Keywords
Pulmonary vein thrombus;Autonomic nervous dysfunction;64-MDCT;CVRR;Dabigatran
Ischemic stroke is severe clinical problem, and preventing ischemic stroke is an important clinical target. A left atrial thrombus can cause an ischemic stroke [1]. In 2015, I reported that pulmonary vein thrombus (PVT) is a root cause of nearly all left atrial thrombi [2], suggesting a link between PVT and ischemic stroke. I published a case report demonstrating that an elderly patient with ischemic stroke had a PVT [3]. However, the relationship between PVT and the autonomic nervous system (ANS) remains unclear.
Cerebral microvascular occlusion leads to chronic cerebral hypoperfusion and consequent hypoxia. It may play a direct role in the pathogenesis of Alzheimers disease or the development of dementia. However, cerebral microvascular occlusion is an underappreciated mechanism of brain pathology. If prompt recanalization of the microvascular occlusion fails, brain circuits can be disrupted and significant functional deficits can occur [4].
In a previous report using mice, microemboli that occluded cerebral microvessels were extruded. After a complete recanalization, the emboli translocated outside the vessel lumen within two to seven days [5]. When microvessels are occluded by microclots, recanalization should also be performed in humans. However, the source of microclots is not yet fully understood.
Pulmonary vein thrombus (PVT) is a life-threatening condition. PVT has been reported to occur after lung cancer, pulmonary resection, lung transplantation, and radiofrequency ablation for atrial fibrillation (AF), but the condition is believed to be rare.
A 64-slice multidetector computed tomography (64-MDCT) can be used to evaluate coronary artery plaques. It allows for the simultaneous assessment of a left atrial appendage (LAA) thrombus, left atrium (LA) thrombus and PVT [6]. I have reported several case reports of PVT in elderly patients with chest pain using a 64-MDCT. Recently, I reported 61% (35 patients) of 57 elderly patients with chest pain had PVT, which indicates PVT is not uncommon [6]. However, few other cases of PVT have been reported. Therefore, the existence of PVT is not widely recognized. Although warfarin has been used as the first choice for patients with AF, dabigatran appears to be a more useful current option. I have reported cases in which dabigatran partially dissolved a PVT [6] ; [7].
The coefficient of variation of the R-wave to R-wave interval (CVRR) is a parameter of heart rate variability (HRV) that is easily obtained using a 24-hour Holter electrocardiogram (ECG), which can be performed non-invasively. CVRR is defined as the standard deviation of all R–R intervals divided by the mean of all R–R intervals, which is then multiplied by 100. Reduced CVRR is an index of sympathetic nerve predominance when the parasympathetic nerve is in a withdrawal state. Reduced CVRR has been reported to predict cardiovascular events, such as sudden death and myocardial infarction, in patients with coronary artery disease and in apparently healthy subjects [8].
This case report presents a patient with PVT who had a decreased CVRR value. This indicates that a PVT may occlude the microvessels of the nervous system and aggravate the ANS.
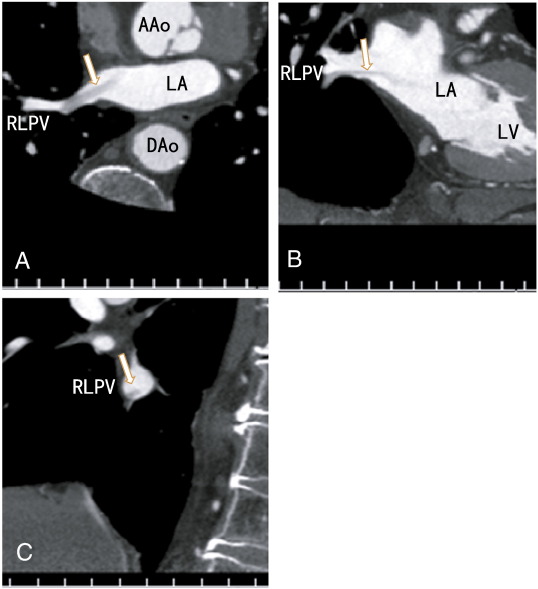
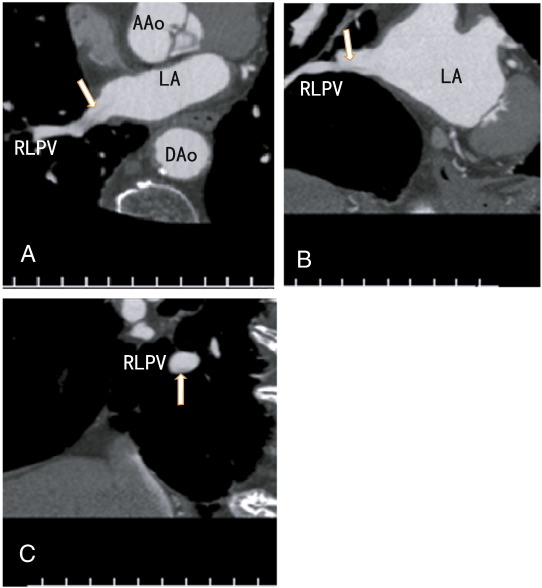
The patient was an 82-year-old male with hypertension and dyslipidemia. The patient presented with chest pain and was referred for a coronary artery assessment. The patients BMI was 22.8 kg/m2. ECG showed a complete right bundle branch block with no significant ST-T changes. The D-dimer level was less than 0.5 μg/ml (normal; < 1.0 μg/ml); the activity of protein S was 85% (normal; 60–150%), and the activity of protein C was 107% (normal; 64–146%). The patient was not treated with anticoagulants. The chest roentgenogram showed no lung cancer. The patient had no symptoms of cough, fever, cerebral infarction, or cancer. The volume of the LA was 22.1 ml. The CVRR value was decreased to 7 (normal range; 18 to 25 for his age), indicating ANS dysfunction. A 64-MDCT scan was performed and showed no coronary artery plaques. However the scan showed a thrombus in the right lower pulmonary vein in the axial (Fig. 1A), coronal (Fig. 1B) and sagittal planes (Fig. 1C). After three months of dabigatran therapy, the images became vague, fine, and clear (Fig. 2A to 2C), suggesting that the thrombus had mostly dissolved and that the contrast enhancements had penetrated the thrombus.
|
|
|
Fig. 1. A: Axial images showing a thrombus in the right lower pulmonary vein with no contrast enhancement (arrow). B: Coronial images showing a thrombus in the right lower pulmonary vein with no contrast enhancement (arrow). C: Sagittal images showing a thrombus in the right lower pulmonary vein attached to the inferior wall with no contrast enhancement (arrow). AAo: ascending aorta, DAo: descending aorta, LA: left atrium, LV: left ventricle, RLPV: right lower pulmonary vein.
|
|
|
|
Fig. 2. A: Axial images vaguely showing an apparently small thrombus in the right lower pulmonary vein with no contrast enhancement (arrow). B: Coronial images showing a decreased thrombus in the right lower pulmonary vein with no contrast enhancement (arrow); the thrombus appears as a fine needle. C: Sagittal images showing a small thrombus on the bottom of the right lower pulmonary vein (arrow). AAo: ascending aorta, DAo: descending aorta, LA: left atrium, RLPV: right lower pulmonary vein.
|
This case report is the first to describe a decreased CVRR in a patient with PVT. Dabigatran appeared to partially dissolve the thrombus. However, the images demonstrate remnants of the thrombus, which may be a fibrin network, after three months on the dabigatran therapy (Fig. 2A to 2C).
A PVT can release microclots that affect the ANS by occluding nervous system microvessels. Presently, there are some candidates for PVT indicators, such as age and total bilirubin [6]. A decreased CVRR may be used as a new indicator. A decreased CVRR is associated with sudden death and cardiac events [8], and it may be caused by occlusions of the cerebral or coronary arteries. These occlusions may be caused by clots that are released by a PVT.
ANS function and viability depend on suitable arterial blood flow through microvessels for adequate oxygen and glucose supply. Mechanisms likely evolved to ensure microvessel patency [5]. Microvessels are believed to be prone to occlusion by microemboli. Microclots released from a PVT can become microemboli. If revascularization of the occluded microvessels fails, then the occluded microvessels can cause hypoxia and undernourishment in the tissue and, thus, injure normal cell functions. Hypoxia and undernourishment result in a decrease of the production of ATP which causes mitochondria dysfunction. PVT is a potentially life-threatening condition that is often underdiagnosed and under-recognized. Basic suspicion is necessary to diagnose a PVT. Although I have published several cases of PVT in elderly patients with chest pain [2]; [3]; [6] ; [7] which suggests that PVT is not uncommon, little is presently known about the condition.
Hypoxia and undernourishment can activate transcription factors, including hypoxia inducible factors (HIFs), nuclear respiratory factor-1 (NRF-1), and metal response transcription factor-1 (MTF-1). HIFs activate several target genes and may activate functional non-coding RNAs. Glycolytic genes are well-known targets of HIFs. Glycolytic functional non-coding RNAs may also be targets. To maintain ATP production, these genetic factors change the energy production of the cell from oxidative phosphorylation to glycolysis. This energetic change results in a decrease in reactive oxygen species (ROS) production from the electron transport chain. ROS is a major source of metabolic damage to cells [9], which can disrupt ANS function.
I previously reported that PVT was dissolved by warfarin [3] and dabigatran [6] ; [7]. In this case, the thrombus was partially dissolved by dabigatran, and some arterial blood flow was restored. Warfarin and new anticoagulants, such as dabigatran, may prevent ischemic stroke in AF patients by dissolving the PVT and the connected left atrial thrombus. Further studies are needed to confirm this benefit.
Anticoagulants, such as warfarin or dabigatran, may partially dissolve a thrombus that is occluding ANS microvessels. Oxygen and glucose can then be restored to the brain. However, the brain cannot necessarily restore normal ANS function. A long-lasting hypoperfusion may change the quality of the ANS cells because of mitochondrial dysfunctions and the activation of HIFs, NRF-1, and MTF-1, which may affect cerebral function via genetic modulations, such as epigenetic changes, and result in ANS dysfunction, dementia, and Alzheimers disease.
The mechanism of PVT formation is not fully understood. Understanding the mechanism is important to prevent the injurious effects of PVT on many organs. Blood stasis and hypercoagulability are two crucial predisposing factors for the development of venous thrombosis. Physically demanding activities increase cardiac output and subsequent cardiac input. Daily body activity may inhibit PVT production by increasing cardiac input through the pulmonary veins, thereby preventing blood stasis in the pulmonary veins. Recently, Kim and their colleagues reported that TSC-1 inhibited chronic artery thrombus formation with no effects on acute thrombus formation [10]. The pulmonary vein includes arterial blood, and TSC-1 may be associated with pulmonary vein thrombus formation. More studies are needed to clarify this relationship. Understanding this relationship may lead to the development of new medicines for ANS dysfunction, dementia and Alzheimers disease.
In brief, for the first time, I reported on a patient with PVT and a decreased CVRR, indicating that a PVT aggravates the ANS. A decreased CVRR may be an indicator of PVT. Microclots released from a PVT may be a source of microemboli that occlude microvessels in all organs, including ANS microvessels. In occluded microvessels, hypoxia and low glucose levels can occur, which can injure normal cell function that lead to not only ANS dysfunction but also dementia and Alzheimers disease. Warfarin and dabigatran dissolved the PVT, indicating that they can inhibit ANS dysfunction by decreasing the release of microclots. A 64-MDCT scan is useful to evaluate a PVT and the effects of warfarin and dabigatran.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- [1] T. Watson, E. Shantsila, G.Y. Lip; Mechanisms of thrombogenesis in atrial fibrillation: Virchows triad revisited; Lancet, 373 (2009), pp. 155–166
- [2] H. Takeuchi; Nearly all left atrial thrombi may be extended from pulmonary vein thrombi; IJC Heart & Vasculature, 7 (2015), p. 9
- [3] H. Takeuchi; A thrombus of the right upper pulmonary vein: detection by the use of a 64-MDCT; BMJ Case Rep (Sep 14 2012) https://doi.org/10.1136/bcr.12.2011.5446 [Published online]
- [4] S.E. Vermeer, N.D. Prins, T. den Heijer, A. Hofman, P.J. Koudstaal, M.M. Breteler; Silent brain infarcts and the risk of dementia and cognitive decline; N Engl J Med, 348 (2003), pp. 1215–1222
- [5] C.K. Lam, T. Yoo, B. Hiner, Z. Liu, J. Grutzendler; Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization; Nature, 465 (7297) (2010), pp. 478–482
- [6] H. Takeuchi; High prevalence of pulmonary vein thrombi in elderly patients with chest pain, which has relationships with aging associated diseases; IJC Heart & Vessels, 4 (2014), pp. 129–134 https://doi.org/10.1016/j.ijchv.2014.05.006 [Published online: 6-JUN-2014]
- [7] H. Takeuchi; Chest pain caused by pulmonary vein thrombi could be curable by dabigatran; BMJ Case Rep (March 13 2014) https://doi.org/10.1126/bcr-2013-203186 [Published online:]
- [8] A. Algra, J.G. Tijssen, J.R. Roelandt, J. Pool, J. Lubsen; Heart rate variability from 24-hour electrocardiography and the 2-year risk for sudden death; Circulation, 88 (1993), pp. 180–185
- [9] G.L. Semenza; Hypoxia-inducible factors in physiology and medicine; Cell, 148 (2012), pp. 399–408
- [10] E.J. Kim, Y.J. Ahn, M.H. Kim, H.H. Kim; Basic cardiovascular sciences (BCVS); P160. Tuberosis sclerosis complex 1 mediated neointima formation and arterial thrombosis following vascular injury, Pris Las Vegas, Las Vegas, Nev, USA (July 14–17 2014)
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?