Abstract
Background
Fragmented QRS (FQRS) in 12 lead ECG was recently correlated with various outcomes in ischemic and non-ischemic heart disease. We studied the relationship between FQRS and ejection fraction (EF) in heart failure patients with QRS < 120 ms.
Methods
Medical records and echocardiograms of 339 patients admitted with CHF were reviewed. ECGs were read twice by a reader blinded to all data.
Results
70 patients with wide QRS were excluded; 63 patients had FQRS and 206 patients did not have FQRS. FQRS group were more likely to be black (OR = 2.17; p = 0.0093), and diabetic (OR = 1.79; p = 0.0451). ROC curve analysis revealed a significant relationship between EF and FQRS (p = 0.002). At EF of 48%, OR for FQRS was 4.36 (95% CI: 2.1–9.05; p < .0001). Adjustment for race and diabetes did not change the OR, or confidence intervals (Adjusted OR for race: 4.08 (95% CI: 1.06–15.67; p = 0.04); for diabetes: 4.13 (95% CI: 1.46–11.69; p = 0.008)). There was a significant difference in EF between patients with FQRS involving ≥ 2 ECG areas and non-FQRS group (p < 0.05), but not between patients with ≥ 2 vs. one area, or 1 area vs. non-FQRS.
Conclusion
In heart failure patients with QRS < 120 ms, FQRS was observed more frequently in persons of black race and in diabetics and was associated with lower EF. This was mainly seen in patients with FQRS involving ≥ 2 ECG areas.
Keywords
Congestive heart failure;Fragmented QRS;Ejection fraction;ECG
1. Introduction
Nearly twenty-five years ago, fragmented QRS (FQRS) electrocardiograms were for the first time recorded in bipolar leads from canine hearts 15 days after experimentally induced acute ischemia. In that study, FQRS correlated with widely separated myofibrils with distorted orientation leading to slow and inhomogeneous activation [1]. Recent studies have confirmed the association between FQRS and myocardial scar [2].
FQRS has also been reported in a variety of cardiac conditions such as ischemic and dilated cardiomyopathy, sarcoidosis, myocarditis, arrhythmogenic ventricular dysplasia [3], and Brugada syndrome. FQRS was found to be an independent risk factor for increased cardiac events after Q wave Myocardial infarction [4] and arrhythmic events in Brugada syndrome [5]. In ischemic and dilated cardiomyopathy patients with Implantable cardioverter defibrillator (ICD), FQRS was associated with increased risk of arrhythmic events and was noticed to be associated with lower ejection fraction (EF) [6].
In this study, we sought to examine the relationship of FQRS and EF in a group of subjects admitted to our institution with congestive heart failure (CHF) without QRS widening, i.e., with a QRS duration < 120 ms.
2. Methods
2.1. Protocol and setting
We retrospectively reviewed records of patients admitted to a 500-bed tertiary-care medical center in northern New Jersey (USA) to determine if FQRS is associated with a decrease in EF. Our cohort included all patients with a diagnosis of CHF, defined as known history of heart failure diagnosis at admission or presentation with symptoms and signs of heart failure and lung congestion on chest x-ray as assessed by an emergency physician, over the two-year period between August 1, 2006 and July 31, 2008, inclusive and a QRS duration < 120 ms. The study was approved by the Institutional Review Board of Trinitas Regional Medical Center, where the study was conducted.
The cohort was bifurcated into those subjects with a non-fragmented QRS and those with FQRS, defined as follows: the presence of RSR′ patterns which include an additional R wave (R prime) or notching of the R wave or notching of the S wave, or the presence of more than two R primes (fragmentation) in two contiguous leads in anterior leads (V1–V5), lateral leads (I, aVL, V6), or inferior leads (II, III, aVF). QRS duration was determined by the longest QRS in any lead. ECGs were analyzed visually without magnification by readers blinded to other data. ECGs were read twice and FQRS was considered present if the two readings agreed.
Baseline data gathered were: age, gender, race (based on data in the medical record) cause of heart failure, diabetes mellitus (DM), defined as patient with previous diagnosis of diabetes or history of oral hypoglycemic drugs or insulin or hemoglobin A1c > 6.5%., hypertension (HTN), defined as documented HTN on admission, or history of antihypertensive medications for no other reasons, ischemic heart disease (IHD), defined as documented history ischemic heart disease or a history coronary artery bypass graft surgery (CABG), coronary stent placement, or cardiac catheterization with at least one lesion > 70% stenosis in coronary arteries, dyslipidemia defined as a documented history of statin or fibrate use, or a serum cholesterol > 200 in fasting samples, body mass index (BMI), chronic kidney disease (CKD); defined as GFR consistently below 60 ml/min using MDRD formula documented in previous hospital or emergency visits, smoking (patient was considered to be a smoker if actively smoking or if patient smoked within the past ten years).
We also attempted to determine the etiology of heart failure from medical records and cardiology consults. Heart failure was considered due to ischemic cardiomyopathy if the patient has a history of established IHD defined as above, HTN was considered if IHD was not present and the patient has longstanding uncontrolled HTN. Dilated cardiomyopathy was defined as heart failure with dilated ventricles on echocardiogram with EF < 50%, normal coronaries (no lesions with > 70% stenosis) in angiography, and the patient has no history of longstanding uncontrolled HTN, alcohol, or cocaine abuse. Alcohol or cocaine were considered the cause of CHF if no other cause was found, and the patient has a history of abuse to these substances.
2.2. Statistical analysis
For this study, α was set at 0.05, thus, a p-value < 0.05 (two-sided) was considered statistically significant. Interval data was tested for normality using the D'Agostino-Pearson omnibus normality test. Because most variables were not normally distributed, non-parametric inferential methods were used (Mann–Whitney test for two-group comparisons; Kruskal–Wallis, with Dunns test, post-hoc for comparison of > 2 groups). Data are expressed as medians and inter-quartile ranges (IQR). Decision levels (cut-offs) to dichotomize interval variables were determined by receiver operating characteristic (ROC) curves. Categorical variables were evaluated by Fishers exact test; because of the retrospective nature of the study, odds ratios (OR) and 95% confidence intervals (CI) are provided. Logistic regression was used to adjust OR for differences in baseline characteristics if the p-value for the difference between the groups was < 0.10. Data were analyzed using Prism® software (GraphPad Corp., San Diego CA) except for logistic regression which was performed using an online routine www.statpages.org/logistic.html (last accessed Dec. 1, 2009).
3. Results
We reviewed the medical records of 339 patients; of these 70 patients were excluded as follows: 3 patients had no echocardiogram, 11 had right bundle branch block, 13 had left bundle branch block, 16 had intraventricular conduction defects and 27 had ventricular paced rhythm 27. We also included 10 ECGs that contained QRS complexes that were read once as fragmented and once as non-fragmented; these were eventually classified as non-FQRS as per protocol. The final analysis included 269 patients, 63 patients (35 men, average age 69.3 years) were found to have FQRS in two or more contiguous leads in one or more ECG lead sets, FQRS was not present in two contiguous leads in 206 patients (100 men, average age 71.8 years).
FQRS was seen in inferior lead set, anterior lead set, and lateral lead set in 73%, 38%, 38% of ECGs, respectively. FQRS involved one ECG lead set in 56% of cases, 2 sets in 41%, and 3 sets in 3% of cases.
The differences in baseline characteristics are shown in Table 1. Black patients were found more frequently in FQRS group (46% black in FQRS group vs. 28% in non-FQRS patients, statistically significant p = .009). In addition; FQRS patients have higher prevalence of diabetes (58% vs. 44% in non-FQRS group; p = 0.045). Because the study included patients who were admitted to the hospital for CHF, NYHA classification – determined usually during stable outpatient follow-up visits – could not be obtained. Other differences in baseline characteristics were not statistically significant at the predetermined level and were, thus, not evaluated as potential confounders.
| Baseline characteristics | FQRS group (63 patients) | Non-FQRS group (206 patients) | p value |
|---|---|---|---|
| Age (median with IQI) | 71.0 (56.3–81.8) | 74.0 (60.0–83.0) | 0.11 |
| Gender (males) | 35 (56%) | 100(49%) | 0.39 |
| Race (blacks) | 29 (46%) | 58 (28%) | 0.009* |
| BMI (median with IQI) | 28.6(24.6–34.6) | 27.5 (24.2–32.9) | 0.15 |
| Ischemic heart disease | 37 (59%) | 110 (53%) | 0.48 |
| Diabetes | 37 (59%) | 91 (44%) | 0.045* |
| Hypertension | 59 (93%) | 187(91%) | 0.61 |
| Dyslipidemia | 37 (59%) | 106(51%) | 0.39 |
| Chronic kidney disease | 32 (51%) | 113(55%) | 0.67 |
| Smoking | 32(51%) | 83 (40%) | 0.15 |
| EF (median with IQI) | 35.0% (30.0–42.0) | 45% (30.0–60.0) | 0.0017* |
| Cause of heart failure | 0.8 | ||
| Ischemic cardiomyopathy | 37(59%) | 110(53%) | |
| Hypertension | 20 (32%) | 78(38%) | |
| Substance abuse | 3 (5%) | 11(5%) | |
| Others | 3(5%) | 7(3%) |
IQI: interquartile intervals.
Regarding the cause of heart failure in both groups: ischemic heart disease was the most common cause of heart failure in both groups (58.7% in FQRS vs. 53.4% in non-FQRS, p = 0.8) followed by HTN (31.7% vs. 37.8%, p = 0.8), idiopathic dilated cardiomyopathy was not presented appreciably in this cohort (5 patients only).
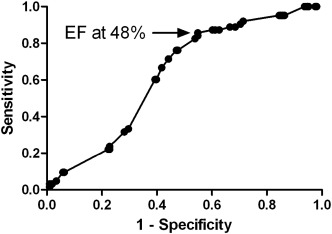
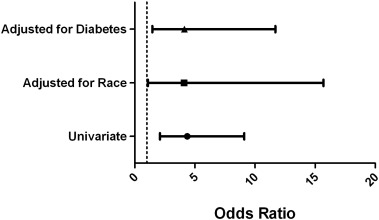
To study the relation between FQRS and EF, we used ROC curve analysis to determine the cut-off; the optimum decision level occurred at 48% (Fig. 1). At this point, the OR for FQRS was 4.36 (95% CI: 2.1–9.05; p < .0001). In logistic regression analysis (Fig. 2), the differences in baseline characteristics (black race and diabetes) considered as potential confounders, did not appreciably change the OR, or confidence intervals (Adjusted OR for race: 4.08 (95% CI: 1.06–15.67; p = 0.04); Adjusted OR for DM: 4.13 (95% CI: 1.46–11.69; p = 0.008)).
|
|
|
Fig. 1. ROC curve for the relation between FQRS and EF. Cut-off at 48% is indicated. |
|
|
|
Fig. 2. Forrest plot of univariate OR obtained at ejection fraction of 48% and ORs adjusted for race and diabetes. |
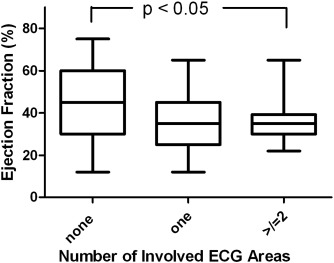
We sought to evaluate differences in EF based upon the number of ECG areas involved. These data are provided in Fig. 3. We observed a statistically significant difference (p = 0.006) among the groups. In the post-hoc analysis there was a significant difference in EF between patients with FQRS involving ≥ 2 ECG lead sets and patients with no FQRS (p < 0.05). This difference was not found between patients with ≥ 2 vs. one ECG sets, or 1 ECG lead set vs. non-FQRS.
|
|
|
Fig. 3. Comparison of number of involved ECG areas and ejection fraction. Overall p-value by the Kruskal–Wallis test was 0.006; p-value shown is for comparison of no areas vs. ≥ 2 areas. |
4. Discussion
Our analysis showed that FQRS is associated with lower ejection fraction in all CHF patients admitted to the hospital with QRS < 120 ms (36% vs. 44% in non-FQRS).The odds ratio of having EF < 48% for FQRS patients is approximately four times than that for patients without FQRS, even after adjustment for two potential confounders, race and diabetes.
The association of lower ejection fraction with FQRS was seen in previous studies with different populations. In a study of 128 patients whose expanded electrocardiographs were recorded by high fidelity, high speed instruments, Flowers et al. demonstrated a correlation between ventricular enlargement documented postmortem and the appearance of slurring or notching of the expanded QRS waves. The numbers of notches were comparable between patients with infracted hearts and those with ventricular enlargement alone [7]. Slurring and broad notching in wide premature ventricular contractions (QRS duration > 160 ms), were also simple and reliable markers of dilated globally hypokinetic left ventricle in another study [8]. Another study included patients referred for nuclear perfusion scan in a cardiac center, and showed that the patients with FQRS had lower ejection fraction [4]. Recently, Das et al. in his study of FQRS and arrhythmic events in a population of dilated and ischemic cardiomyopathy with AICD (EF < 40% was part of the inclusion criteria) lower ejection fraction was again seen in patients with FQRS [6].
In a study by Mahenthiran et al. FQRS pattern sensitivity was 75% and specificity was 94% for a corresponding regional scar by myocardial perfusion imaging (MPI) [9]. SPECT analysis showed similar correlations (sensitivity of 85%, specificity > 97% in anterior segment, 90–92% in lateral and inferior segments) [2]. Stein Ørn et al. studied long term survivors of myocardial infarction using cardiac MRI, and showed a strong linear relation between scar size and LV ejection fraction (r: − 0.74, p < 0.0001). Anterior scars were associated with lower ejection fraction than non-anterior scars (43% vs. 51%, p = 0.01)[10].
Diabetes was also seen more frequently in our FQRS group in comparison to non-FQRS. This association was seen before in a study that included patients with fragmented wide QRS undergoing cardiac evaluation in comparison to patients with wide non-FQRS, but not in other FQRS studies[11]. In addition; race was a confounding factor in this study which revealed that FQRS are more prevalent in blacks admitted with CHF. To the best of the authors' knowledge, most – if not all – studies about FQRS did not look at race as part of the demographic characteristics.
In our post-hoc analysis, finding of FQRS in ≥ 2 ECG areas was associated with lower ejection fraction – but not one area alone vs. non FQRS – could be attributed to high frequency of FQRS in inferior leads in our cohort, which means that in patients with one FQRS area, there is a higher chance for this area to be inferior rather than anterior. In this area, FQRS is less specific for a scar, and there is increased incidence of non-specific conduction abnormalities [2]. Furthermore, scars in this segment are less likely to affect the ejection fraction in comparison to anterior segments. Involvement of 2 areas means a higher chance of the anterior area of being included in the analysis and thus lower ejection fraction. We believe that our study is underpowered to detect a difference in EF between one and 2 area involvement. It would be interesting for further FQRS studies to look into the site and the number ECG area involvements to correlate with the studied outcome.
5. Limitations
The study limitations include retrospective design, dependence on the echocardiogram to measure the ejection fraction and absence of data regarding NYHA classification. In addition; dilated cardiomyopathy was not presented well in this cohort. Dilated cardiomyopathy patients may have been categorized under other groups if they had uncontrolled HTN or substance abuse.
6. Conclusion
In CHF patients with QRS < 120 ms, FQRS is more likely to be found in blacks and diabetics; and to be associated with lower EF. This was mainly seen in patients with FQRS involving ≥ 2 ECG areas.
Acknowledgments
None of the authors have any conflicts of interest, financial or other disclosures to acknowledge.
References
- [1] P.I. Gardner, P.C. Ursell, J.J. Fenoglio, A.L. Wit; Electrophysiologic and anatomic basis for fractionated electrocardiograms recorded from healed myocardial infarcts; Circulation, 72 (1985), pp. 596–611
- [2] M.K. Das, B. Khan, S. Jacob, A. Kumar, J. Mahenthiran; Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease; Circulation, 113 (2006), pp. 2495–2501
- [3] S. Peters, M. Trummel, B. Koehler; QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy; Heart Rhythm, 5 (2008), pp. 1417–1421
- [4] M.K. Das, C. Saha, H. El Masry, J. Peng, G. Dandamudi, J. Mahenthiran, P. McHenry, D.P. Zipes; Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease; Heart Rhythm, 4 (2007), pp. 1385–1392
- [5] H. Morita, K. Kengo, D. Miura, S. Nagase, K. Nakamura, S. Morita, T. Ohe, D.P. Zipes, J. Wu; Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome; Circulation, 118 (2008), pp. 1697–1704
- [6] M.K. Das, W. Maskoun, C. Shen, M. Michael, H. Suradi, M. Desai, R. Subbarao, B. Bhakta; Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and non-ischemic cardiomyopathy; Heart Rhythm, 1 (2010), pp. 74–80
- [7] N. Flowers, L. Horan, J.R. Thomas, W.J. Tolleson; The anatomic basis of high frequency components in the electrocardiogram; Circulation, 39 (1969), pp. 531–539
- [8] K.P. Moulton, T. Medcalf, R. Lazzara; Premature ventricular complex morphology: a marker for left ventricular structure and function; Circulation, 81 (1990), pp. 1245–1251
- [9] J. Mahenthiran, B.R. Khan, S.G. Sawada, M.K. Das; Fragmented QRS complexes not typical of a bundle branch block: a marker of greater myocardial perfusion tomography abnormalities in coronary artery disease; J. Nucl. Cardiol., 3 (2007), pp. 347–353
- [10] S. Ørn, C. Manhenke, I. Anand, I. Squire, E. Nagel, T. Edvardsen, K. Dickstein; Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction; Am. J. Cardiol., 99 (2007), pp. 1109–1114
- [11] M.K. Das, H. Suradi, W. Maskoun, M. Michael, C. Shen, J. Peng, G. Dandamudi, J. Mahenthiran, et al.; Fragmented wide QRS on a 12-lead ECG — a sign of myocardial scar and poor prognosis; Circ. Arrhythm. Electrophysiol., 1 (2008), pp. 258–268
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?