Highlights
- Tests a frequency of exposure hypothesis for attentional bias towards alcohol cues
- Findings suggest an impact of exposure dependent upon drinking history.
- Suggests differential impact of passive and active cue exposure on attentional bias
Abstract
Aims
To examine whether a group of social drinkers showed longer response latencies to alcohol-related stimuli than neutral stimuli and to test whether exposure to 1) an alcohol-related environment and 2) consumption related cues influenced the interference from alcohol-related stimuli.
Methods
A 2 × 2 × 2 × 5 factorial design with Exposure Group (high, low) and Consumption Group (high, low) as between-participant factors and Word Type (alcohol, neutral) and Block (1–5) as within-participant factors was used. Forty-three undergraduate university students, 21 assigned to a high exposure group and 22 to a low exposure group, took part in the experiment. Exposure Group was defined according to whether or not participants currently worked in a bar or pub. Consumption Group was defined according to a median split on a quantity–frequency measure derived from two questions of the Alcohol Use Disorders Identification Test (AUDIT) questionnaire. A modified computerised Stroop colour naming test was used to measure response latencies.
Results
Exposure and consumption factors interacted to produce greater interference from alcohol-related stimuli. In particular, the low consumption group showed interference from alcohol-related stimuli only in the high exposure condition. Exposure did not affect the magnitude of interference in the high consumption group.
Conclusions
Attentional bias is dependent upon exposure to distinct types of alcohol-related cues.
Keywords
Attentional bias;Cues;Exposure;Social drinkers
1. Introduction
A defining characteristic of incentive-motivational models of addictive behaviours is that ongoing use and misuse of substances leads to an increase in the salience of drug-related cues (Franken, 2003 ; Robinson and Berridge, 1993). It has been argued that with repeated behavioural enactment an attentional bias towards these concern-related stimuli develops, meaning that they are detected automatically (without conscious awareness), which results in the desire to undertake associated behaviour (see Field et al., 2009 ; Franken, 2003). Utilising various experimental tasks (e.g. modified Stroop, eye tracking technology, flicker induced change blindness, dot probe), attentional biases for concern-related stimuli have been identified in a variety of habitual behaviours including alcohol use (e.g. Sharma, Albery, & Cook, 2001), cannabis use (e.g. Cane, Sharma, & Albery, 2009), smoking (e.g. Attwood, O'Sullivan, Leonards, Mackintosh, & Munafò, 2008), and sex-related activity (Fromberger et al., 2012). The role of automatic processes for the cognition of addiction-related cues has been the subject of theoretical debate (Albery et al., 2006; McCusker, 2001; McCusker, 2006; Moss and Albery, 2009 ; Tiffany, 1990). It is argued that problem drinkers have a memory structure for alcohol-related concepts that is generated at an implicit level (Stacy, 1997; Stacy and Weirs, 2006; Weinstein and Cox, 2006 ; Wiers et al., 2006). In other words, alcohol users, and other substance abusers, do not have control over attention to relevant stimuli and activation of appropriate memory structures that, in turn, may guide behavioural responses to such cues (Ingjaldsson et al., 2003a; Leung and McCusker, 1999; Munafò et al., 2003 ; McCusker and Gettings, 1997). If this is the case then alcohol users should show greater pre-occupation with alcohol-related stimuli compared to non-alcohol-related stimuli. This effect has been shown to be consistent across studies using free association memory activation paradigms amongst alcohol users and other substance users (e.g. Leung and McCusker, 1999 ; Stacy, 1995), psychobiological measures (e.g. Ingjaldsson et al., 2003a ; Ingjaldsson et al., 2003b) and other implicit correlates of alcohol-related problems (e.g. Bruce and Jones, 2004; Cox et al., 2003; Field et al., 2005; Field et al., 2004; Jones et al., 2003; Moss et al., 2011; Pothos and Cox, 2002 ; Townsend and Duka, 2001; see Bruce & Jones, 2006).
In work which has utilised a modified Stroop task (Stroop, 1935), in which participants are asked to ignore a presented word and respond to the colour in which the word appears, it is found that alcohol-related words show increased response latencies in comparison to neutral words amongst problem drinkers (e.g. Bauer and Cox, 1998 ; Sharma et al., 2001). Theoretically, this effect has been explained in terms of the automatic activation of a semantic network related to alcohol (e.g. Cox et al., 2006; Field, 2006; Franken, 2003 ; Sharma et al., 2001). If this explanation were reasonable it would predict that such an effect would also be apparent amongst a sub-group of non-problem drinkers. Few studies have addressed this issue by comparing high and low consuming non-problem drinkers. Cox, Yeates, and Regan (1999) and Cox et al. (2003) reported no interference from alcohol-related words in either group whereas Sharma et al. (2001) and Bruce and Jones (2004) demonstrated that within a high consuming group of non-problem drinkers there was significant interference. One aim of the present study was to provide further evidence for an alcohol Stroop effect amongst high consuming social drinkers.
Although the preferred explanation for interference amongst problem and non-problem social drinkers is that repeated engagement in drinking behaviour strengthens the semantic network related to alcohol, other not incompatible explanations are possible. One relates to the frequency of exposure to alcohol-related stimuli. This frequency of exposure explanation suggests that problem drinkers have a greater pre-exposure to alcohol-related stimuli that acts to prime the related semantic network which manifests itself in increased interference compared to non-problem drinkers. Using a modified Stroop, Sharma et al. (2001) have provided some evidence against this hypothesis showing that amongst problem drinkers there was no increase in the interference (reaction time to alcohol stimuli minus neutral stimuli) when alcohol-related stimuli were repeated. This data suggested a reduction in this interference with repetition, which supports a habituation response, and is consistent with evidence from other studies that show a reduction in the modified Stroop effect (and other measures of attention) after intervention through repetition (see Waters & Leventhal, 2006; see Williams, Mathews, & Macleod, 1996). For example, Marissen et al. (2006) showed a decrease in attentional bias (using a modified Stroop) for heroin-dependent individuals after cue exposure treatment or placebo conditions. Similarly, Schoenmakers, Wiers, Jones, Brice, and Jansen (2007) found a decrease in attentional bias (measured with the dot probe task) amongst heavy drinkers who had undertaken an attentional retraining programme. This issue has also been investigated by comparing spouses of patients with a control group since spouses are assumed to have been exposed more frequently to concern-related cues than control participants. McCusker and Gettings (1997) showed no greater interference for gambling-related stimuli in a group of spouses of gamblers and a control group. The current paper attempts to address this version of the frequency of exposure explanation by comparing two groups of social drinkers. A control group of social drinkers was compared to an experimental group who worked in an alcohol-related environment. It is predicted that if frequency of exposure moderates the interference from alcohol-related stimuli then the experimental group should show greater interference than the control group.
A second explanation relates specifically to the drinking behaviour of individuals as a measure of frequency of exposure rather than to exposure to general alcohol cues in the environment. If drinking behaviour is a viable exposure cue there should be increased interference for alcohol related stimuli in comparison to neutral stimuli for those individuals who drink more alcohol on more occasions. In the present study a quantity–frequency measure of drinking behaviour was used to compare social drinkers. If interference from alcohol-related stimuli is greater amongst those who consumed greater amounts of alcohol on more occasions when compared to those who consume less on fewer occasions, it could be argued that drinking behaviour per se as a measure of frequency of exposure moderates any interference effects.
2. Method
2.1. Participants
Forty-five undergraduate university students took part in the study. Participants were divided into low exposure (N = 22) and high exposure (N = 21) groups on the basis of whether participants currently worked in a bar or pub. The high exposure group (mean = 18.14 h per week, SE = .90, range 11–26 h per week) reported a significantly greater number of hours spent in bars/nightclubs/pubs (including work time) than the low exposure group (mean = 7.77 h per week, SE = .61, 1–10 h per week), t(41) = 9.62, p < .001. For analyses involving the specific effects of participants' alcohol consumption a median split was carried out on a quantity–frequency measure of alcohol consumption derived from the multiple of two questions of the AUDIT questionnaire (i.e. ‘How often do you have a drink containing alcohol?’ (scored 0–4) and ‘How many drinks containing alcohol do you have on a typical day when you are drinking?’ (each scored 0–4)). Possible range of scores for this measure was 0–16. Participants were divided into either high consumption (N = 21, mean = 7.38, SE = .55, range 4–12) or low consumption (N = 22, mean = 1.22, SE = .25, range 0–3) groups accordingly.
2.2. Design
A 2 × 2 × 2 × 5 factorial design with Exposure Group (high, low) and Consumption Group (high, low) as between-participant factors and Word Type (alcohol, neutral) and Block (1–5) as within-participant factors was used. The first five neutral words were presented as part of one block, the second five as part of another block and so on for a total of five blocks (see the Materials section for the words used). In each of the five blocks a different set of five words were used. Words across the five blocks were counterbalanced using a Latin square design. Each of the words was presented in each of four ink colours, red, green, blue and brown giving 20 stimuli per block. These twenty stimuli were randomised with the restriction that an identical word or colour could not repeat itself on consecutive trials. This formed one block in the stimulus array. Five such blocks were formed to produce 100 neutral category stimuli. The same design was used for the alcohol related words producing 100 alcohol related stimuli. For half the participants the alcohol stimuli were presented before the neutral stimuli and for the other half the neutral stimuli were presented before the alcohol stimuli. There was a short break of about 1 min at the end of one stimulus set and the beginning of the second stimulus set.
2.3. Materials
The words used in the experiment were all presented in capital letters and were as follows.
Neutral category (environmental features) words: BOG, RAVINE, VALLEY, BRIDGES, PEBBLE, COVE, CRAGS, LEAVES, PLAIN, GEYSER, TRENCH, CANAL, INLET, HARBOR, TREE, SWAMP, MOSS, HILL, TUNNEL, CLIFF, HOLLOW, MEADOW, WINDS, FOG, OCEAN.
Alcohol words: PUB, LIQUEUR, WINE, COCKTAIL, BREWERY, BREW, CIDER, SPIRITS, LIQUOR, TAVERN, MEAD, STOUT, BOOZE, DRUNK, BITTER, SCOTCH, SHERRY, BAR, BOURBON, SALOON, ALCOHOL, WHISKEY, PORT, GIN, BEER.
Neutral words were selected from the category of environmental features, as used previously by McKenna and Sharma (1995) and Sharma et al. (2001). The words used for the environmental features and alcohol categories were selected as follows: First, a number of words that the authors thought might belong to this category were selected and then rated by four judges on a five point (0–4, bad–good) scale as to category membership. Using a criterion of at least three out of four judges giving a rating of 2 or more, the selected words were then matched for word frequency and word length using Kucera and Francis (1967). Mean word frequency did not significantly differ between alcohol (mean = 20.6, sd = 23.10) and neutral (mean = 21.04, sd = 23.01) words, t(48) = 0.067, p > 0.9, and word length did not differ significantly between alcohol (mean = 5.36, sd = 1.44) and neutral (mean = 5.16, sd = 1.03) words, t(48) = 0.565, p > 0.5. Also word frequency and word length were matched between the five blocks.
All stimuli were presented using a Viglen 386 PC computer. A turbo basic programme controlled stimulus presentation and collected response latencies with an accuracy of 1 ms. Participants sat approximately 60 cm from the computer screen with each word of the dimensions 0.6 cm high (0.6° of visual angle) and approximately 2 cm wide (2° of visual angle).
2.4. Procedure
The procedure was identical to that reported by Sharma et al. (2001). The task involved presenting a single colour-word at the centre of a white coloured video screen. Each stimulus remained on the screen until a response was made. Following the participants' response the next stimulus was presented immediately.
Participants were introduced to the task as a colour perception task. They were instructed to ignore the words and make a key-press response to the colour of the ink as quickly and as accurately as possible. If any errors were made they were asked not to correct themselves. Before conducting the experiment all participants were given two practice sessions involving 100 repeated letter strings (e.g. XXXX). A short break was given between each of the two practice sessions and the beginning of the experimental session.
Prior to the experimental session participants were informed that real words were going to be presented but were not informed of the nature of these words. All responses were made using one of four buttons by positioning the index and middle fingers from each hand on top of each of the buttons. Each button was labelled with one of four words written in black ink, BLUE, BROWN, RED and GREEN. Half the participants received the red and green labels on the left hand and the blue and brown labels on the right hand and the other half in reverse order.
After completing the alcohol Stroop task participants were asked to complete two questionnaires. The AUDIT (Saunders, Aasland, Babor, De La Feunte, & Grant, 1993) was used to measure harmful drinking. Because previous work has shown that state anxiety and trait anxiety to be associated with increased interference (Williams et al., 1996) the State–Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) was also presented.
3. Results
3.1. Analysis of response latencies
The mean correct reaction times (RT) were analysed using a 4-way ANOVA with Exposure Group (low, high) and Consumption Group (low, high) as between-participant factors and Word Type (alcohol, neutral) and Block (1–5) as within-participant factors. Results showed that the colour identification of alcohol words (1031 ms) took longer than neutral words (981 ms), F(1,39) = 9.97, p < .003, Eta2 = .87. A significant Word Type × Exposure Group interaction showed increased interference scores (RT alcohol–RT neutral) for the high exposure group compared to the low exposure group, F(1,39) = 4.36, p < .05, Eta2 = .53 (see Table 1).
| Exposure Group | Word Type | Interference score (alcohol–neutral) | |
|---|---|---|---|
| Neutral | Alcohol | ||
| Low exposure | 1006.57 (27.58) | 1020.19 (31.97) | 13.62 (17.38) |
| High exposure | 966.44 (35.51) | 1052.91 (42.87) | 86.47 (25.44) |
| Low consumption | 1009.02 (27.02) | 959.82 (36.65) | 50.88 (22.30) |
| High consumption | 952.80 (75.78) | 1026.58 (62.63) | 50.81 (24.51) |
| Total | 984.93 (22.76) | 1035.80 (26.77) | 50.87 (14.99) |
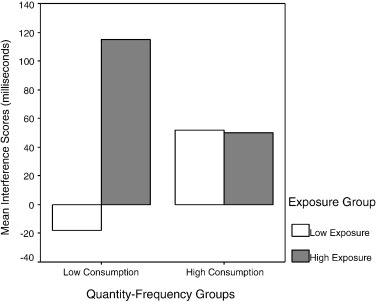
In addition a Word Type × Exposure Group × Consumption Group interaction was found, F(1,39) = 4.62, p < .04, Eta2 = .55. To explore this finding interference scores for the Exposure Group × Consumption Group interaction term were calculated and included in a two-way ANOVA. This interaction was shown to be significant, F(1,39) = 4.62, p < .03, Eta2 = .55 (see Fig. 1 and Table 2). Simple effects analyses within the low consumption group showed increased interference for the high exposure group relative to the low exposure group, F(1,20) = 12.19, p < .01, Eta2 = .91. No effect was shown within the high consumption group by exposure, F(1,19) = .002, p > .05, Eta2 = .05. In addition, the simple effect within the low exposure group showed increased interference for the high consumption group, F(1,20) = 4.75, p < .05, Eta2 = .55, while the simple main effect of consumption was not significant in the high exposure group, F(1, 19) = 1.41, p > .05, Eta2 = .02. Moreover, within the high consumption group the magnitude of the overall interference was shown to be significant, one-sample t(20) = 2.07, p < .05.
|
|
|
Fig. 1. Mean correct interference scores (alcohol RT–neutral RT) for consumption groups and exposure groups. |
| Exposure–Consumption Group | Word Type | Interference score (alcohol–neutral) | |
|---|---|---|---|
| Neutral | Alcohol | ||
| Low consumption–low exposure (N = 12) | 1027.01 (44.73) | 1008.78 (52.49) | − 18.23 (29.62) |
| Low consumption–high exposure (N = 10) | 975.90 (48.95) | 1090.62 (57.68) | 114.72 (32.43) |
| High consumption–low exposure (N = 10) | 982.06 (49.00) | 1033.89 (57.50) | 51.83 (32.44) |
| High consumption–high exposure (N = 11) | 939.61 (46.72) | 989.49 (54.82) | 49.88 (30.93) |
In addition, there was no significant interaction between Exposure Group, Consumption Group, Word Type and Block showing that there was no increase or decrease in interference scores with repeated exposure to alcohol words for either high or low consumption groups or high and low exposure groups, F(4, 156) = .233, p = .92, Eta2 = .10. All other main and interaction effects were not significant (ps > .05).
3.2. Analysis of errors
Errors were not subjected to inferential statistics since very few were made. The error rates were: High exposure group (alcohol words = 1.9%, neutral words = 1.8%); low exposure group (alcohol words = 2.7%, neutral words = 1.6%); high consumption group (alcohol words = 1.8%, neutral words = 1.5%); low consumption group (alcohol words = 2.5%, neutral words = 1.5%).
3.3. Analysis of AUDIT, state anxiety and trait anxiety
AUDIT, state anxiety and trait anxiety scores were analysed using 2-way ANOVAs with Exposure Group (low, high) and Consumption Group (low, high) as between-participant factors. Results showed no differences between low exposure and high exposure for AUDIT score, F(1, 39) = .36, p = .55, Eta2 = .09, state anxiety, F(1, 39) = .04, p = .85, Eta2 = .05, or trait anxiety, F(1, 39) = .63, p = .43, Eta2 = .12. Low consumption and high consumption groups were also not shown to differ for state anxiety, F(1, 39) = .01, p = .92, Eta2 = .05, or trait anxiety, F(1, 41) = .012, p = .913, Eta2 = .05. The finding that AUDIT scores differed between consumption groups, F(1, 39) = 30.35, p < .001, Eta2 = 1.00, confirms the differentiation in terms of a median split adopted for this factor (see Table 3). All interaction effects were not significant (ps > .05).
| Exposure–Consumption Group | Questionnaire measures | ||
|---|---|---|---|
| AUDIT | State anxiety | Trait anxiety | |
| Low consumption–low exposure | 6.42 (1.28) | 35.25 (3.29) | 42.42 (3.35) |
| Low consumption–high exposure | 5.70 (1.41) | 41.10 (3.56) | 44.00 (3.69) |
| High consumption–low exposure | 14.00 (1.42) | 40.10 (3.60) | 47.20 (3.67) |
| High consumption–high exposure | 13.09 (1.34) | 35.55 (3.44) | 40.00 (3.50) |
4. Discussion
Results of this study demonstrate an attentional bias towards alcohol cues amongst heavy non-problem social drinkers. It is interesting to note that the group of non-problem drinkers in the present sample had AUDIT scores comparable to the non-problem drinkers reported in Sharma et al. (2001). This replicates findings reported by Sharma et al. (2001) and supports the view that there is a sub-group of social drinkers for whom a semantic network related to alcohol can be activated automatically. The purpose of this study was to test a frequency of exposure hypothesis, which suggests that mere exposure to alcohol-related cues might be sufficient to generate an attentional bias.
We tested this hypothesis in two ways. We first asked the question, does mere exposure to alcohol related cues over time (i.e. across experimental blocks) in the modified Stroop task produce increased interference from alcohol words. If this were the case the argument would be that repeated exposure to alcohol stimuli creates such interference. Our results showed this not to be the case. There was no evidence that interference significantly increased with repeated exposure to alcohol stimuli during the experiment and supports earlier findings (Sharma et al., 2001 ; Williams et al., 1996).
We then examined whether there was greater interference from alcohol-related stimuli in a group of individuals who are exposed more frequently to an environment rich in alcohol-related cues, operationalised here as number of hours spent in bars/pubs/nightclubs per week. Results showed that this measure of frequency of exposure interacted with drinker type to produce greater interference from alcohol words. The pattern of results indicates that frequency of exposure per se is not important in understanding attentional disruption from alcohol stimuli amongst social drinkers. Amongst the heavy social drinkers, there was no difference between those in the high and low exposure groups. This finding is consistent with other evidence not showing an increase in interference with increased exposure (McCusker and Gettings, 1997 ; Sharma et al., 2001). However, we found that light drinkers who are exposed to environmental cues through spending more time in a bar showed significantly greater interference than those who had not been exposed to such cues. This group of light drinking, high exposure participants demonstrated a statistically equivalent interference from alcohol related stimuli to the heavy drinkers. It seems therefore that the role of environmental cue exposure for attentional disruption is especially important for those individuals who do not consume much alcohol.
The question now becomes, why should this be the case? One possible answer concerns the nature of the cues to which a person is exposed. Drinking behaviour and spending time in a bar differ in as much as the former can be seen as involving a person actively manipulating alcohol-related cues, while the latter can be seen as a person being the passive recipient of alcohol-related cues. Amongst the heavy drinkers, it seems that the active engagement with the alcohol-related cues in their environment (i.e. drinking) is sufficient to lead to the development of an attentional bias. The finding that increased passive exposure to such cues did not lead to increased interference amongst this group suggests a threshold model, whereby these individuals are less sensitive to passive environmental cues. This is supported by the differential levels of interference observed amongst light drinkers who were passively exposed to alcohol-related cues. Previous research has demonstrated that the active engagement in drinking is not sufficient to produce interference effects amongst this group (Sharma et al., 2001; Townshend & Duka, 2001). The implication of the present findings is that, for light drinkers, passive environmental cue exposure can lead to the development of attentional biases for alcohol-related cues. Importantly, passive and active exposure does not seem to produce a cumulative increase in interference.
As this study reports findings from a relatively small sample, future research should explore the role of passive exposure and active drinking engagement to further understand this effect. In addition, while passive exposure was operationalised here as the number of weekly hours spent in bars and nightclubs, it would be useful to examine the nature of this exposure — for instance, whether individuals are working or socialising in these environments. Previous research exploring attentional biases amongst non-drinkers demonstrated that such biases can be detected amongst individuals who abstain for religious reasons (Moss et al., 2012), so it would be interesting to examine whether groups of individuals such as healthcare professionals – who are frequently exposed to the negative consequences of alcohol – develop biases which differ from other professionals such as bar staff. This kind of further study will add to our understanding of the nature of attentional biases in this field, in terms of the extent to which they directly or indirectly motivate prospective drinking behaviour.
Author disclosures
The authors of this manuscript have no conflicts of interest to declare. No funding was received for the conduct of this research.
IA, DS and SN designed the study described in this manuscript. IA and DF conducted the analysis. IA and AM produced the final draft of the manuscript.
References
- Albery et al., 2006 I.P. Albery, D. Sharma, A. Niazi, A.C. Moss; Theoretical perspectives and approaches; M. Munafò, I.P. Albery (Eds.), Cognition and addiction, Oxford University Press, Oxford (2006), pp. 1–29
- Attwood et al., 2008 A.S. Attwood, H. O'Sullivan, U. Leonards, B. Mackintosh, M. Munafò; Attentional bias training and cue reactivity in cigarette smokers; Addiction, 103 (2008), pp. 1875–1882
- Bauer and Cox, 1998 D. Bauer, W.M. Cox; Alcohol-related words are distracting to both alcohol abusers and non-abusers in the Stroop color-naming task; Addiction, 93 (1998), pp. 1539–1542
- Bruce and Jones, 2004 G. Bruce, B. Jones; A pictorial Stroop paradigm reveals an alcohol attentional bias in heavier as compared to lighter social drinkers; Journal of Psychopharmacology, 18 (2004), pp. 527–533
- Bruce and Jones, 2006 G. Bruce, B. Jones; Methods, measures, and findings of attentional bias in substance use, abuse, and dependence; R.W. Wiers, A.W. Stacy (Eds.), Handbook of implicit cognition and addiction (2006), pp. 135–150
- Cane et al., 2009 J.E. Cane, D. Sharma, I.P. Albery; The addiction Stroop task: examining the fast and slow effects of smoking and marijuana-related cues; Journal of Psychopharmacology, 23 (2009), pp. 510–519
- Cox et al., 2003 W.M. Cox, M.A. Brown, L.J. Rowlands; The effects of alcohol cue exposure on non-dependent drinkers' attentional bias for alcohol-related stimuli; Alcohol and Alcoholism, 38 (2003), pp. 45–49
- Cox et al., 2006 W.M. Cox, J.S. Fadardi, E.M. Pothos; The addiction-Stroop test: theoretical considerations and procedural recommendations; Psychological Bulletin, 132 (2006), pp. 443–476
- Cox et al., 1999 W.M. Cox, G.N. Yeates, C.M. Regan; Effects of alcohol cues on cognitive processing in heavy and light drinkers; Drug and Alcohol Dependence, 55 (1999), pp. 85–89
- Field, 2006 M. Field; Attentional biases in drug abuse and addiction: cognitive mechanisms, causes, consequences and implications; M. Munafò, I.P. Albery (Eds.), Cognition and addiction, Oxford University Press, Oxford (2006), pp. 73–99
- Field et al., 2005 M. Field, K. Mogg, B.P. Bradley; Craving and cognitive biases for alcohol cues in social drinkers; Alcohol and Alcoholism, 40 (2005), pp. 504–510
- Field et al., 2004 M. Field, K. Mogg, J. Zetteler, B.P. Bradley; Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention; Psychopharmacology, 176 (2004), pp. 88–93
- Field et al., 2009 M. Field, M. Munafo, I.H.A. Franken; A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse; Psychological Bulletin, 35 (2009), pp. 589–607
- Franken, 2003 I.H.A. Franken; Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches; Progress in Neuropsychopharmacology and Biological Psychiatry, 27 (2003), pp. 563–579
- Fromberger et al., 2012 P.J. Fromberger, K. Herder, J. Steinkrauss, H. Nemetschek, R. Stolpmann, G. Müller, J. Leo; Initial orienting towards sexually relevant stimuli: preliminary evidence from eye movement measures; Archives of Sexual Behavior, 41 (2012), pp. 919–928
- Ingjaldsson et al., 2003a J.T. Ingjaldsson, J.F. Thayer, J.C. Laberg; Craving for alcohol and pre-attentive processing of alcohol stimuli; International Journal of Psychophysiology, 49 (2003), pp. 29–39
- Ingjaldsson et al., 2003b J.T. Ingjaldsson, J.F. Thayer, J.C. Laberg; Preattentive processing of alcohol stimuli; Scandinavian Journal of Psychology, 44 (2003), pp. 161–165
- Jones et al., 2003 B.T. Jones, B.C. Jones, H. Smith, N. Copley; A flicker paradigm for inducing change blindness reveals alcohol and cannabis information processing bias in social users; Addiction, 98 (2003), pp. 235–244
- Kucera and Francis, 1967 H. Kucera, W. Francis (Eds.), Computational analysis of present-day American English, Brown University Press, Providence (1967)
- Leung and McCusker, 1999 K.S. Leung, C.G. McCusker; Accessibility and availability of smoking-related associations in smokers; Addiction Research, 7 (1999), pp. 213–226
- Marissen et al., 2006 M.A.E. Marissen, I.H.A. Franken, A.J. Waters, P. Blanken, W. van den Brink, V.M. Hendriks; Attentional bias predicts relapse following treatment; Addiction, 101 (2006), pp. 1306–1312
- McCusker, 2001 C.G. McCusker; Cognitive biases and addiction: an evolution in theory and method; Addiction, 96 (2001), pp. 47–56
- McCusker, 2006 C.G. McCusker; Towards understanding loss of control: an automatic network theory of addictive behaviours; M. Munafò, I.P. Albery (Eds.), Cognition and addiction, Oxford University Press, Oxford (2006), pp. 117–145
- McCusker and Gettings, 1997 C.G. McCusker, B. Gettings; Automaticity of cognitive biases in addictive behaviours: further evidence with gamblers; British Journal of Clinical Psychology, 36 (1997), pp. 543–554
- McKenna and Sharma, 1995 F.P. McKenna, D. Sharma; Intrusive cognitions: an investigation of the emotional Stroop task; Journal of Experimental Psychology: Learning, Memory and Cognition, 21 (1995), pp. 1595–1607
- Moss and Albery, 2009 A.C. Moss, I.P. Albery; A dual process model of the alcohol–behaviour link for social drinking; Psychological Bulletin, 135 (2009), pp. 516–530
- Moss et al., 2011 A.C. Moss, I.P. Albery, D. Sharma; Development of a repeated-measures affective change blindness task; Behavior Research Methods, 43 (2011), pp. 826–833
- Moss et al., 2012 A.C. Moss, I.P. Albery, I. Siddiqui, N. Rycroft; Attentional bias for alcohol-related stimuli among belief based and non-belief based non-drinkers; European Addiction Research, 19 (6) (2012), pp. 299–302
- Munafò et al., 2003 M. Munafò, K. Mogg, S. Roberts, B.P. Bradley; Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task; Journal of Psychopharmacology, 17 (2003), pp. 311–317
- Pothos and Cox, 2002 E.M. Pothos, M. Cox; Cognitive bias for alcohol-related information in inferential processes; Drug and Alcohol Dependence, 66 (2002), pp. 235–241
- Robinson and Berridge, 1993 T.E. Robinson, K.C. Berridge; The neural basis of drug craving: an incentive-sensitization theory of addiction; Brain research reviews, 18 (3) (1993), pp. 247–291
- Saunders et al., 1993 J.B. Saunders, O.G. Aasland, T.F. Babor, J.R. De La Feunte, M. Grant; Development of the Alcohol Use Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption: II; Addiction, 88 (1993), pp. 791–804
- Schoenmakers et al., 2007 T. Schoenmakers, R.W. Wiers, B.T. Jones, G. Brice, A.T.M. Jansen; Attentional retraining decreases attentional bias in heavy drinkers without generalization; Addiction, 102 (2007), pp. 399–405
- Sharma et al., 2001 D. Sharma, I.P. Albery, C. Cook; Selective attentional bias to alcohol-related stimuli in problem drinkers and non-problem drinkers; Addiction, 96 (2001), pp. 285–295
- Spielberger et al., 1983 C.D. Spielberger, R.L. Gorsuch, R. Lushene, P. Vagg, G.A. Jacobs (Eds.), Manual for the State–Trait Anxiety Inventory, Consulting Psychological Press, Palo Alto, CA (1983)
- Stacy, 1995 A.W. Stacy; Memory association and ambiguous cues in models of alcohol and marijuana use; Experimental and Clinical Psychopharmacology, 3 (1995), pp. 183–194
- Stacy, 1997 A.W. Stacy; Memory activation and expectancy as prospective predictors of alcohol and marijuana use; Journal of Abnormal Psychology, 106 (1997), pp. 61–73
- Stacy and Weirs, 2006 A.W. Stacy, R.W. Weirs; An implicit cognition, associative memory framework for addiction; M. Munafò, I.P. Albery (Eds.), Cognition and addiction, Oxford University Press, Oxford (2006), pp. 31–71
- Stroop, 1935 J.R. Stroop; Studies of interference in serial verbal reactions; Journal of Experimental Psychology, 18 (1935), pp. 643–662
- Tiffany, 1990 S.T. Tiffany; A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes; Psychological Review, 97 (1990), pp. 147–168
- Townsend and Duka, 2001 J.M. Townshend, T. Duka; Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers; (2001)
- Waters and Leventhal, 2006 A.J. Waters, A.M. Leventhal; Clinical relevance of implicit cognition in addiction; M. Munafò, I.P. Albery (Eds.), Cognition and addiction, Oxford University Press, Oxford (2006), pp. 249–277
- Weinstein and Cox, 2006 A. Weinstein, W.M. Cox; Cognitive processing of drug-related stimuli: the role of memory and attention; Journal of Psychopharmacology, 20 (2006), pp. 850–859
- Wiers et al., 2006 R.W. Wiers, K. Houben, F.T.Y. Smulders, P.J. Conrod, B.T. Jones; To drink or not to drink: the role of automatic and controlled cognitive processes in the etiology of alcohol-related problems; R.W. Wiers, A.W. Stacy (Eds.), Handbook of implicit cognition and addiction (2006), pp. 339–361
- Williams et al., 1996 J.M.G. Williams, A. Mathews, C. Macleod; The emotional Stroop task and psychopathology; Psychological Bulletin, 120 (1996), pp. 3–24
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?