which is characterized by a high rainfall enhancing oocysts dispersion.
Our results showed also that the infection rate was higher for goats with abortion history (P = 0.01). Since congenital toxoplasmosis is one of the main causes of abortion in goats (Duncanson et al. 2001), the consumption of raw milk from infected goats may present a real risk to public health.
There was no concordance between the two techniques (4/77; 5.2%). Positive PCR and negative ELISA samples could be due to the occurrence of early infections (Bezerra et al. 2013). Further studies are needed to quantify and estimate the viability of T. gondii excreted in the milk. Our results provided a molecular evidence of T. gondii DNA presence in raw goats’ milk. Considering these results, authorities must integrate health education guidance to encourage milk consumers to avoid drinking raw milk. Toxoplasmosis infection must therefore be added to the list of pathogens transmitted by raw milk with Brucella melitensis (Gupta et al. 2006).
Acknowledgements
The authors thank Mr. Bechir Guesmi, and Mr. Taoufik Lahmar for their support, and all goat farmers who agreed to let us handle their animals. The work was funded by the laboratory “Epidemiology of endemic infections of herbivores in Tunisia: application to the control”. Ministry of Higher Education, Scientific Research and Information Technology and Communication, Tunisia.
Source of funding
The work was funded by the laboratory of “Laboratoire d’épidémiologie des infections enzootiques des herbivores en Tunisie: application à la lutte” (Ministère de l'enseignement supérieur et de la recherche scientifique, Tunisia).
Conflicts of interest
The authors declare no conflict of interests in relation to this work.
Contributions
SA, MG, MRR and MM conceived and designed the experiments. SA and MR performed the experiments. HN involved in the collection of samples. LS contributed to the analysis of tools. SA and MG wrote the manuscript.
References
- Baril L., Ancelle T., Thulliez P., Goulet V., Tirard V. & Carme B. (1995) Facteurs de risque d'acquisition de la toxoplasmose chez les femmes enceintes en France. Bulletin Épidémiologique Hebdomadaire16, 73–75.
- Ben Othman H. (1986). La toxoplasmose animale: enquête sérologique dans un troupeau caprin. Détermination des seuils significatifs de la réaction d'agglutination directe par comparaison avec l'immunofluorescence indirecte. Thèse de Doctorat en Médecine Vétérinaire de Sidi Thabet, Tunisie.
- Benkirane A., Essamkaoui S., El Idrissi A., Lucchese L. & Natale A. (2015) A sero-survey of major infectious causes of abortion in small ruminants in Morocco. Veterinaria Italiana51, 25–30.
- Bezerra M.J., Kim P.C., Moraes E.P., Sá S.G., Albuquerque P.P., Silva J.G.et al. (2013) Detection of Toxoplasma gondii in the milk of naturally infected goats in the Northeast of Brazil. Transboundary and Emerging Diseases62, 421–424.
- Bouratbine A., Siala E., Chahed M.K., Aoun K. & Ben Ismail R. (2001) Profil séro- épidémiologique de la toxoplasmose au Nord de la Tunisie. Parasite8, 61–66.
- Costa D.G., Marvulo M.F., Silva J.S., Santana S.C., Magalhães F.J., Filho C.D.et al. (2012) Seroprevalence of Toxoplasma gondii in domestic and wild animals from the Fernando de Noronha, Brazil. The Journal of Parasitology98, 679–680.
- Dehkordi F.S., Borujeni M.R., Rahimi E. & Abdizadeh R. (2013) Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR Methods in Iran. Foodborne Pathogens and Disease10, 120–125.
- Dubey J.P. (1994) Toxoplasmosis. Journal of the American Veterinary Medical Association205, 1593–1598.
- Dubey J.P. (1996) Strategies to reduce transmission of Toxoplasma gondii to animals and humans. Veterinary Parasitology64, 65–70.
- Dubey J.P. (2010) Toxoplasmosis of Animals and Humans, 2nd edn, CRC Press: Boca Rotan, pp 336.
- Dubey J.P. & Adams D.S. (1990) Prevalence of Toxoplasma gondii antibodies in dairy goats from 1982 to 1984. Journal of the American Veterinary Medical Association196, 295–296.
- Dubey J.P. & Jones J.L. (2008) Toxoplasma gondii infection in humans and animals in the United States. International Journal for Parasitology38, 1257–1278.
- Duncanson P., Terry R.S., Smith J.E. & Hide G. (2001) High levels of congenital transmission of Toxoplasma gondii in a commercial sheep flock. International Journal for Parasitology31, 1699–1703.
- EFSA (2007) Scientific opinion of the panel on biological hazards on a request from EFSA on surveillance and monitoring of Toxoplasma in humans, foods and animals. EFSA Journal583, 1–64.
- Ghoneim N.H., Shalaby S.I., Hassanain N.A., Zeedan G.S., Soliman Y.A. & Abdalhamed A.M. (2010) Comparative study between serological and molecular methods for diagnosis of toxoplasmosis in women and small ruminants in Egypt. Foodborne Pathogens and Disease7, 17–22.
- Gupta V.K., Verma D.K. & Rout P.K. (2006) Polymerase chain reaction (PCR) for detection of Brucella melitensis in goat milk. Small Ruminant Research65, 79–84.
- Havelaar A.H., van Rosse F., Bucura C., Toetenel M.A., Haagsma J.A., Kurowicka D.et al. (2010) Prioritizing emerging zoonoses in The Netherlands. PLoS One5, e13965. doi: 10.1371/journal.pone.0013965.
- Held T.K., Kruger D., Switala A.R., Beyer J., Kingreen D., Busemann C.et al. (2000) Diagnosis of toxoplasmosis in bone marrow transplant recipients: comparison of PCR based results and immunohistochemistry. Bone Marrow Transplantation25, 1257–1262.
- Hurtado A., Aduriz G. & Moreno B. (2001) Single tube nested PCR for the detection of Toxoplasma gondii in fetal tissues from naturally aborted ewes. Veterinary Parasitology102, 17–27.
- Jittapalapong S., Sangvaranond A., Pinyopanuwat N., Chimnoi W., Khachaeram W., Koizumi S. & Maruyama S. (2005) Seroprevalence of Toxoplasma gondii infection in domestic goats in Satun Province, Thailand. Veterinary Parasitology127, 17–22.
- Jung B.Y., Gebeyehu E.B., Lee S.H., Seo M.G., Byun J.W., Oem J.K.et al. (2014) Detection and determination of Toxoplasma gondii seroprevalence in native Korean goats (Capra hircus coreanae). Vector Borne and Zoonotic Diseases14, 374–377.
- Kamani J., Mani A.U. & Egwu G.O. (2010) Seroprevalence of Toxoplasma gondii infection in domestic sheep and goats in Borno state, Nigeria. Tropical Animal Health and Production42, 793–797.
- Kompalic-Cristo A., Frotta C., Suárez-Mutis M., Fernandes O. & Britto C. (2007) Evaluation of a real-time PCR assay based on the repetitive B1 gene for the detection of Toxoplasma gondii in human peripheral blood. Parasitology Research101, 619–625.
- Liu Z.K., Li J.Y. & Pan H. (2015) Seroprevalence and risk factors of Toxoplasma gondii and Neospora caninum infections in small ruminants in China. Preventive Veterinary Medicine118, 488–492.
- Lovu A., Györke A., Mircean V., Gavrea R. & Cozma V. (2012) Seroprevalence of Toxoplasma gondii and Neospora caninum in dairy goats from Romania. Veterinary Parasitology186, 470–474.
- Mancianti F., Nardoni S., D'Ascenzi C., Pedonese F., Mugnaini L., Franco F. & Papini R. (2013) Seroprevalence, detection of dna in blood and milk, and genotyping of Toxoplasma gondii in a goat population in Italy. BioMed Research International. doi:10.1155/2013/905326.
- Montoya J.G. & Liesenfeld O. (2004) Toxoplasmosis. Lancet363, 1965–1976.
- Paştiu A.I., Ajzenberg D., Györke A., Şuteu O., Balea A., Rosenthal B.M.et al. (2015) Traditional goat husbandry may substantially contribute to human toxoplasmosis exposure. Veterinary Parasitology101, 45–49.
- Patton S., Johnson S. & Puckett K. (1990) Prevalence of Toxoplasma gondii antibodies in nine populations of dairy goats: compared titers using modified direct agglutination and indirect hemagglutination. International Journal for Parasitology76, 74–77.
- Prelozov P., Koinarski V. & Georgieva D. (2008) Seroprevalence of Toxoplasma gondii infection among sheep and goats in the Stara Zagora region. Bulgarian Journal of Veterinary Medicine11, 113–119.
- Psifidi A., Dovas C.I. & Banos G. (2010) A comparison of six methods for genomic DNA extraction suitable for PCR-based genotyping applications using ovine milk samples. Molecular and Cellular Probes24, 93–98.
- Ramzan M., Akhtar M., Muhammad F., Hussain I., Hiszczynska-Sawicka E., Haq A.U.et al. (2009) Seroprevalence of Toxoplasma gondii in sheep and goats in Rahim Yar Khan (Punjab), Pakistan. Tropical Animal Health and Production41, 1225–1229.
- Riemann H.P., Meyer M.E., Theis J.H., Kelso G. & Behymer D.E. (1975) Toxoplasmosis in an infant fed unpasteurized goat milk. Journal of Pediatrics87, 573–576.
- Saadatnia G. & Golkar M. (2012) A review on humans toxoplasmosis. Scandinavian Journal of Infectious Diseases44, 805–814.
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L.et al. (2011) Foodborne illness acquired in the United States-major pathogens. Emerging Infectious Diseases17, 7–15.
- Schwartz D. (1993). Méthodes Statistiques à l'usage Des Médecins et Des Biologistes, 3ème édn. Flammarion: Paris.
- Skinner L.J., Timperley A.C., Wightman D., Chatterton J.M.W. & Ho-Yen D.O. (1990) Simultaneous diagnosis of toxoplasmosis in goats and goat owners family. Scandinavian Journal of Infectious Diseases22, 359–361.
- Tenter A.M. (2009) Toxoplasma gondii in animals used for human consumption. Memorias do Instituto Oswaldo Cruz104, 364–369.
- Toma B., Dufour B., Sanaa M. & Bénet J.J. (2007) Epidémiologie Appliquée à la Lutte Collective Contre les Maladies Animales Transmissibles Majeures. Association pour l’étude de l’épidémiologie des maladies animales: Paris.
- Wang Y., Tian R.M., Gao Z.M., Bougouffa S., Qian P.Y. (2014) Optimal eukaryotic 18S and universal 16S/18S ribosomal RNA primers and their application in a study of Symbiosis. PLoS One9, e90053. doi: 10.1371/journal.pone.0090053.
- Yaglom H.D., Rottinghaus A.A. & Pithua P. (2014) Evidence of Toxoplasma gondii exposure in Boer goat herds in Missouri, USA. Zoonoses Public Health61, 395–397.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?